Abstract
Purpose
Evidence accumulates that an active lifestyle positively influences cancer treatment outcome. A “smartphone application” (app) such as “RunKeeper,” to self-monitor physical activity (PA) might be helpful. This study aimed to examine whether using RunKeeper to increase self-reported PA is feasible in cancer patients and to evaluate patients’ opinion about using RunKeeper in a 12-week program.
Methods
Adult patients (n = 32), diagnosed with cancer, were randomized between usual care (n = 16) or a 12-week intervention with instructions to self-monitor PA with RunKeeper (n = 16). Changes in PA were determined with the Physical Activity Scale for the Elderly (PASE) at baseline (T0), 6 weeks (T1), and 12 weeks (T2). Usability and patients’ experiences were tested at T2 with the System Usability Scale (SUS) and a semi-structured interview.
Results
Patient mean age was 33.6 years. Between T0 and T1, an increase in PA of 51% (medium estimated effect size r = 0.40) was found in PASE sum score in the intervention group compared with usual care. In addition, total minutes of PA increased with 46% (r = 0.37). These effects decreased over time (T2). Sedentary time decreased with 19% between T0 and T1 and 27% between T0 and T2. Usability was rated “good” and most patients found RunKeeper use helpful to improve PA.
Conclusions
Self-monitoring PA with RunKeeper was safe and feasible in cancer patients. The RunKeeper use resulted in an increase in PA after 6 weeks. RunKeeper usability was rated good and can be used to study PA in cancer patients.
Trial registration
Electronic supplementary material
The online version of this article (10.1007/s00520-018-4263-5) contains supplementary material, which is available to authorized users.
Keywords: Cancer, Physical activity, Smartphone application, Exercise, RunKeeper, Self-monitoring, Healthy lifestyle
Introduction
In the USA, it is estimated that approximately 60% of yearly cancer deaths are preventable [1, 2]. In the last decades, tobacco use, obesity, sun exposure, infectious agents, and diet are associated with the development of cancer [1, 2]. Approximately 30% of cancer deaths in the USA can be accredited to tobacco use [3], an additional one third by being overweight or obese in combination with physical inactivity and an unhealthy diet [2, 4, 5].
Therefore, a healthy lifestyle, e.g., smoking cessation, increasing physical activity (PA), protection against sun exposure, a healthy diet, can be considered as primary prevention of cancer [6, 7]. In addition, evidence accumulates that lifestyle influences cancer treatment outcome, and changes the recurrence rates of the disease [8]. Several studies indicate that weight gain after cancer treatment increases the risk of cancer recurrence and cancer-related mortality [4, 9]. Moreover, an increase in PA might decrease cancer-related mortality [10–12]. Maintaining PA during active cancer treatment appears to be safe and feasible, reduces cancer-related fatigue, limits weight gain, and increases quality of life (QoL) [13].
Unfortunately, cancer patients have a tendency to decrease their PA due to impaired cardiopulmonary function, diminishing skeletal muscles, and cancer-related fatigue [14, 15]. Over the last two decades, several studies examined whether PA programs could reduce or prevent these negative effects of cancer treatment [13, 16, 17]. PA programs have proven to enhance patients’ physical fitness, with improving QoL and reducing cancer-related fatigue [18, 19].
Studies among cancer patients and survivors regularly encounter difficulties in recruiting and retaining patients in PA programs [20, 21]. Furthermore, patients experience difficulties in remaining physically active after completion of participation in a PA program. This might be due to lack of motivation or coaching [22]. A smartphone application (app) may be a low-threshold tool to counteract these problems [23]. Universally, smartphones are widespread and incorporated in our daily living. Various studies that examined apps designed to self-monitor PA showed promising results in improving PA [24–27]. However, none of these studies examined an app to self-monitor PA in cancer patients.
Therefore, the primary aim of this study was to determine the feasibility of the RunKeeper app use on self-reported PA during and after cancer treatment in comparison with usual care in a 12-week follow-up. The secondary aim was to explore the usability and patients’ experiences of when using the RunKeeper app.
Methods
Participants
This feasibility study was conducted at the University Medical Center Groningen (UMCG) in Groningen, The Netherlands. From February until April 2015, adult cancer patients who received systemic cancer treatment with curative intent, or in the follow-up period (cancer survivors), were recruited and enrolled at the department of Medical Oncology. Eligibility criteria included patients diagnosed with cancer (regardless of type/staging) ≥ 18 years of age with a World Health Organization (WHO)-performance score ≤ 1. Patients were not eligible when they were unable to read or comprehend the Dutch language, unable to handle or were not in possession of a smartphone, diagnosed with a cardiac illness, psychiatric illness, severe kidney or liver impairment, pancytopenia or when they were participating in a supervised PA program.
RunKeeper app
The “RunKeeper app,” RunKeeper version 4.8.1, founded by Mr. Jason Jacobs, 2008, FitnessKeeper Inc. (RunKeeper) is a free, widely spread and well-known app for self-monitoring PA with over 26 million users on the smartphone so called platforms Android and iPhone’s iOS. In a recent review, RunKeeper was rated best on using behavioral change techniques to stimulate a healthy lifestyle [28].
Study procedure
Eligible patients were identified by the investigator who screened the medical records of patients from the Medical Oncology department of the UMCG. After a hospital visit, the investigator informed eligible patients with permission of their treating oncologist. Patients were given an information letter with an attached informed consent form in twofold and a pre-paid return envelope. When there was no response within 1 week, the investigator contacted the corresponding patient. After written informed consent was obtained, patients were equally divided by computerized randomization in a 1:1 ratio to “Group A,” intervention group or “Group B,” control group. Patients randomized in Group A received a brief user’s guide for the RunKeeper app use and were instructed to self-monitor PA (see Appendix).
Usual care and study intervention
In the UMCG, all patients who are currently treated for cancer or in follow-up are informed that regular PA during and after multimodality cancer treatment is safe, feasible and enhances recovery and physical health. Patients are advised to stay physically active for at least 30 min per day, 5 days per week. This is care as usual. Patients randomized in Group B were advised according to usual care. Patients in the intervention group, Group A, were additionally instructed to self-monitor PA (e.g., cycling, hiking, running) by GPS or stopwatch with RunKeeper. Furthermore, patients were requested to activate the “training reminder” option in the RunKeeper app. One investigator (HO) was available for answering questions about the RunKeeper use by telephone or e-mail.
Measurements
At T0, medical characteristics (treatment status, end of systemic cancer treatment (months) and cancer type) were obtained by reviewing patients’ medical record and socio-demographic, physical and behavioral characteristics (age, gender, marital status, BMI, smoking habits and alcohol consumption) were gathered by a short self-report questionnaire. Alcohol consumption was classified as non-drinker, occasional drinker (alcohol intake < 3 beverages/week), light regular drinker (alcohol intake ≥ 3 but < 6 beverages/week), moderate regular drinker (≥ 6 beverages/week) and excessive drinker (alcohol intake ≥ 14 beverages/week for women and ≥ 21 for men) [29]. Patient-reported outcome (PRO) was evaluated at baseline [T0], 6 weeks [T1], and after 12 weeks [T2], in both the control and intervention group (see Supplementary Fig. S1 for the design of the SMART-trial). Patients were contacted by a telephone call to return the questionnaires when there was no response within 1 week after each measurement. Adverse events (intervention-related) were closely monitored by investigators during the course of the study.
PRO’s included the validated (Dutch) translated Physical Activity Scale for the Elderly (PASE) questionnaire, a 7-day recall questionnaire [30, 31]. The PASE is designed to measure PA extensively by identifying leisure-time-, household-, and work-related activities. The three subscales of the PASE questionnaire, PASE sum score, total minutes of PA (minutes/week), and sedentary time (minutes/week), were classified to be valuable for clinical practice with a small standard error of measurement (SEM) [31]. In addition, the PASE sum score and total minutes of PA were subdivided in leisure-time PA [32].
At T2, each patient of Group A filled out the (Dutch) translated validated System Usability Scale (SUS) to measure the usability of the RunKeeper app [33]. The SUS is a standardized 10 item questionnaire to quantify the usability of the used systems (RunKeeper app) in terms of effectiveness, efficacy, and satisfaction [33].
In addition, patients in Group A were questioned about how they experienced the RunKeeper use in a semi-structured interview by telephone. Medical records were screened and one investigator (HO) asked if any adverse events occurred during the 12-week intervention and three questions regarding how patients experienced the RunKeeper use. These three questions were: “How did you experience the RunKeeper app use?” “How frequent did u use the RunKeeper app and are you planning to continue using RunKeeper?” and “Did you become more physically active due to the use of RunKeeper?”
Statistical analysis
Data from T0, 1 and 2 within groups and between both groups were analyzed. The Shapiro-Wilk normality test and Q-Q plots were used to evaluate whether continuous data was normally distributed. Between groups, comparisons were performed using independent samples T tests or Mann-Whitney U tests when data was not normally distributed. Means, pooled standard deviations (SDp) and 95% confidence intervals of the PASE subscales were calculated. When the data was not normally distributed, the medians and associated inter quartile ranges were calculated. Estimated effect sizes were calculated using the formula of Cohen’s d and Pearson’s correlation coefficient r for the interpretability and were classified as described by the guidelines of Cohen, et al. with 0.20, 0.50 and 0.80 for “small,” “medium” and “large” effects, respectively [34]. IBM SPSS Statistics package for Mac (version 22.0, SPSS Inc., Chicago, USA) was used to analyze data. The score of the usability questionnaire SUS was calculated with mean, SDp and 95% confidence intervals or median with inter quartile range. All analyses were intention-to-treat with all data available.
Results
Study sample
In total, 78 patients were assessed for eligibility by screening the medical records of patients visiting the Medical Oncology department of the UMCG. Nine patients did not have a smartphone, one patient was participating in oncologic rehabilitation and one was an active user of the RunKeeper app (see Fig. 1). Seven were ineligible due to medical reasons. Of the 60 eligible patients, 28 declined (too busy n = 4, already active n = 5, no reply n = 4 and not interested n = 15) and 32 agreed to participate. Patients were randomized in Group A, intervention group (n = 16) or Group B (n = 16), control group. No adverse events related to the study intervention occurred.
Fig. 1.
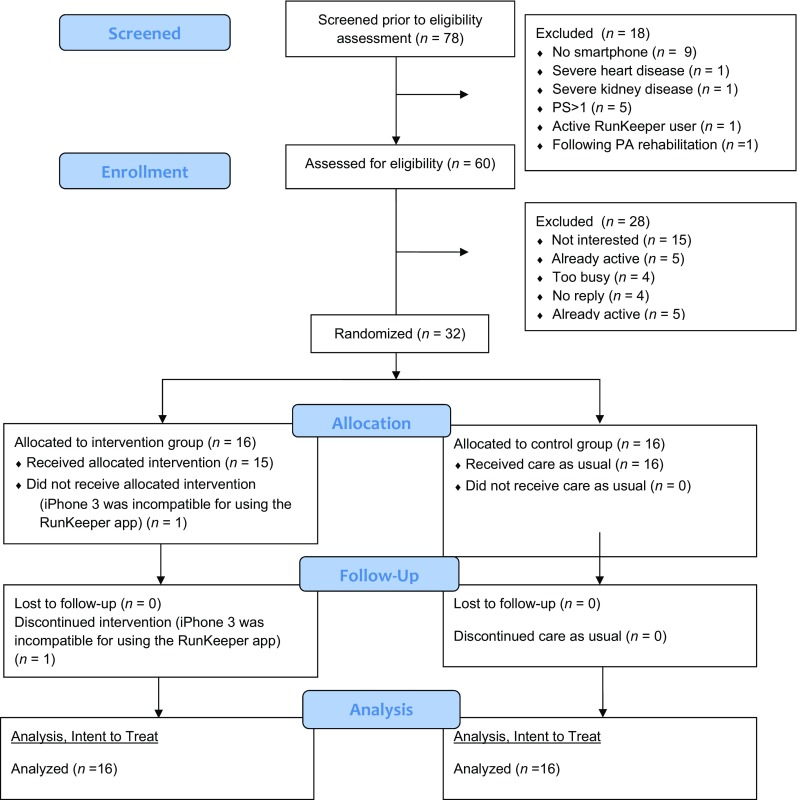
Flowchart of the SMART-trial (n = 32). PA, physical activity; PS, performance score; app, smartphone application
Effect of intervention on outcomes
Baseline characteristics of both groups were well balanced (see Table 1). Of the 32 patients included in the analysis, 87.5% was male, the median age was 33.6 years. All patients were treated with a curative intent. At baseline, four patients were still receiving systemic cancer treatment. The majority of patients (n = 23) was in follow-up after systemic cancer treatment, with a median of 22 months after completing systemic cancer treatment (inter quartile range = 35.5). The average BMI of patients was 25.7 kg/m2. The majority of patients (75%) was unmarried.
Table 1.
PA at baseline as measured by the PASE in the SMART-trial (n = 32)
| IG (n = 16) | CG (n = 16) | |||||
|---|---|---|---|---|---|---|
| Characteristic | No. | % | No. | % | ||
| Demographic characteristics | ||||||
| Age at inclusion (y) | ||||||
| Mean | 35.3 | 31.9 | ||||
| SD | 12.9 | 9.4 | ||||
| Gender | ||||||
| Male | 14 | 87.5 | 14 | 87.5 | ||
| Female | 2 | 12.5 | 2 | 12.5 | ||
| Married | 5 | 31.3 | 3 | 18.8 | ||
| Physical characteristics | ||||||
| BMI (kg/m2) | ||||||
| Mean | 26.1 | 25.3 | ||||
| SD | 3.2 | 3.4 | ||||
| Behavioral characteristics | ||||||
| Smoking habits | ||||||
| Non-smoker | 14 | 87.5 | 16 | 100 | ||
| Smoker | 2 | 12.5 | 0 | 0 | ||
| Alcohol consumption | ||||||
| Non-drinker | 4 | 25.0 | 5 | 31.3 | ||
| Occasional drinker | 2 | 12.5 | 1 | 6.3 | ||
| Light regular drinker | 5 | 31.3 | 6 | 37.5 | ||
| Moderate regular drinker | 4 | 25.0 | 3 | 18.8 | ||
| Excessive drinker | 1 | 6.3 | 1 | 6.3 | ||
| Medical characteristics | ||||||
| Treatment status | ||||||
| Active systemic cancer treatment | 2 | 12.5 | 2 | 12.5 | ||
| Follow-up | 13 | 81.3 | 10 | 62.5 | ||
| No systemic cancer treatment | 1 | 6.3 | 4 | 25.0 | ||
| End of systemic cancer treatment (months) | (n = 13) | (n = 10) | ||||
| Mdn | 20 | 28.5 | ||||
| IQR | 37.5 | 36.5 | ||||
| Cancer type | ||||||
| Testicular cancer | 14 | 87.5 | 13 | 81.3 | ||
| Breast cancer | 2 | 12.5 | 2 | 12.5 | ||
| Osteosarcoma | 0 | 0 | 1 | 6.3 | ||
Differences between both groups were assessed by independent samples T tests when data was continuous, Mann-Whitney U tests when data was not normally distributed and chi-squared tests or Fisher’s exact test with categorical data with two-sided P values (*P ≤ 0.05; **P ≤ 0.01)
Abbreviations: IG, intervention group; CG, control group; SD, standard deviation; BMI, body mass index; IQR, inter quartile range
One patient randomized in the intervention group was unable to use RunKeeper, due to the possession of an iPhone 3, the new RunKeeper version was compatible only for models iPhone 4 or higher. At baseline, there were no significant differences in self-reported PA between groups as measured by the three subscales of the PASE: PASE sum score, total minutes of PA and sedentary time (Table 2).
Table 2.
Outcome PASE questionnaire at baseline (n = 32)
| IG (n = 16) | CG (n = 16) | P a | Quartiles of the Dif. | ||||
|---|---|---|---|---|---|---|---|
| PASE subscales | Mdn | IQR | Mdn | IQR | Q1 | Q3 | |
| PASE sum score (score) | 133 | 102 | 116 | 172 | 0.851 | 76.8 | 188 |
| Total minutes of PA (min/wk) | 2693 | 2096 | 2498 | 3218 | 0.546 | 1534 | 3776 |
| Sedentary time (min/wk) | 735 | 540 | 630 | 979 | 0.985 | 540 | 1080 |
aDifferences between both groups were assessed by Mann-Whitney U tests with two-sided P values (*P ≤ 0.05; **P ≤ 0.01)
Abbreviations: IG, intervention group; CG, control group; PASE, Physical Activity Scale for the Elderly; PA, physical activity; min, minutes; wk., week; Mdn, median; Dif., difference; IQR, inter quartile range; Q1, 25th quartile; Q3, 75th quartile
Median scores and effect sizes of different subscales of the PASE questionnaire at T0, T1, and T2, which were completed by all patients of both groups (n = 32) are presented in Table 3. The intervention group as compared to the control group showed a significant increase in self-reported PA of 51% from T0 to T1, as measured by the subscale PASE sum score (r = 0.40). The leisure-time PA scores are displayed in Supplementary Table S2. A similar result was observed by the PASE subscale total minutes of PA, which indicated that patients’ total minutes of weekly PA increased significantly by 46% compared to the control group (r = 0.37). Though, these improvements were not sustained at T2. Analysis of the subscale sedentary time between T0 and T1 revealed a 19% difference in decrease of patients’ sedentary time which favored the intervention group (r = 0.071). Between T0 and T2, the difference in sedentary time increased to 27% (r = 0.11).
Table 3.
PA change scores as measured by the PASE in the SMART-trial (n = 32)
| Δ IG versus CG median change with IQR | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (T0) | Week 6 (T1) | Week 12 (T2) | 6 weeks (T0–T1) | 12 weeks (T0–T2) | ||||||||
| PASE subscales | Mdn | IQR | Mdn | IQR | Mdn | IRQ | Mdn | Q1 to Q3 | P a | Mdn | Q1 to Q3 | P a |
| PASE sum score (score) | ||||||||||||
| IG (n = 16) | 133 | 102 | 206 | 87.7 | 175 | 176 | 12.1 | − 31.2 to 73.9 | 0.024* | 8.84 | − 68.0 to 92.6 | 0.651 |
| CG (n = 16) | 116 | 172 | 121 | 97.8 | 134 | 92 | ||||||
| Total minutes of PA (min/wk) | ||||||||||||
| IG (n = 16) | 2693 | 2096 | 3773 | 2351 | 2843 | 2565 | 345 | − 660 to 1376 | 0.038* | − 105 | − 1144 to 1605 | 0.763 |
| CG (n = 16) | 2498 | 3218 | 2348 | 1568 | 2490 | 1405 | ||||||
| Sedentary time (min/wk) | ||||||||||||
| IG (n = 16) | 735 | 540 | 540 | 1181 | 540 | 1215 | 0 | − 394 to 0 | 0.686 | 0 | − 416 to 0 | 0.532 |
| CG (n = 16) | 630 | 979 | 585 | 799 | 630 | 900 | ||||||
aDifferences between both groups were assessed by Mann-Whitney U tests with two-sided P values (*P ≤ 0.05; **P ≤ 0.01)
Abbreviations: IG, intervention group; CG, control group; PASE, Physical Activity Scale for the Elderly; PA, physical activity; min, minutes; wk., week; IQR, inter quartile range; Q1, 25th quartile; Q3, 75th quartile
The usability of the RunKeeper app was quantitatively and qualitatively analyzed. At T2, 15 patients in Group A rated the usability as measured by the SUS, mean = 79.3 (SDp = 13.1; 95% confidence interval = 72.1–86.6). In addition, 14 qualitative semi-structured interviews were performed (see Supplementary Table S3). Two patients did not respond on our telephone calls and did not participate in this part. Most patients (n = 12) were enthusiastic about the RunKeeper app use. Eleven patients were still frequently using the RunKeeper app to self-monitor PA at T2. Majority of patients (n = 8) became more active due to the RunKeeper use and were planning to continue the use of RunKeeper.
Discussion
This study clearly demonstrates the feasibility of self-monitoring PA with the RunKeeper app in cancer patients and survivors in a 12-week program. Cancer patients and survivors were enthusiastic about the use of the RunKeeper app and found its use of additional value to care as usual. This exploratory study was not powered to detect differences between both groups.
Despite the accumulating evidence of importance of PA in cancer treatment, studies reveal that recruiting and retaining patients in PA programs is challenging [13, 20]. Possible hurdles are the intensity and frequency of training sessions, being unfamiliar with PA, travel distances and being too busy (work, children at home) [35]. There is an urgent need of low-threshold approaches to improve and sustain PA in patients diagnosed with cancer. Our results provide information to further develop PA programs for cancer patients and survivors. Patients increased their PA by using the RunKeeper app compared to usual care from baseline to 6 weeks as measured by the PASE sum score and total minutes of PA. However, no difference was detected between baseline and 12 weeks. This phenomenon might be due to the novelty of using the RunKeeper app, which diminishes over time and reduces interest in using the app to self-monitor PA [27]. In addition, patients randomized to the control group were expected to slightly increase their PA due to the participation in a study and the PA advice given as care as usual. The usability of RunKeeper was scored “good” as measured by the SUS, which was endorsed by the findings of the qualitative semi-structured interview.
The current study has several limitations. First, PA was measured by a self-report questionnaire, which is less optimal as compared to an objective measurement, like accelerometry [31]. In addition, patients could have overestimated or underestimated their self-reported PA and the measurement of PA with a 7-day recall questionnaire could have been biased by recall. Potentially, measurement of PA deviations caused by the use of the RunKeeper app were confounded by the components “household”-, and “work-related activities,” which are part of the measurement of self-reported PA by the PASE questionnaire [32]. In future studies, it might be worthwhile to consider household income and employment status.
Over the last few years, studies showed that self-monitoring PA with an app could improve PA [24–27]. Though, less is known about the use of publicly available smartphone apps to stimulate PA in cancer patients. Recently, Puszkiewicz, et al. [36] showed that the use of a publicly available app “GAINFitness” to self-monitor strengthening exercises during 6 weeks was positively received and used by small population of cancer survivors (n = 11). The positive attitude of patients against using an app to self-monitor PA is corresponding with findings in our study.
The scores of the PASE questionnaire subscales in our study population at baseline are comparable with other studies that used the PASE in patients with hip osteoarthritis [37], knee pain, ischemic stroke [38] and cancer [31]. A recent qualitative study showed that patients are interested and enthusiastic about the use of apps, which is in line with findings of our study [39]. However, the median age of patients that participated in the study of 33.6 years is low in comparison with the average age of cancer patients. Probably, the high number of patients with testicular cancer resulted in this younger age of the study population, which limits the generalizability. Cancer patients of older age might encounter difficulties in using advanced technological devices.
Furthermore, there were no additional costs and there was no travel burden for patients by participation in this study which contributed to the adherence. Travel burden is one of the main reasons for non-adherence to PA programs [40]. Potentially, the low-threshold character of our intervention encouraged the less motivated patients to become more physically active. However, half of eligible patients decided not to participate in the study due to several reasons. This was also observed in various PA studies across different cancer patient populations, resulting in lower recruitment rates [17].
In the semi-structured qualitative interview, several patients mentioned barriers for using the app to self-monitor PA. Some patients mentioned they forgot to use the app during their activities. In addition, few patients suggested that tailored feedback on their PA data would increase their adherence to the PA program [41]. This in accordance with findings of Phillips, et al. [42], they explored preferences of breast cancer survivors with online questionnaires and found that the majority were interested in using an app to self-monitor PA and to receive coaching from a distance with personalized feedback. Healthy, overweight participants adhered better to a weight loss program when individual feedback was provided as compared to participants who received a link to educational websites only in a home-based setting [43]. Moreover, tailored feedback through biweekly telephone calls in addition to self-monitoring on a mobile system was significantly more effective in sustained weight loss than the intervention without tailored feedback [44]. Also, both telephone counseling and coaching are effective weight loss strategies in breast cancer survivors [45].
In conclusion, this study shows that self-monitoring PA with RunKeeper is safe and feasible in cancer patients. Further research is needed to investigate whether the RunKeeper app use improves and sustains PA in cancer patients and survivors. The use of RunKeeper is of clinical interest as a low-threshold tool to stimulate PA in cancer patients in a home-based setting.
Electronic supplementary material
(DOCX 257 kb)
(DOCX 17.5 kb)
(DOCX 14.5 kb)
(DOCX 1728 kb)
Acknowledgements
The authors would like to sincerely thank the patients who participated in this feasibility study. Furthermore, the authors gratefully thank all medical oncologists and Gerry Sieling of the Medical Oncology department, UMCG, for their support, recruitment, data management and randomization.
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the medical ethical committee of the UMCG (ethical approval) and with the 1964 Declaration of Helsinki and its later amendments. Written informed consent was obtained from all participating patients. The study was registered in ClinicalTrials.gov (NCT02391454).
References
- 1.Schottenfeld D, Beebe-Dimmer JL, Buffler PA, Omenn GS. Current perspective on the global and United States cancer burden attributable to lifestyle and environmental risk factors. Annu Rev Public Health. 2013;34:97–117. doi: 10.1146/annurev-publhealth-031912-114350. [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Wei EK. Preventability of cancer: the relative contributions of biologic and social and physical environmental determinants of cancer mortality. Annu Rev Public Health. 2012;33:137–156. doi: 10.1146/annurev-publhealth-031811-124627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cokkinides V, Bandi P, McMahon C, Jemal A, Glynn T, Ward E. Tobacco control in the United States—recent progress and opportunities. CA Cancer J Clin. 2009;59:352–365. doi: 10.3322/caac.20037. [DOI] [PubMed] [Google Scholar]
- 4.Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62:242–274. doi: 10.3322/caac.21142. [DOI] [PubMed] [Google Scholar]
- 5.Masters GA, Krilov L, Bailey HH, Brose MS, Burstein H, Diller LR, Dizon DS, Fine HA, Kalemkerian GP, Moasser M, Neuss MN, O'Day SJ, Odenike O, Ryan CJ, Schilsky RL, Schwartz GK, Venook AP, Wong SL, Patel JD. Clinical cancer advances 2015: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2015;33:786–809. doi: 10.1200/JCO.2014.59.9746. [DOI] [PubMed] [Google Scholar]
- 6.Vineis P, Wild CP. Global cancer patterns: causes and prevention. Lancet. 2014;383:549–557. doi: 10.1016/S0140-6736(13)62224-2. [DOI] [PubMed] [Google Scholar]
- 7.Moore SC, Lee I-M, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P, Adami HO, Blair CK, Borch KB, Boyd E, Check DP, Fournier A, Freedman ND, Gunter M, Johannson M, Khaw KT, Linet MS, Orsini N, Park Y, Riboli E, Robien K, Schairer C, Sesso H, Spriggs M, van Dusen R, Wolk A, Matthews CE, Patel AV. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Intern Med. 2016;176:816–825. doi: 10.1001/jamainternmed.2016.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy SM, Sadim M, Li J, Yi N, Agarwal S, Mantzoros CS, Kaklamani VG. Clinical and genetic predictors of weight gain in patients diagnosed with breast cancer. Br J Cancer. 2013;109:872–881. doi: 10.1038/bjc.2013.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, Navarro Rosenblatt D, Thune I, Vieira R, Norat T. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. doi: 10.1093/annonc/mdu042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ekelund Ulf, Ward Heather A, Norat Teresa, Luan Jian’an, May Anne M, Weiderpass Elisabete, Sharp Stephen J, Overvad Kim, Østergaard Jane Nautrup, Tjønneland Anne, Johnsen Nina Føns, Mesrine Sylvie, Fournier Agnès, Fagherazzi Guy, Trichopoulou Antonia, Lagiou Pagona, Trichopoulos Dimitrios, Li Kuanrong, Kaaks Rudolf, Ferrari Pietro, Licaj Idlir, Jenab Mazda, Bergmann Manuela, Boeing Heiner, Palli Domenico, Sieri Sabina, Panico Salvatore, Tumino Rosario, Vineis Paolo, Peeters Petra H, Monnikhof Evelyn, Bueno-de-Mesquita H Bas, Quirós J Ramón, Agudo Antonio, Sánchez María-José, Huerta José María, Ardanaz Eva, Arriola Larraitz, Hedblad Bo, Wirfält Elisabet, Sund Malin, Johansson Mattias, Key Timothy J, Travis Ruth C, Khaw Kay-Tee, Brage Søren, Wareham Nicholas J, Riboli Elio. Physical activity and all-cause mortality across levels of overall and abdominal adiposity in European men and women: the European Prospective Investigation into Cancer and Nutrition Study (EPIC) The American Journal of Clinical Nutrition. 2015;101(3):613–621. doi: 10.3945/ajcn.114.100065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293–1311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 12.O’Donovan G, Lee I-M, Hamer M, Stamatakis E. Association of “weekend warrior” and other leisure time physical activity patterns with risks for all-cause, cardiovascular disease, and cancer mortality. JAMA Intern Med. 2017;177:335–342. doi: 10.1001/jamainternmed.2016.8014. [DOI] [PubMed] [Google Scholar]
- 13.van Waart H, Stuiver MM, van Harten WH, Geleijn E, Kieffer JM, Buffart LM, de Maaker-Berkhof M, Boven E, Schrama J, Geenen MM, Meerum Terwogt JM, van Bochove A, Lustig V, van den Heiligenberg SM, Smorenburg CH, Hellendoorn-van Vreeswijk JAJH, Sonke GS, Aaronson NK. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 14.Speed-Andrews AE, Courneya KS. Effects of exercise on quality of life and prognosis in cancer survivors. Curr Sports Med Rep. 2009;8:176–181. doi: 10.1249/JSR.0b013e3181ae98f3. [DOI] [PubMed] [Google Scholar]
- 15.Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. Physical activity levels before and after a diagnosis of breast carcinoma. Cancer. 2003;97:1746–1757. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buffart LM, Galvão DA, Brug J, Chinapaw MJM, Newton RU. Evidence-based physical activity guidelines for cancer survivors: current guidelines, knowledge gaps and future research directions. Cancer Treat Rev. 2014;40:327–340. doi: 10.1016/j.ctrv.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Courneya KS, McKenzie DC, Mackey JR, et al. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. JNCI J Natl Cancer Inst. 2013;105:1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 18.Knols R, Aaronson NK, Uebelhart D, Fransen J, Aufdemkampe G. Physical exercise in cancer patients during and after medical treatment: a systematic review of randomized and controlled clinical trials. J Clin Oncol. 2005;23:3830–3842. doi: 10.1200/JCO.2005.02.148. [DOI] [PubMed] [Google Scholar]
- 19.Midtgaard J, Christensen JF, Tolver A, Jones LW, Uth J, Rasmussen B, Tang L, Adamsen L, Rørth M. Efficacy of multimodal exercise-based rehabilitation on physical activity, cardiorespiratory fitness, and patient-reported outcomes in cancer survivors: a randomized, controlled trial. Ann Oncol. 2013;24:2267–2273. doi: 10.1093/annonc/mdt185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courneya KS, McKenzie DC, Reid RD, et al. Barriers to supervised exercise training in a randomized controlled trial of breast cancer patients receiving chemotherapy. Ann Behav Med. 2008;35:116–122. doi: 10.1007/s12160-007-9009-4. [DOI] [PubMed] [Google Scholar]
- 21.Ottenbacher AJ, Day RS, Taylor WC, Sharma SV, Sloane R, Snyder DC, Lipkus IM, Jones LW, Demark-Wahnefried W. Long-term physical activity outcomes of home-based lifestyle interventions among breast and prostate cancer survivors. Support Care Cancer. 2012;20:2483–2489. doi: 10.1007/s00520-011-1370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trinh L, Mutrie N, Campbell AM, Crawford JJ, Courneya KS. Effects of supervised exercise on motivational outcomes in breast cancer survivors at 5-year follow-up. Eur J Oncol Nurs. 2014;18:557–563. doi: 10.1016/j.ejon.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Burke LE, Styn MA, Sereika SM, Conroy MB, Ye L, Glanz K, Sevick MA, Ewing LJ. Using mHealth technology to enhance self-monitoring for weight loss. Am J Prev Med. 2012;43:20–26. doi: 10.1016/j.amepre.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turner-McGrievy GM, Beets MW, Moore JB, et al. Comparison of traditional versus mobile app self-monitoring of physical activity and dietary intake among overweight adults participating in an mHealth weight loss program. J Am Med Informatics Assoc. 2013;20:513–518. doi: 10.1136/amiajnl-2012-001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turner-McGrievy G, Tate D. Tweets, apps, and pods: results of the 6-month mobile pounds off digitally (mobile POD) randomized weight-loss intervention among adults. J Med Internet Res. 2011;13:e120. doi: 10.2196/jmir.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spook JE, Paulussen T, Kok G, Van Empelen P. Monitoring dietary intake and physical activity electronically: feasibility, usability, and ecological validity of a mobile-based ecological momentary assessment tool. J Med Internet Res. 2013;15:e214. doi: 10.2196/jmir.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.King AC, Hekler EB, Grieco LA, Winter SJ, Sheats JL, Buman MP, Banerjee B, Robinson TN, Cirimele J. Harnessing different motivational frames via mobile phones to promote daily physical activity and reduce sedentary behavior in aging adults. PLoS One. 2013;8:e62613. doi: 10.1371/journal.pone.0062613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Middelweerd A, Mollee JS, van der Wal CN, Brug J, te Velde SJ. Apps to promote physical activity among adults: a review and content analysis. Int J Behav Nutr Phys Act. 2014;11:97. doi: 10.1186/s12966-014-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perreault K, Bauman A, Johnson N, Britton A, Rangul V, Stamatakis E. Does physical activity moderate the association between alcohol drinking and all-cause, cancer and cardiovascular diseases mortality? A pooled analysis of eight British population cohorts. Br J Sports Med. 2017;51:651–657. doi: 10.1136/bjsports-2016-096194. [DOI] [PubMed] [Google Scholar]
- 30.Washburn RA, Smith KW, Jette AM, Janney CA. The physical activity scale for the elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 31.Liu RD, Buffart LM, Kersten MJ, et al. Psychometric properties of two physical activity questionnaires, the AQuAA and the PASE, in cancer patients. BMC Med Res Methodol. 2011;11:30. doi: 10.1186/1471-2288-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE) J Clin Epidemiol. 1999;52:643–651. doi: 10.1016/S0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 33.Bangor A, Kortum PT, Miller JT. An empirical evaluation of the system usability scale. Int J Hum Comput Interact. 2008;24:574–594. doi: 10.1080/10447310802205776. [DOI] [Google Scholar]
- 34.Cohen J. Things I have learned (so far) Am Psychol. 1990;45:1304–1312. doi: 10.1037/0003-066X.45.12.1304. [DOI] [Google Scholar]
- 35.van Waart H, van Harten WH, Buffart LM, Sonke GS, Stuiver MM, Aaronson NK. Why do patients choose (not) to participate in an exercise trial during adjuvant chemotherapy for breast cancer? Psychooncology. 2016;25:964–970. doi: 10.1002/pon.3936. [DOI] [PubMed] [Google Scholar]
- 36.Puszkiewicz P, Roberts AL, Smith L, Wardle J, Fisher A. Assessment of cancer survivors’ experiences of using a publicly available physical activity mobile application. JMIR Cancer. 2016;2:e7. doi: 10.2196/cancer.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svege I, Kolle E, Risberg MA. Reliability and validity of the physical activity scale for the elderly (PASE) in patients with hip osteoarthritis. BMC Musculoskelet Disord. 2012;13:26. doi: 10.1186/1471-2474-13-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindahl M, Hansen L, Pedersen A, Truelsen T, Boysen G. Self-reported physical activity after ischemic stroke correlates with physical capacity. Adv Physiother. 2008;10:188–194. doi: 10.1080/14038190802490025. [DOI] [Google Scholar]
- 39.Robertson MC, Tsai E, Lyons EJ, Srinivasan S, Swartz MC, Baum ML, Basen-Engquist KM. Mobile health physical activity intervention preferences in cancer survivors: a qualitative study. JMIR mHealth uHealth. 2017;5:e3. doi: 10.2196/mhealth.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ormel HL, van der Schoot GGF, Sluiter WJ, Jalving M, Gietema JA, Walenkamp AME. Predictors of adherence to exercise interventions during and after cancer treatment: a systematic review. Psychooncology. 2018;27:713–724. doi: 10.1002/pon.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt ME, Wiskemann J, Ulrich CM, Schneeweiss A, Steindorf K. Self-reported physical activity behavior of breast cancer survivors during and after adjuvant therapy: 12 months follow-up of two randomized exercise intervention trials. Acta Oncol (Madr) 2017;56:618–627. doi: 10.1080/0284186X.2016.1275776. [DOI] [PubMed] [Google Scholar]
- 42.Phillips SM, Conroy DE, Keadle SK, Pellegrini CA, Lloyd GR, Penedo FJ, Spring B. Breast cancer survivors’ preferences for technology-supported exercise interventions. Support Care Cancer. 2017;25:3243–3252. doi: 10.1007/s00520-017-3735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tate DF. Using internet technology to deliver a behavioral weight loss program. JAMA. 2001;285:1172–1177. doi: 10.1001/jama.285.9.1172. [DOI] [PubMed] [Google Scholar]
- 44.Spring B, Duncan JM, Janke EA, Kozak AT, McFadden HG, DeMott A, Pictor A, Epstein LH, Siddique J, Pellegrini CA, Buscemi J, Hedeker D. Integrating technology into standard weight loss treatment. JAMA Intern Med. 2013;173:105–111. doi: 10.1001/jamainternmed.2013.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Y, Playdon M, Li F, Irwin ML. Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: the lifestyle, exercise, and nutrition (LEAN) study. J Clin Oncol. 2016;34:669–676. doi: 10.1200/JCO.2015.61.6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 257 kb)
(DOCX 17.5 kb)
(DOCX 14.5 kb)
(DOCX 1728 kb)


