Abstract
Purpose
The introduction of ligands targeting prostate-specific membrane antigen (PSMA), especially 68Ga-PSMA-11, has changed the management of patients with prostate cancer (PCa). 18F-Labelled ligands can be produced in larger amounts and therefore can improve availability for a larger group of patients. The aim of this study was to evaluate the diagnostic performance of the recently introduced 18F-PSMA-1007 in patients with recurrent PCa.
Methods
This retrospective analysis included 100 consecutive patients with biochemical relapse (mean age 68.75 ± 7.6 years) referred for PSMA PET/CT. Whole-body PET/CT imaging (from the lower limbs to the skull) was performed in all patients 120 min after injection of 338 ± 44.31 MBq 18F-PSMA-1007. Prostatectomy, radiation beam therapy of the prostate bed and androgen-deprivation therapy had been performed in 92%, 45% and 27% of the patients, respectively. Radiation beam therapy of the prostate bed had been performed in addition to surgery in 38 patients (38%) and 10 patients (10%) had received all three therapy modalities. The probability of a 18F-PSMA-1007 PET/CT scan suggestive of pathology was compared with the Gleason score (GS) and PSA level.
Results
Of the 100 patients, 95 (95%) showed at least one pathological finding on 18F-PSMA-1007 PET/CT. The overall median PSA level was 1.34 ng/ml (range 0,04–41.3 ng/ml). The rates of pathological scans were 86%, 89%, 100% and 100% among patients with PSA levels ≤0.5, 0.51–1.0, 1.1–2.0 and > 2.0 ng/ml, respectively. The median GS was 7 (range 5–10). The majority of patients (70) with a GS available had a score in the range 7–9. The rate of pathological scans in these patients was 93% (65/70). The median SUVmax values of the pathological findings were 10.25, 14.32, 13.16 and 28.87 in patients with PSA levels ≤0.5, 0.51–1.0, 1.1–2.0 and >2.0 ng/ml, respectively. The median SUVmax in patients with a PSA level of >2.0 ng/ml was significantly higher than in all other PSA groups.
Conclusion
18F-PSMA-1007 PET/CT can detect recurrent PCa in a high percentage of patients with biochemical relapse. The probability of a pathological 18F-PSMA-1007 PET/CT scan seems to be high even in patients with a low PSA level ≤0.5 ng/ml, and this may have a significant impact on the management of this relevant group of patients.
Keywords: Prostate cancer, PSMA-1007, Biochemical relapse
Introduction
Prostate cancer (PCa) is the most frequent tumour disease in men worldwide [1]. Biochemical relapse is frequent after initial curative treatment, especially in patients with high-risk PCa. Conventional imaging with, for example, computed tomography (CT) and magnetic resonance imaging (MRI), are insufficient in detecting tumour lesions due to their low sensitivity and specificity. During recent years targeted imaging of prostate-specific membrane antigen (PSMA), also known as glutamate carboxypeptidase II, N-acetyl-α-linked acidic dipeptidase I or folate hydrolase, has opened a new chapter in the diagnostic management of patients with PCa [2–6]. PSMA is a type II transmembrane glycoprotein that is strongly overexpressed in PCa cells. The level of PSMA expression rises with increasing tumour dedifferentiation and is higher in hormone-refractory cancers [7–9].
Because of the larger activity amount of cyclotron-produced 18F compared with the limited activity of 68Ga derived from 68Ge/68Ga generator elution, and its longer half-life and higher physical spatial resolution, 18F has advantages over 68Ga [10]. Furthermore, the very low urinary activity in 18F-PSMA-1007 PET/CT scans seems to be another advantage, which makes this new imaging pharmaceutical highly interesting for the differentiation of lymph node metastases of recurrent PCa from urinary activity in the ureter or for the differentiation of local relapse from the urinary bladder [11].
The first clinical experience in single cases or case series with 18F-PSMA-1007 has shown the potential of this new radiopharmaceutical in patients with PCa [11–14]. The aim of the present study was to evaluate the performance of 18F-PSMA-1007 in detecting PCa recurrence or metastases in patients with biochemical relapse.
Materials and methods
Patients
From October 2017 to May 2018, 212 patients were examined with 18F-PSMA-1007 PET/CT as part of clinical routine. Of these patients, 100 were referred for the detection of recurrent PCa. The patients received primary therapy for PCa a median of 44.3 months before PSMA imaging (range 2–314 months). In seven patients, the exact time of the first therapy for PCa was not available. Some of the patients had undergone CT, MRI or bone scan before PSMA imaging. However, a systematic comparison of these data was not possible since the intervals were highly variable and different procedures had been performed. All other imaging studies performed before PET/CT had been negative. Of the 100 patients, 28 had been analysed previously in a dual time scanning analysis [14].
Patient characteristics are given in Table 1. Patients with no primary therapy with curative intent or patients referred for PSMA radioligand therapy were not included in the current analysis (Fig. 1). Prostatectomy, radiation beam therapy and androgen-deprivation therapy had been performed in 92%, 45% and 27% of the patients. Radiation beam therapy in addition to surgery had been performed in 38 patients (38%) and 10 patients (10%) had received all three therapy modalities. All patients received detailed information about the imaging procedures and provided signed informed consent according institutional guidelines.
Table 1.
Characteristics of the 100 included patients
| Characteristic | Value |
|---|---|
| Age (years) | |
| Mean ± SD | 68.75 ± 7.6 |
| Median (range) | 70.44 (47.36–85.82) |
| Administered activity (MBq) | |
| Mean ± SD | 338.02 ± 33.31 |
| Median (range) | 334 (200–412) |
| Gleason scorea | |
| Mean ± SD | 7.5 ± 1.01 |
| Median (range) | 7 (5–10) |
| PSA level at PET (ng/ml) | |
| Mean ± SD | 3.36 ± 6.11 |
| Median (range) | 1.34 (0.04–41.3) |
| Prior therapies, n (%) | |
| Prostatectomy | 92 (92) |
| Radiation beam therapy to prostate bed | 45 (45) |
| Androgen-deprivation therapy | 27 (27) |
SD standard deviation, PSA prostate-specific antigen
aIn 76 patients; data from 24 patients missing
Fig. 1.
Flow chart of patient selection
Imaging procedures and preparation of 18F-PSMA-1007
18F-PSMA-1007 was produced in a GE TracerLab MX synthesizer according to the one-step procedure described by Cardinale et al. and standard operation procedures described previously [14, 15] including sterile filtration of the final batch solution. 18F-PSMA-1007 precursor, cassettes and reagents for the synthesis of 18F-PSMA-1007 as well as the synthesis sequence for fully automatic production with a GE TracerLab MX module were obtained from ABX GmbH (Radeberg, Germany). The final injection solution of the 18F-PSMA-1007 batch was clear, colourless and particle-free, and had a mean radiochemical purity of 96.5 ± 1.1% (range 95–99%) as determined by high-performance liquid chromatography. Unreacted 18F-fluoride or 18F-fluoride resulting from compound cleavage was not detected by thin-layer chromatography. The pH of the batch solution ranged from 5.9 to 7.8 and the endotoxin content was <5.0 endotoxin units/ml. The concentrations of ethanol and dimethyl sulfoxide (DMSO) as residual solvents were measured by gas chromatography (ethanol 29.6–31.6 mg/ml; DMSO 0.25–0.68 mg/ml). The osmolality ranged from 1,110 to 1,300 mOsmol/kg.
Patients received 4 MBq/kg body weight with a maximum of 400 MBq per patient (mean injected activity 338 ± 44.31 MBq). Scanning was performed 120 min after injection from the lower limbs to the skull. Patients were asked to empty their bladder before the scan. Images were acquired with a scan time of 3 min per bed position on a Siemens mCT scanner (Siemens Healthcare, Knoxville, TN). Images were reconstructed using the standard software provided by the manufacturer. For attenuation correction, a low-dose CT scan was performed in accordance with the PET imaging. Contrast-enhanced CT of the abdomen and pelvis was only performed if no recent CT or MRI scans were available.
Image analysis
Coregistered images were analysed using syngovia software, version: VB20A (Siemens Healthcare). According to institutional procedures all scans were analysed by two board-certified nuclear medicine physicians and radiologists at an interdisciplinary conference for reporting. Absence of morphological changes was regularly found and were classified as bone metastases and local recurrence. The sensitivity of CT for these types of metastases is known to be limited, and thus high focal tracer uptake in these areas was considered suggestive of bone metastases or local recurrence despite a lack of morphological correlation. In lesions characteristic of PCa, volumes of interest were placed on the plane with highest uptake, and maximum standardized uptake values (SUVmax) were measured and documented. Focal tracer uptake above local background in morphologically visible lesions on CT was considered as PSMA-positive. Any visible PCa lesions were analysed unless the patients had more than three lesions, in which case, a maximum of three lesions were analysed. This kind of selection avoids overestimation of SUVs as otherwise dominant lesions would be preferentially selected. Typical pitfalls such as PSMA uptake in sacral and coeliac ganglia or in the stellate ganglia were frequently observed but were not considered pathological [16].
Statistical analysis
SPSS Statistics 25 (IBM Inc. Armonk, NY, USA) was used for statistical analysis. Descriptive statistics are absolute and relative frequencies, mean or median and standard deviation or range, were used to characterize the study population. For subgroup analysis, patients were divided into four groups depending on their prostate-specific antigen (PSA) level: ≤0.5, 0.51–1.0, 1.1–2.0 and >2.0 ng/ml. The Mann-Whitney test was used to evaluate the significant differences of the median SUVmax (of the lesion with maximum uptake from each patient) between PSA groups. A P value <0.05 was considered significant and noticeable.
Results
The analysis included 100 consecutive patients (mean age 68.75 ± 7.6 years) who were referred for 18F-PSMA-1007 PET/CT for evaluation and localization of biochemical relapse. None of the patients showed any kind of adverse event or clinically detectable pharmacological effect following injection of 18F-PSMA-1007. In 95 of the 100 patients (95%) at least one lesion suggestive of PCa was detected on 18F-PSMA-1007 PET/CT. The patient-based sensitivity was therefore 95% (95% confidence interval 91–99%). In total, 213 lesions characteristic of PCa (37 local relapses, 107 lymph node metastases, 67 bone metastases and 2 soft tissue metastases) were detected in 95 patients, and in 29 patients relapse was exclusively lymph node metastases, in 16 patients exclusively bone metastases and in 15 patients exclusively local relapse. Table 2 presents the average SUVmax of all included lesions. Examples of a bone lesion, a lymph node and a local relapse are given in Figs. 2, 3 and 4.
Table 2.
Average SUVmax of PCa lesions of all types
| Lesion type | No. of lesions | SUVmax | |||
|---|---|---|---|---|---|
| Mean ± SD | Minimum | Maximum | Median | ||
| All lesions | 213 | 21.52 ± 29.8 | 2.49 | 248.28 | 11.45 |
| Local relapse | 37 | 18.29 ± 23.78 | 3.87 | 137.94 | 10.64 |
| Lymph node metastases | 107 | 26.03 ± 34.51 | 2.59 | 248.28 | 16.1 |
| Bone metastases | 67 | 16.57 ± 23.59 | 2.49 | 136.18 | 7.5 |
| Soft tissue metastases | 2 | 6.82 ± 3.7 | 4.19 | 9.46 | 6.8 |
SD standard deviation, PCa prostate cancer
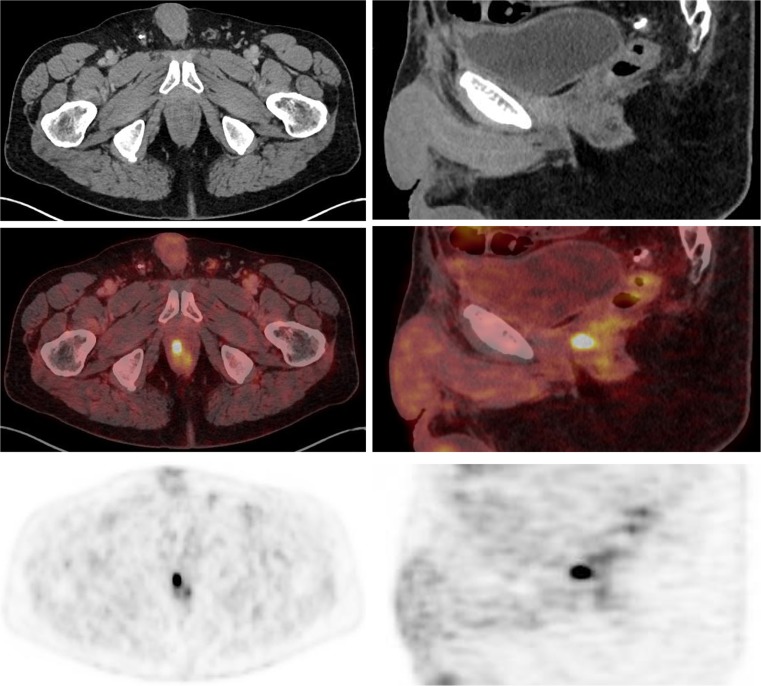
Fig. 2.
18F-PSMA-1007 PET/CT imaging (left axial images, right sagittal images) in a 72-year-old patient with biochemical relapse (PSA level 0.86 ng/ml) and with a Gleason score of 7b (4 + 3). The images show focal right-sided tracer uptake in the prostate bed
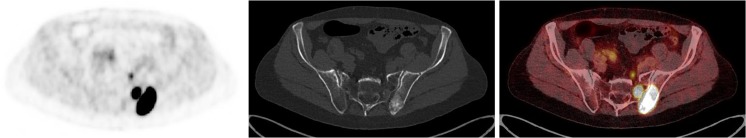
Fig. 3.
18F-PSMA-1007 PET/CT imaging (axial images: left PET, centre CT, right fused PET/CT) in a 73-year-old patient with biochemical relapse (PSA level 1.05 ng/ml) after radical prostatectomy and prior antiandrogen therapy and radiation to prostate bed (Gleason score not available). The images show bone metastases in the left pelvis and in the adjacent os sacrum. An additional presacral lesion is seen on the left side suggestive of lymph node metastasis
Fig. 4.
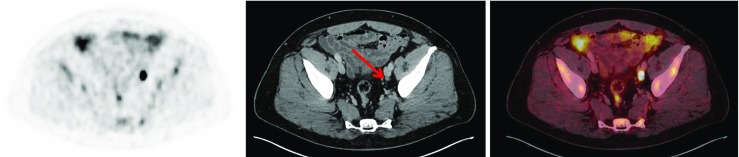
18F-PSMA-1007 PET/CT imaging (axial images: left PET, centre CT, right fused PET/CT) in a 59-year-old patient with biochemical relapse (PSA level 0.54 ng/ml) after radical prostatectomy and with a Gleason score of 7b (4 + 3). Images show a 5-mm lymph node lesion (arrow) with high tracer uptake along the left iliac artery
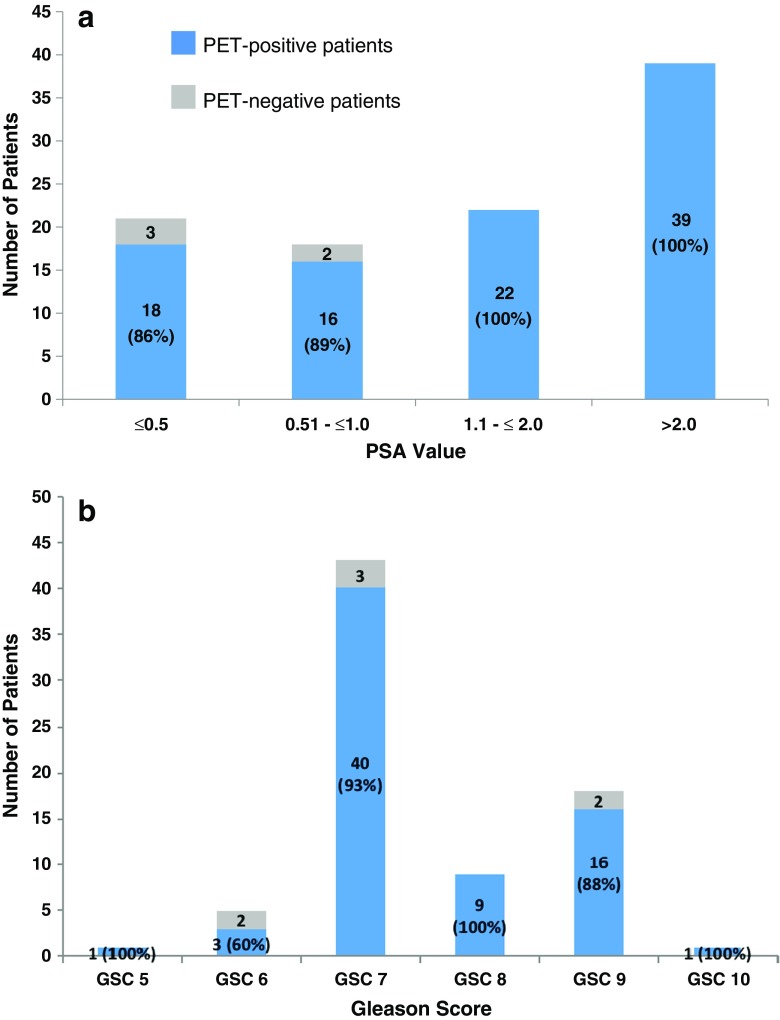
Of patients with a PSA level ≤2.0 ng/ml, 92% were PET-positive. The rates of pathological 18F-PSMA-1007 PET/CT scans in relation to PSA level and Gleason score (GS) are presented in Fig. 5. In total, 18F-PSMA-1007 PET/CT was negative in five patients, three with a PSA level ≤0.5 ng/ml and two with a PSA level in the range 0.51–1.0 ng/ml.
Fig. 5.
Probability of pathological 18F-PSMA-1007 PET/CT in relation to PSA level (a) in 100 patients and in relation to Gleason score (b) in 76 patients. Blue columns show the number and percentage of patients with a pathological 18F-PSMA-1007 PET/CT scan. Grey columns show the number and percentage of patients with a negative PET scan
The majority of patients (70) with a GS available had a score in the range 7–9. The detection rate in these patients was 93% (65/70). The median SUVmax values of the pathological findings in patients with PSA levels ≤0.5, 0.51–1.0, 1.1–2.0 and > 2.0 ng/ml were 10.25 (range 3.98–48.99), 14.32 (range 4.36–38.61), 13.16 (range 3.14–136.18) and 28.87 (range 5.03–248.27), respectively. The median SUVmax in patients with a PSA level >2.0 ng/ml was significantly higher than that in patients in the other PSA groups (>2.0 ng/ml vs. ≤0.5 ng/ml, P = 0.001; vs. 0.51–1.0 ng/ml, P = 0.005; vs. 1.1–2.0 ng/ml, P = 0.027). There were no significant differences among the other PSA groups.
Discussion
PSMA-targeted imaging has achieved a leading role in the management of PCa patients during recent years overcoming the challenging lack of sensitivity and specificity of conventional imaging modalities. In the present study the performance of 18F-PSMA-1007 in detecting PCa lesions in patients with biochemical relapse was evaluated.18F-PSMA-1007 PET/CT scans in 100 consecutive patients referred for evaluation and localization of biochemical relapse were retrospectively analysed. Of all the patients included in this analysis, 95% showed at least one lesion with characteristics suggestive of PCa on 18F-PSMA-1007 PET/CT. A recent case series of 12 patients revealed a detection rate of 75% [17], which is lower than in the present study, but the detection rate found in that study might have been due to the small number of patients. In the study mentioned, 62% of the patients who had a PSA level ≤2.0 ng/ml had a pathological scan, whereas in our study 92% of the patients (56/61) with a PSA level of ≤2.0 ng/ml had a pathologic scan.
The PET-positive rate in the present study was higher than the detection rate in a study investigating the performance of 68Ga-PSMA-11 PET/CT in 1007 patients with biochemical relapse (the largest group investigated so far) [3]. In that study the detection rate was 79.5% in patients with a median PSA level of 2.2 ng/ml compared with 95% in the present study and a median PSA level of 1.34 ng/ml. In another study also using 68Ga-PSMA-11, the detection rate was 89.5% in 248 patients with biochemical relapse and a median PSA level of 1.99 ng/ml [18]. In that study, the detection rate in patients with a PSA level <0.5 ng/ml was 57.9% (11/19) compared with a rate of 86% (18/21) in our study. Although the patient groups are not directly comparable (e.g. different proportions of patients with previous surgical treatment), our results could indicate that 18F-PSMA-1007 has a higher sensitivity than 68Ga-PSMA-11. However, further studies including more patients are mandatory to further analyse the sensitivity of 18F-PSMA-1007.
In the present study, local relapse was detected in 37% of patients (37/100), which is considerably higher than found in a recent study using 68Ga-PSMA-11 in which local relapse was detected in 4% of patients (13/319) [2]. Our results suggest that 18F-PSMA-1007 might be superior to 68Ga-PSMA-11, especially in patients with a low PSA level. However, this suggestion must be further evaluated in a head to head comparison, which is not yet available. Furthermore, it is well known from 68Ga-PSMA-11 and 18F-PSMA-1007 studies that PCa lesions are shown with better contrast and higher tracer uptake after longer uptake times (e.g. 3 h rather than 1 h after injection) [2, 6, 14]. This indicates that imaging with 18F-PSMA-1007 is probably more advantageous at 2 h than at 1 h after injection, which has become common with 68Ga-PSMA-11 at most centres. The longer half-life and higher injected activities allow high-quality delayed images, higher lesion uptake and superior clearance of background activity. The higher detection rate may therefore be because of the superior differentiation of ureter and bladder activity from local recurrence and locoregional lymph node metastasis [11, 14]. The confidence in diagnosing local recurrence is thus enhanced. Particularly in patients with a low PSA level, radiotherapy of local recurrences may induce secondary complete remission. A reliable imaging procedure for use in patients with increasing but still low PSA levels is thus of the utmost clinical relevance. The nonsignificant differences in median SUVmax among the subgroups of patients with a PSA level ≤2.0 ng/ml underlines the high detection rates revealed in the present analysis in patients with a very low PSA level (e.g. ≤0.5 ng/ml). A lower PSA threshold level can thus not be recommended since tumour lesions can be visualized with high sensitivity due to high uptake.
High liver and bowel uptake of 18F-PSMA-1007 has been reported. This was also found in the present group of patients. Since isolated liver metastases occur infrequently and bowel metastases very rarely, we consider that the high uptake in these organs would not cause a marked deterioration in the sensitivity of the tracer. In the present study, the sensitivity of 18F-PSMA-1007 PET/CT seemed to be independent of the GS. Prior studies using 68Ga-PSMA-11 have only shown a probable tendency for higher detection rates with increasing GS [3].
The lack of histopathology was a limitation of the present study. Therefore, we cannot exclude false-positive lesions, although the images were analysed by physicians with long experience of PET imaging, especially PCa imaging with PSMA-targeted radioligands.
Conclusion
18F-PSMA-1007 PET/CT can detect recurrent PCa in a high percentage of patients with biochemical relapse. The probability of a pathological 18F-PSMA-1007 PET/CT seems to be high even in patients with a low PSA level of ≤0.5 ng/ml, which may have a significant impact in the further clinical management of patients. Prospective controlled trials are mandatory to validate these data.
Acknowledgments
We thank the Radiochemistry Group at the Department of Nuclear Medicine for their highly reliable production of 18F-PSMA-1007, and the technologists for their support.
Funding
The publication of this article was supported by funds from the European Association of Nuclear Medicine (EANM).
Conflicts of interest
The University of Münster received consulting fees from ABX GmbH, Radeberg, Germany, for K.R. and M.B. Additionally K.R. is a scientific consultant/advisor to ABX GmbH. The authors declare they have no conflict of interest according to the subject and matter of the present article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This article does not describe any studies with animals performed by any of the authors. According to data protection guidelines, formal ethical approval for retrospective studies is not necessary.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, et al. The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42(2):197–209. doi: 10.1007/s00259-014-2949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afshar-Oromieh A, Holland-Letz T, Giesel FL, Kratochwil C, Mier W, Haufe S, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44(8):1258–1268. doi: 10.1007/s00259-017-3711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahbar K, Weckesser M, Huss S, Semjonow A, Breyholz HJ, Schrader AJ, et al. Correlation of Intraprostatic tumor extent with 68Ga-PSMA distribution in patients with prostate cancer. J Nucl Med. 2016;57(4):563–567. doi: 10.2967/jnumed.115.169243. [DOI] [PubMed] [Google Scholar]
- 5.Afshar-Oromieh A, Zechmann CM, Malcher A, Eder M, Eisenhut M, Linhart HG, et al. Comparison of PET imaging with a (68)Ga-labelled PSMA ligand and (18)F-choline-based PET/CT for the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41(1):11–20. doi: 10.1007/s00259-013-2525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40(4):486–495. doi: 10.1007/s00259-012-2298-2. [DOI] [PubMed] [Google Scholar]
- 7.Israeli RS, Powell CT, Corr JG, Fair WR, Heston WD. Expression of the prostate-specific membrane antigen. Cancer Res. 1994;54(7):1807–1811. [PubMed] [Google Scholar]
- 8.Sweat SD, Pacelli A, Murphy GP, Bostwick DG. Prostate-specific membrane antigen expression is greatest in prostate adenocarcinoma and lymph node metastases. Urology. 1998;52(4):637–640. doi: 10.1016/S0090-4295(98)00278-7. [DOI] [PubMed] [Google Scholar]
- 9.Wright GL, Jr, Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48(2):326–334. doi: 10.1016/S0090-4295(96)00184-7. [DOI] [PubMed] [Google Scholar]
- 10.Kesch C, Kratochwil C, Mier W, Kopka K, Giesel FL. 68Ga or 18F for prostate cancer imaging? J Nucl Med. 2017;58(5):687–688. doi: 10.2967/jnumed.117.190157. [DOI] [PubMed] [Google Scholar]
- 11.Rahbar K, Weckesser M, Ahmadzadehfar H, Schafers M, Stegger L, Bogemann M. Advantage of 18F-PSMA-1007 over 68Ga-PSMA-11 PET imaging for differentiation of local recurrence vs. urinary tracer excretion. Eur J Nucl Med Mol Imaging. 2018;45(6):1076–1077. doi: 10.1007/s00259-018-3952-0. [DOI] [PubMed] [Google Scholar]
- 12.Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44(4):678–688. doi: 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giesel FL, Kesch C, Yun M, Cardinale J, Haberkorn U, Kopka K, et al. 18F-PSMA-1007 PET/CT detects micrometastases in a patient with biochemically recurrent prostate cancer. Clin Genitourin Cancer. 2017;15(3):e497–e499. doi: 10.1016/j.clgc.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 14.Rahbar K, Afshar-Oromieh A, Bogemann M, Wagner S, Schafers M, Stegger L, et al. (18)F-PSMA-1007 PET/CT at 60 and 120 minutes in patients with prostate cancer: biodistribution, tumour detection and activity kinetics. Eur J Nucl Med Mol Imaging. 2018;45(8):1329–1334. doi: 10.1007/s00259-018-3989-0. [DOI] [PubMed] [Google Scholar]
- 15.Cardinale J, Schafer M, Benesova M, Bauder-Wust U, Leotta K, Eder M, et al. Preclinical evaluation of 18F-PSMA-1007, a new prostate-specific membrane antigen ligand for prostate cancer imaging. J Nucl Med. 2017;58(3):425–431. doi: 10.2967/jnumed.116.181768. [DOI] [PubMed] [Google Scholar]
- 16.Backhaus P, Noto B, Avramovic N, Grubert LS, Huss S, Bogemann M, et al. Targeting PSMA by radioligands in non-prostate disease-current status and future perspectives. Eur J Nucl Med Mol Imaging. 2018;45(5):860–877. doi: 10.1007/s00259-017-3922-y. [DOI] [PubMed] [Google Scholar]
- 17.Giesel FL, Will L, Kesch C, Freitag M, Kremer C, Merkle J, et al. Biochemical recurrence of prostate cancer: initial results with [(18)F]PSMA-1007 PET/CT. J Nucl Med. 2018;59(4):632–635. doi: 10.2967/jnumed.117.196329. [DOI] [PubMed] [Google Scholar]
- 18.Eiber M, Maurer T, Souvatzoglou M, Beer AJ, Ruffani A, Haller B, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56(5):668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]