Abstract
Background
Evidence of humanistic detriments of Clostridium difficile infection (CDI) remains limited.
Aims
To assess humanistic burden associated with CDI.
Methods
Self-reported National Health and Wellness Survey data between 2013 and 2016 were analyzed for the USA, five European countries, China, and Brazil. Outcome measures included SF-36v2® for health-related quality of life (HRQoL) and Work Productivity and Activity Impairment questionnaire. Respondents (≥ 18 years old) were classified as (1) currently treated doctor-diagnosed CDI (C-CDI), (2) doctor-diagnosed prior CDI (P-CDI), or (3) never experienced CDI (NO-CDI). Regression modeling assessed the association between CDI status and outcomes, adjusting for potential confounders.
Results
Of 352,780 respondents, 299, 2111, and 350,370 met the criteria for C-CDI, P-CDI, and NO-CDI, respectively, with 45% of the total from the USA. C-CDI and P-CDI respondents were older, were less often employed and had more comorbidities than those with NO-CDI. After adjustment for covariates, C-CDI and P-CDI had significantly lower HRQoL relative to NO-CDI for mental (MCS 39, 43 vs. 46) and physical (PCS 39, 41 vs. 46) component summary scores, and health utility (SF-6D 0.58, 0.64 vs. 0.71) (all p < 0.05), meeting common thresholds for minimally important differences. Those with C-CDI and P-CDI reported missing more work (21, 16 vs. 8%), greater impairment while working (43, 34 vs. 22%), and more activity impairment (61, 49 vs. 34%) than those with NO-CDI (all p < 0.05), respectively.
Conclusions
CDI is associated with meaningfully worse HRQoL and greater impairment to work and activities compared with NO-CDI. The impairment directly attributable to CDI requires further evaluation.
Keywords: Clostridium difficile, Quality of life, Work productivity, Outcomes, Questionnaire, Survey
Introduction
Clostridium difficile is the most common cause of infectious diarrhea in hospitalized patients and the leading cause of healthcare-associated infections in hospitals [1–3]. A population study estimates that the annual volume of Clostridium difficile infection (CDI) in the USA tops 450,000 cases [2]. Due to this statistic, the United States of America (US) Centers for Disease Control and Prevention has classified CDI as an urgent public health threat [4]. In addition to healthcare-acquired CDI, community-acquired CDI is increasingly a concern as rates increase [5–7]. Though lower in incidence than in the USA, CDI remains a large and growing concern in both Europe and Asia; however, published estimates may undercount the actual burden given the lack of testing, underdiagnoses, and potentially missed community-acquired cases [8–12].
Numerous studies have documented clinical and economic consequences of CDI. Patients with CDI have longer lengths of hospital stay [13–17], are more frequently readmitted to the hospital, are less likely to be discharged directly home from the hospital [13, 18], and incur higher hospital costs [19, 20] than those without CDI. Nursing home residents with CDI have been found to incur greater direct medical costs including hospital costs, nonhospital-based outpatient medical costs, and prescription costs [21] compared to those without CDI. The total economic impact is considerable, with CDI estimated to contribute nearly $5 billion in excess healthcare costs in the USA [19] and nearly €3 billion in Europe [22].
In contrast, few studies have examined the impact of CDI on health-related quality of life (HRQoL). Some of the studies published to date that demonstrate potentially serious negative HRQoL consequences of CDI [23–25] present multiple methodological challenges that may limit their interpretation. To address this evidence gap, we assessed the HRQoL, work productivity, and activity impacts of CDI using a large international survey.
Methods
Data Source
We used data from the National Health and Wellness Survey (NHWS; Kantar Health, New York, NY, USA), a cross-sectional, primarily Internet-based commercial survey of adults (aged 18 years and older) fielded annually in the USA and periodically in nine other countries. The majority of potential respondents to NHWS are identified through commercial survey panels (e.g., MySurvey.com and Lightspeed GMI), which recruit their members through convenience methods such as targeted advertisements, e-newsletters, and online banner placements. The NHWS sample is derived from these panels through random sampling stratified by key characteristics to attempt a sample derivation that is representative of the adult population of each participating country. These include age and sex for all countries with the addition of white/nonwhite race in the USA. Online recruitment is supplemented by offline recruitment for certain age-groups in countries with lower Internet penetration among the older population (Brazil, China; 2013 France, Germany, Italy, Spain; 2016 Germany, Italy). The 2013 surveys received Institutional Review Board (IRB) approval from the Essex IRB (Lebanon, New Jersey, USA). The 2015 and 2016 surveys were approved by the Pearl IRB (Indianapolis, Indiana, USA). All respondents provided informed consent prior to participating.
Study Cohort
The surveys used to create the cohort were from the USA (2013, 2015), Europe (EU5: UK, France, Germany, Italy, and Spain; 2013, 2016), Brazil (2015), and China (2013, 2015). Depending on the year and country of the survey, 4–16% of those who were invited to the survey completed the entire NHWS. These response rates were largely due to individuals not opening the invitation or not clicking on the survey link; most of those who clicked the link and completed the screening questions (61–79%, again depending on country and year) completed the survey. Because the NHWS is a cross-sectional survey and multiple years of data are included, a few respondents might have participated in multiple years. To avoid having these respondents unduly impact the results, only 1 year of data for such respondents were included in the analysis. For respondents who completed the survey twice, data of the first year were used if they reported a history of CDI in the first year. The data of the second (i.e., most recent) year of survey were used if the respondent reported a history of CDI in only the second year or if the respondent indicated no history of CDI in either year.
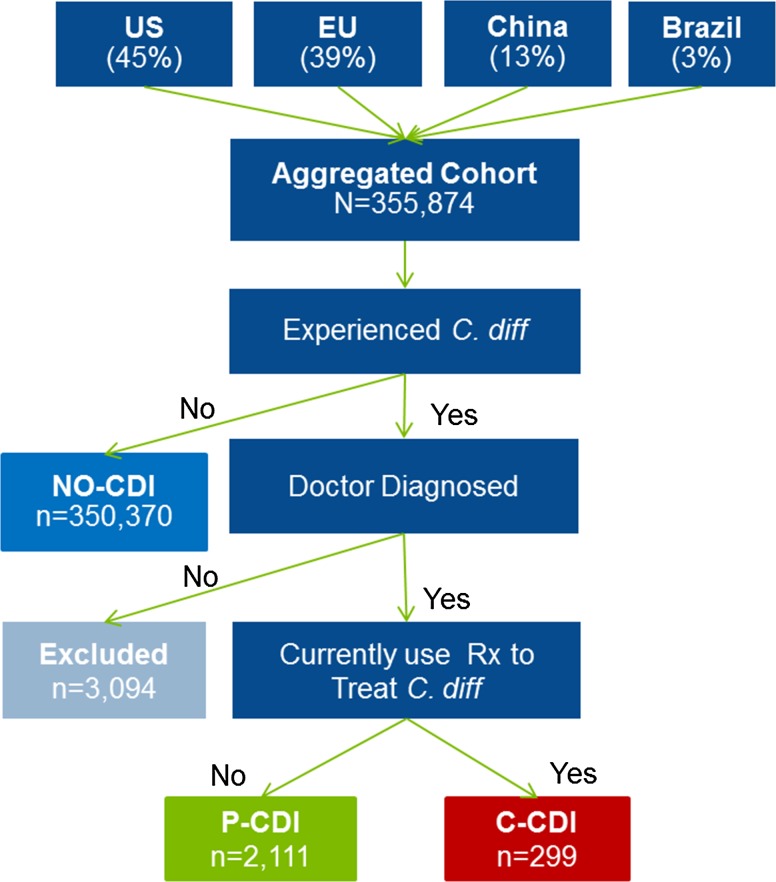
The cohort was divided into three groups according to CDI status based on self-reports: current CDI (C-CDI), prior CDI (P-CDI), and never experienced CDI (NO-CDI) (Fig. 1). To be categorized as C-CDI, the respondent had to say “yes” to all of the following: (1) having ever experienced “C. diff (antibiotic associated colitis which is diarrhea from antibiotic use),” (2) having a physician diagnosis of CDI, and (3) currently using prescription medication to treat CDI. P-CDI respondents reported all of the following: (1) ever experienced CDI, (2) a physician diagnosis for CDI, and (3) not currently using prescription medication to treat CDI. Treatment status was used in an attempt to differentiate respondents whose CDI had resolved versus those who were currently experiencing infection. NO-CDI respondents were limited to those who did not report ever experiencing CDI.
Fig. 1.
Sample diagram
Demographic and Health Characteristic Measures
Demographic variables from the NHWS included age, education, gender, insurance status, employment status, and ethnicity (USA, Brazil, and China only). As response options for ethnicity, health insurance, and education vary among countries and across different years of the NHWS, these variables were harmonized to allow for inclusion in the analysis. Ethnicity was categorized into either majority or minority categories at the country level, with majority defined as the most frequently reported ethnicity within that specific country, with all others categorized as minority. Insurance was dichotomized as either reporting having any kind of insurance (public and/or private), or reporting having no insurance of any kind. The education variable was categorized as the highest level of education completed based on the 2011 International Standard Classification of Education developed by the United Nations Educational, Scientific and Cultural Organization (UNESCO) as below upper secondary education, upper secondary or post-secondary nontertiary, tertiary, or decline to answer [26].
Patient health characteristics included the Charlson comorbidity index (CCI) calculated from self-reported comorbidities [27], antibiotic use, and healthcare resource utilization (HCRU). As the NHWS does not collect information on paraplegia or liver disease severity, the CCI used herein did not include these conditions. All HCRU variables used 6-month recall except in the case of surgery, where 12-month recall was required. The descriptive statistics for the number of HCRU visits were calculated to include respondents with no reported visits.
Outcomes Measures
The Medical Outcomes Study 36-Item Short-Form Health Survey version 2 (SF-36v2®) standard (4-week recall) form was used in the NHWS to measure HRQoL [28]. The SF-36v2® provides scores for 8 subscales (domains) which are comprised of mutually exclusive questions: general health (GH), social functioning (SF), vitality (VT), bodily pain (BP), mental health (MH), physical functioning (PF), role-emotional (RE) and role-physical (RP), which are further combined into two scores summarizing mental (mental component summary score [MCS]) and physical (physical component summary score [PCS]) health. Consistent with usual practice, the scores presented here are the norm-based scores, which are standardized so that each scale has a mean score of 50 and standard deviation of 10 in the US general population. Lower values indicate worse outcomes. The SF-36v2® also provides health utility scores with the SF-6D, a single preference-based measure of health using general population values.
Impairment to work and other activities was measured using the Work Productivity and Activity Impairment questionnaire, general health version (WPAI-GH), with a 7-day recall [29]. Outcomes calculated from responses to the WPAI-GH included degree of activity impairment due to health (activity impairment), degree of impairment while working due to health (presenteeism), percent of work missed due to health (absenteeism), and degree of overall work impairment due to health (overall work impairment, a combination of both presenteeism and absenteeism). Presenteeism, absenteeism, and overall work impairment assessments were restricted to respondents who reported part-time, full-time, or self-employment. Activity impairment was assessed among all respondents. Higher values indicate a greater degree of impairment.
Statistical Analysis
Unadjusted descriptive summary statistics were calculated for the aggregated cohort by CDI status for all demographic and outcome variables. Demographic and outcome variables were compared using the t test for continuous variables and the Chi-square test for categorical variables. Mean norm-based health domain scales were described and compared between CDI groups for the aggregated cohort using the equal or unequal variance t-test depending on the variance equality test result.
Regression modeling assessed the association between CDI status and outcomes after adjusting for CCI category (0, 1 or 2, 3 or more), education level, age category (18–49, 50–64, and ≥ 65 years), sex, and country. Estimates and standard errors for activity impairment, presenteeism, absenteeism, and overall work impairment were exponentiated and derived from a generalized linear model with negative binomial distribution with log link function. All HRQoL outcomes were assessed between groups using a general linear model with normal distribution.
For all comparisons, p values less than 0.05 were considered statistically significant. Minimally important differences (MIDs) were defined based on criteria from the developers of each measure. For the SF-36v2, MIDs are defined as differences in norm-based scores of at least 2 points for PCS, GH, and VT; 3 points for each of MCS, PF, BP, RP, SF, and MH; and 4 points for RE [28]. For SF-6D, MID was defined as the difference in average health utility of at least 0.041 [30].
All analyses were performed using Statistical Analysis System (SAS), version 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Among the 352,780 respondents included in the analysis, 299 (0.08%) met the criteria for C-CDI and 2111 (0.60%) for P-CDI. A plurality of respondents (45%) were from the USA, 39% from the EU5, 13% from China, and 3% from Brazil (Table 1). Of those reporting C-CDI, nearly one-half were from the EU5 (48%), while the majority of the P-CDI respondents were in the USA (59%). Compared to those with NO-CDI and P-CDI, respondents with C-CDI were older, were less often employed, and had a higher mean CCI score (Table 1). Fewer people in the C-CDI and NO-CDI groups were female than in P-CDI (49, 53 vs. 67%; p values < 0.0001). Education levels differed significantly among groups, with P-CDI responders less likely than the other two groups to have tertiary education (Table 1). There were a significantly greater number of healthcare provider visits, emergency room visits, and hospitalizations among persons with C-CDI than among those with NO-CDI or P-CDI (Table 2). A greater proportion of the C-CDI group reported surgeries and at least one physician visit, with the mean number of healthcare encounters among those with at least one visit also significantly higher in C-CDI than in NO-CDI or P-CDI groups (Table 2).
Table 1.
Descriptive characteristics
| Never experienced CDI (NO-CDI) n = 350,370 |
Experienced doctor-diagnosed CDI | p value C-CDI versus P-CDI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not currently treated (P-CDI) n = 2111 |
Currently treated (C-CDI) n = 299 |
|||||||||||
| p value versus NO-CDI | p value versus NO-CDI | |||||||||||
| Country (n, %) | ||||||||||||
| Brazil | 11,933 | 3.4% | 12 | 0.6% | < .0001 | 14 | 4.7% | 0.1243 | < .0001 | |||
| China | 45,833 | 13.1% | 16 | 0.8% | < .0001 | 21 | 7.0% | 0.0019 | < .0001 | |||
| USA | 157,285 | 44.9% | 1245 | 58.9% | < .0001 | 121 | 40.5% | 0.2239 | < .0001 | |||
| EU | 135,319 | 38.6% | 838 | 39.7% | 0.3118 | 143 | 47.8% | 0.0011 | 0.0074 | |||
| Age [mean; median (SD)] | 45.9 | 45.0 | (16.47) | 52.5 | 54.0 | (15.97) | < .0001 | 48.4 | 48.0 | (16.44) | 0.0122 | < .0001 |
| Age group (n, %) | < .0001 | 0.15 | 0.0002 | |||||||||
| 18–49 | 203,673 | 58.1% | 887 | 42.0% | 164 | 54.9% | ||||||
| 50–64 | 83,364 | 23.8% | 622 | 29.5% | 68 | 22.7% | ||||||
| ≥ 65 | 63,333 | 18.1% | 602 | 28.5% | 67 | 22.4% | ||||||
| Employed (n, %) | 206,821 | 59.0% | 909 | 43.1% | < .0001 | 149 | 49.8% | 0.0012 | < .0001 | |||
| Education level (n, %) | < .0001 | 0.2663 | 0.0056 | |||||||||
| Below upper secondary | 39,899 | 11.4% | 202 | 9.6% | 43 | 14.4% | ||||||
| Upper secondary or post-secondary | 124,778 | 35.6% | 893 | 42.3% | 103 | 34.5% | ||||||
| Tertiary | 184,407 | 52.6% | 1011 | 47.9% | 152 | 50.8% | ||||||
| Female (n, %) | 183,939 | 52.5% | 1420 | 67.3% | < .0001 | 146 | 48.8% | 0.2041 | < .0001 | |||
| Majority ethnicitya (n, %) | 167,478 | 78.1% | 1143 | 89.9% | < .0001 | 134 | 85.9% | 0.0181 | 0.1289 | |||
| Health insurance status—insured (n, column %) | 307,005 | 87.6% | 1985 | 94.0% | < .0001 | 273 | 91.3% | 0.1316 | 0.1294 | |||
| Adapted CCI score [mean; median (SD)] | 0.2 | 0.0 | (0.7) | 0.9 | 0.0 | (1.6) | < .0001 | 2.6 | 1.0 | (4.6) | < .0001 | < .0001 |
| Adapted CCI score category (n, %) | < .0001 | < .0001 | < .0001 | |||||||||
| 0 | 309,829 | 88.4% | 1255 | 59.5% | 128 | 42.8% | ||||||
| 1 or 2 | 34,980 | 9.9% | 607 | 28.8% | 92 | 30.8% | ||||||
| ≥ 3 | 5561 | 1.6% | 249 | 11.8% | 79 | 26.4% | ||||||
Unless otherwise noted, the percentage is calculated as the respondents for that category divided by the total nonmissing or declined-to-answer responses for that question
aRespondents from Europe are not included in ethnicity as it is not collected for European countries in the NHWS
Table 2.
Healthcare resource utilization by CDI status
| Never experienced CDI (NO-CDI) n = 350,370 |
Experienced doctor-diagnosed CDI | p value C-CDI versus P-CDI | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not currently treated (P-CDI) n = 2111 |
Currently treated (C-CDI) n = 299 |
|||||||||||
| p value versus NO-CDI | p value versus NO-CDI | |||||||||||
| Surgery in past 12 months (n, %) | 44,087 | 12.6% | 664 | 31.5% | < .0001 | 131 | 43.8% | < .0001 | < .0001 | |||
| Traditional HCP visits [mean; median (SD)] | 3.8 | 2.0 | (5.92) | 10.1 | 7.0 | (12.1) | < .0001 | 14.7 | 8.0 | (30.4) | 0.0092 | 0.0092 |
| Emergency room visits [mean; median (SD)] | 0.3 | 0.0 | (1.18) | 0.7 | 0.0 | (2.1) | < .0001 | 1.6 | 0.0 | (3.4) | < .0001 | < 0.0001 |
| Hospitalizations [mean; median (SD)] | 0.1 | 0.0 | (0.99) | 0.5 | 0.0 | (1.8) | < .0001 | 1.1 | 0.0 | (2.6) | < .0001 | < 0.0001 |
| Gastroenterologist visits [mean; median (SD)] | 0.06 | 0.00 | (0.40) | 0.33 | 0.00 | (1.02) | < .0001 | 0.61 | 0.00 | (1.48) | < .0001 | 0.0023 |
| Infectious disease specialist visits [mean; median (SD)] | 0.01 | 0.00 | (0.25) | 0.10 | 0.00 | (0.91) | < .0001 | 0.30 | 0.00 | (1.31) | 0.0001 | 0.0086 |
| Internist visits [mean; median (SD)] | 0.16 | 0.00 | (0.80) | 0.38 | 0.00 | (1.31) | < .0001 | 0.57 | 0.00 | (1.40) | < .0001 | 0.0197 |
Unless otherwise noted, the percentage is calculated as the respondents for that category divided by the total nonmissing or declined-to-answer responses for that question
Unless otherwise noted, healthcare resource utilization recall is past 6 months
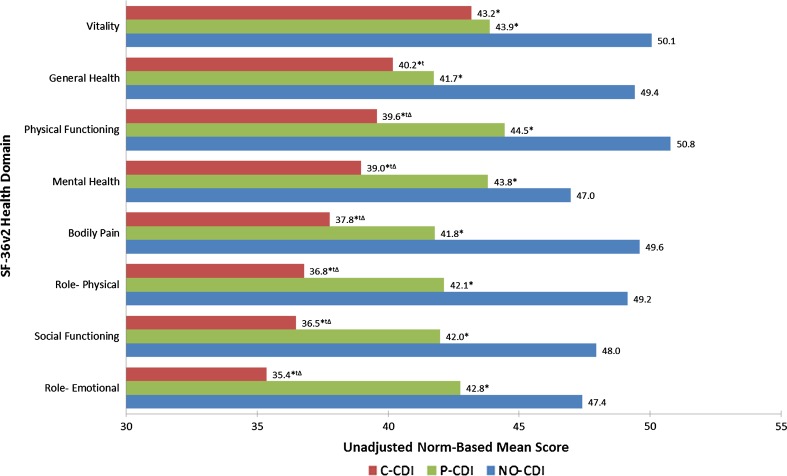
Respondents with C-CDI and P-CDI reported significantly lower unadjusted mean scores across the eight SF-36 domains compared with those with NO-CDI (Fig. 2). The C-CDI group had significantly lower scores than the P-CDI group on all domains except vitality. Results of country-level unadjusted analyses mirrored the aggregated analysis (data not shown). Unadjusted differences in individual health domains between groups are shown in Fig. 2.
Fig. 2.
Unadjusted SF-36v2® norm-based mean health domain scores by CDI status. *Statistically significant difference meeting MID criteria versus NO-CDI, tstatistically significant difference versus P-CDI, Δmeeting MID criteria versus P-CDI
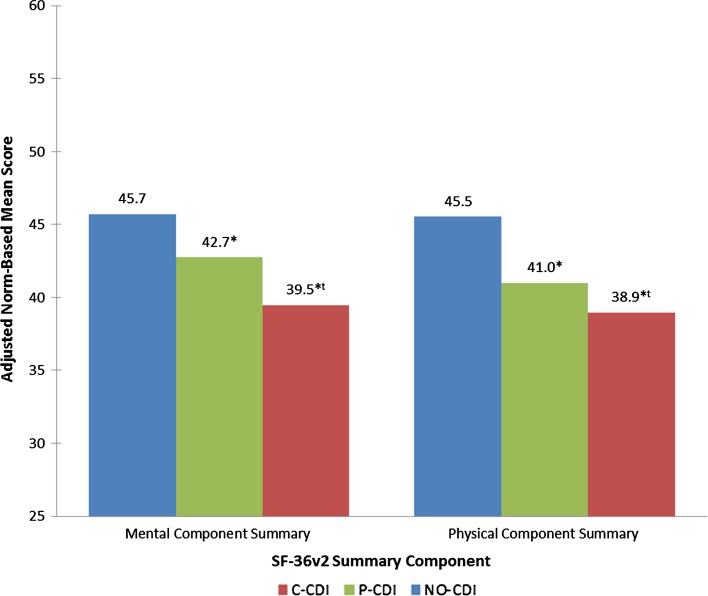
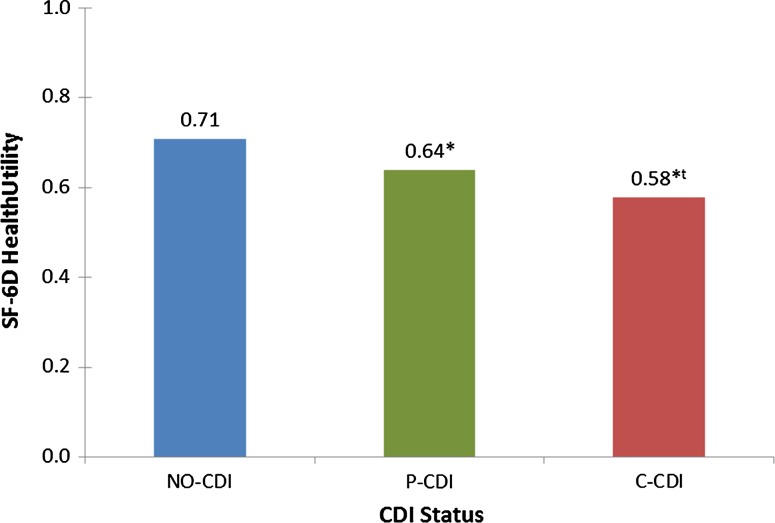
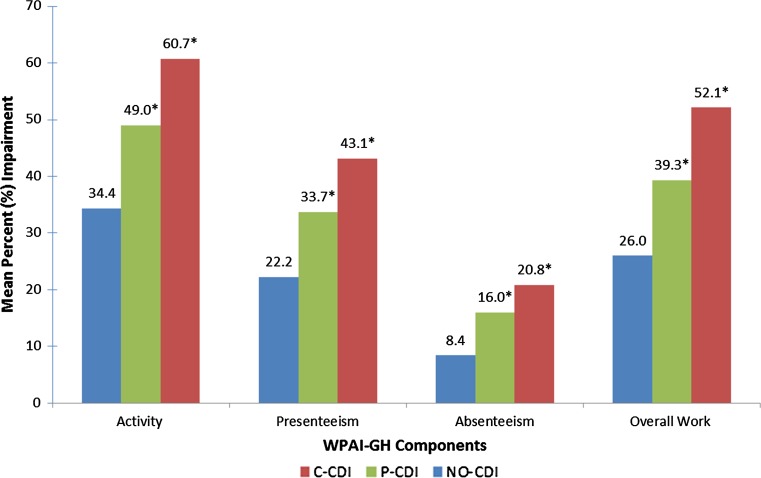
After adjustment for CCI scores, age, sex, education level, and country, C-CDI and P-CDI were associated with significantly lower (all p values < 0.05) mean scores for MCS (39.5, 42.7 vs. 45.7), PCS (38.9, 40.9 vs. 45.5), and health utility (0.58, 0.64 vs. 0.71) vs. NO-CDI, respectively (Figs. 3 and 4). Persons with C-CDI had significantly lower mean scores on the MCS, PCS, and health utility versus those with P-CDI. C-CDI and P-CDI groups reported significantly greater percent of work time missed (20.8, 16.0 vs. 8.4%), degree of impairment while working (43.1, 33.7 vs. 22.2%), degree of overall work impairment (52.1, 39.3 vs. 26.0%), and degree of activity impairment (60.7, 49.0 vs. 34.4%) due to health than NO-CDI group, respectively (C-CDI or P-CDI vs. NO-CDI, all p values < 0.05; Fig. 5). There were no significant differences between C-CDI and P-CDI groups with respect to work productivity and activity impairment measures (Fig. 5).
Fig. 3.
Adjusted mean SF-36v2® summary scores by CDI status. * Statistically significant difference meeting MID criteria versus NO-CDI, t statistically significant difference meeting MID criteria versus P-CDI
Fig. 4.
Adjusted mean health utility by CDI status. * Statistically significant difference meeting MID criteria versus NO-CDI, t statistically significant difference meeting MID criteria versus P-CDI
Fig. 5.
Adjusted mean work and activity impairment by CDI status. * Statistically significant difference versus NO-CDI, differences between P-CDI and C-CDI were not statistically significant
Discussion
In this large and geographically diverse analysis of the impact of self-reported CDI on HRQoL, we have found that individuals either currently treated for CDI or with a past history but no current treatment for CDI have significantly worse HRQoL, greater impairment in their daily activities, and reduced work productivity than those with no history of CDI. These differences persist even after adjusting for age, sex, CCI scores, education, and country. In addition to worsened HRQoL, C-CDI respondents suffer diminished work productivity, with the rate of absenteeism 2.5 times higher than among those with no history of CDI. The productivity loss among those attending work (i.e., presenteeism) associated with C-CDI is nearly double that of respondents with no CDI history. Additionally, after adjustment for covariates, differences between groups in PCS, MCS, and health utility met MID criteria (Figs. 3 and 4). Compared to NO-CDI respondents, C-CDI and P-CDI persons reported significantly lower unadjusted mean scores across the eight SF-36v2 domains meeting MID criteria (Fig. 2). When comparing P-CDI and C-CDI persons, MIDs persisted for all health domains except for VT and GH where the difference did not meet the thresholds for MID (Fig. 2).
One prior study has noted a substantial degree of impairment to HRQoL, work, and activities in CDI using the same instruments. In an analysis of the 2010 US NHWS, Peery and colleagues noted that patients who had ever experienced CDI had a mean MCS of 45, mean PCS of 39, mean degree of activity impairment of 49%, and mean degree of overall work impairment of 50% [24]. Although difficult to interpret without a comparator, this study’s results hinted at the magnitude of CDI-associated impairment. Our results build on Peery’s work by confirming the overall CDI-related decrements and further clarify the differential impacts of prior and current CDI on people in comparison with those who have never experienced this affliction. In addition, whereas Peery’s analysis did not attempt to deal with confounding, our adjusted analyses demonstrate persistent magnitude of CDI-associated HRQoL impairment.
Taken in concert, our results may indicate that the impact of CDI on HRQoL is different from other diarrheal diseases. A study conducted in the US sample of NHWS found, after adjusting for confounders, that patients with irritable bowel syndrome (IBS), diarrhea subtype (IBS-D), reported significantly lower HRQoL (mean MCS 45.2 vs. 49.5 p < 0.001; mean PCS 47.3 vs. 50.7; p < 0.001; mean SF-6D 0.68 vs. 0.74; p < 0.001) and greater absenteeism (5.1 vs. 2.9%; p = 0.004), presenteeism (17.9 vs. 11.3%; p < 0.001), overall work productivity loss (20.7 vs. 13.2%, p < 0.001), and activity impairment (29.6 vs. 18.9%; p < 0.001) than those without IBS-D [31]. Another study using the US NHWS found that persons with any form of IBS reported mean unadjusted MCS, PCS, activity impairment, and overall work impairment scores of 43, 42, 43, and 30%, respectively [24]. A key threat to the validity of comparing these studies to ours is the chronicity of IBS, which contrasts with the generally acute nature of CDI. In a small hospital-based matched case–control study, domains of the SF-36 were found to be lower among patients with antibiotic-associated diarrhea (n = 18) than among those without (n = 36); however, while the differences met MID criteria, no statistically significant differences were found, most likely due to the small sample size [32]. The same study also assessed HRQoL using a validated gastrointestinal disease-specific instrument, finding significantly worse HRQoL among those in the hospital with antibiotic-associated diarrhea, when compared to the published norms for active IBS patients in the USA [32].
Since diarrhea is the most frequent symptom of CDI [33] and diarrheal diseases are known to impair HRQoL, CDI’s impact on HRQoL is not surprising. Multiple patient populations, such as those with renal transplant or patients with HIV, have reported worsened HRQoL in association with acute diarrhea, some showing a dose–response effect, where the HRQoL is diminished in proportion to greater diarrheal symptoms [34, 35].
Health utilities for noninfectious diarrhea from symptomatic prostate cancer, colorectal cancer chemotherapy-induced diarrhea, or ulcerative colitis have been used as proxies for CDI in cost-effectiveness modeling [36–39]. Given that our results suggest greater impairments to HRQoL than have been noted in other gastrointestinal afflictions, a CDI-specific instrument may be useful in improving the accuracy and precision of these estimates. To date, no CDI-specific HRQoL instrument published has been fully validated, although CDI-DaySyms™ and Cdiff32 are known to be in development [23, 40].
Our study has a number of limitations, most notably the likelihood of selection bias. While the NHWS is constructed to be representative of the general population of each country in terms of age, sex and, in the USA, racial and ethnic mix, the self-selected nature of the respondents due to the opt-in nature and the low response rates almost guarantee some degree of selection bias, likely leaving out in particular people who are either too sick to participate, or too healthy to bother. Therefore, it is likely that our results cannot be generalized to persons suffering a current bout of more severe CDI. However, it is also likely that those people, if included in our study, would have substantially increased the magnitude of HRQoL impairments associated here with current CDI. The fact that we identified an important HRQoL impairment in a group of patients with what is most likely mild-to-moderate disease demonstrates CDI’s detrimental impact on these outcomes. Additionally, the majority of respondents in our analysis are from the USA or EU5, which may limit the generalizability of results outside of these geographic areas. Classification of respondents by CDI status relied on individuals being aware of and reporting their medical diagnosis. It is possible that respondents may not have identified their antibiotic-associated diarrhea as CDI. To partially address this, respondents who indicated “C. difficile (antibiotic associated colitis which is diarrhea from antibiotic use),” but did not indicate their condition was doctor-diagnosed were excluded; however, the possibility remains that true CDI respondents were misclassified as NO-CDI leading to more conservative results. Our results may also be subject to residual confounding as, though we adjusted for age, comorbidities, sex, education, and country, we could not adjust for differences in HCRU between groups due to unknown temporality. Additionally, there were likely unmeasured or unknown confounders that could have affected the results. As CDI diagnosis and treatment status were self-reported and not independently verified, the possibility of misclassification due to a recall bias exists. Variable mapping allowed us to combine data across countries for a larger sample size, but may have introduced measurement error in the covariates as the variable categories in the aggregated cohort were collapsed into fewer categories for health insurance, race/ethnicity, and education level. It should be noted that this increase in error variance would tend to reduce power (type 2 error) in the adjusted analysis rather than result in spurious findings (type 1 error). As clinically important differences have not been defined for diarrheal diseases, this study used common benchmarks for MIDs as defined by the developers of the measures; however, MIDs and clinically important differences are known to vary between populations and at different levels of HRQoL. Finally, as with any cross-sectional study, causality should not be inferred. That is, it is possible that at least in a subsample of the responders it was the worsened HRQoL that led to a positive response on the CDI questions, at least in the absence of current treatment.
Its limitations notwithstanding, our study highlights the global humanistic burden of CDI and raises important concerns regarding the degree to which CDI is associated with HRQoL, work, and activity impairments. Understanding the precise extent of CDI’s impact on its sufferers’ HRQoL and impairment to work is essential in order to quantify the indirect costs and benefits of treatment and prevention strategies for this potentially debilitating infection.
Funding
This study was sponsored by Pfizer, Inc.
Compliance with ethical standards
Conflict of interest
HK, YH, VJ, CR, and HJ are employees and stockholders of Pfizer, Inc., the study sponsor. ZM has received compensation as a consultant to Pfizer, Inc., including for the development of this manuscript. She has also received research and/or consulting funding from Merck, The Medicines Company, Astellas, Melinta, Tetraphase, and Paratek. Editorial support was provided by Scott Vuocolo of Pfizer, Inc.
Contributor Information
Kirstin Heinrich, Email: Kirstin.heinrich@pfizer.com.
James Harnett, Email: James.harnett@pfizer.com.
Jeffrey Vietri, Email: Jeffrey.Vietri@pfizer.com.
Richard Chambers, Email: Richard.Chambers@Pfizer.com.
Holly Yu, Email: Holly.Yu@Pfizer.com.
Marya Zilberberg, Email: evimedgroup@gmail.com.
References
- 1.Keller JM, Surawicz CM. Clostridium difficile infection in the elderly. Clin Geriatr Med. 2014;30:79–93. doi: 10.1016/j.cger.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller BA, Chen LF, Sexton DJ, Anderson DJ. Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol. 2011;32:387–390. doi: 10.1086/659156. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention [CDC] Antibiotic resistance threats in the United States. Washington, DC: Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 5.Chitnis A, Holzbauer S, Belflower R, et al. Epidemiology of community-associated Clostridium difficile infection, 2009–2011. JAMA Intern Med. 2013;173:1359–1367. doi: 10.1001/jamainternmed.2013.7056. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2015 Annual Report from the Emerging Infections Program for Clostridium difficile Infection. Department of Health and Human Services; 2015. https://www.cdc.gov/hai/eip/pdf/cdiff/2015-CDI-Report.pdf. Accessed 21 Mar 2018.
- 7.Khanna S, Pardi D, Aronson S, Kammer P, Orenstein R, Sauver JS. The epidemiology of community-acquired Clostridium difficile infection: a population-based study. The American Journal of Gastroenterology. 2012;107:89–95. doi: 10.1038/ajg.2011.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbut F, Mastrantonio P, Delmee M, et al. Prospective study of Clostridium difficile infections in Europe with phenotypic and genotypic characterisation of the isolates. Clin Microbiol Infect. 2007;13:1048–1057. doi: 10.1111/j.1469-0691.2007.01824.x. [DOI] [PubMed] [Google Scholar]
- 9.Borren NZ, Ghadermarzi S, Hutfless S, Ananthakrishnan AN. The emergence of Clostridium difficile infection in Asia: a systematic review and meta-analysis of incidence and impact. PLoS ONE. 2017;12:e0176797. doi: 10.1371/journal.pone.0176797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins DA, Hawkey PM, Riley TV. Epidemiology of Clostridium difficile infection in Asia. Antimicrob Resist Infect Control. 2013;2:21. doi: 10.1186/2047-2994-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies KA, Longshaw CM, Davis GL, et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalised patients with diarrhoea (EUCLID) Lancet Infect Dis. 2014;14:1208–1219. doi: 10.1016/S1473-3099(14)70991-0. [DOI] [PubMed] [Google Scholar]
- 12.Lopardo G, Morfin-Otero R, Moran V, II, et al. Epidemiology of Clostridium difficile: a hospital-based descriptive study in Argentina and Mexico. Braz J Infect Dis. 2015;19:8–14. doi: 10.1016/j.bjid.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elixhauser A (AHRQ), Steiner C (AHRQ), Gould C (CDC). Readmissions following hospitalizations with Clostridium difficile infections. HCUP Statistical Brief #145. December 2012. Agency for Healthcare Research and Quality, Rockville, MD; 2009. Available at http://www.hcup-us.ahrq.gov/reports/statbriefs/sb145.pdf. Accessed 21 Mar 2018. [PubMed]
- 14.Lucado J, Gould C, Elixhauser A. Clostridium difficile infections (CDI) in Hospital Stays. HCUP Statistical Brief #124. January 2012. Agency for Healthcare Research and Quality, Rockville, MD; 2009. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf. Accessed 21 Mar 2018. [PubMed]
- 15.Eckmann C, Wasserman M, Latif F, Roberts G, Beriot-Mathiot A. Increased hospital length of stay attributable to Clostridium difficile infection in patients with four co-morbidities: an analysis of hospital episode statistics in four European countries. Eur J Health Econ. 2013;14:835–846. doi: 10.1007/s10198-013-0498-8. [DOI] [PubMed] [Google Scholar]
- 16.Kwon JH, Olsen MA, Dubberke ER. The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect Dis Clin North Am. 2015;29:123–134. doi: 10.1016/j.idc.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Tabak YP, Zilberberg MD, Johannes RS, Sun X, McDonald LC. Attributable burden of hospital-onset Clostridium difficile infection: a propensity score matching study. Infect Control Hosp Epidemiol. 2013;34:588–596. doi: 10.1086/670621. [DOI] [PubMed] [Google Scholar]
- 18.Dubberke ER, Butler AM, Reske KA, et al. Attributable outcomes of endemic Clostridium difficile-associated disease in nonsurgical patients. Emerg Infect Dis. 2008;14:1031–1038. doi: 10.3201/eid1407.070867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55:S88–S92. doi: 10.1093/cid/cis335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghantoji SS, Sail K, Lairson DR, DuPont HL, Garey KW. Economic healthcare costs of Clostridium difficile infection: a systematic review. J Hosp Infect. 2010;74:309–318. doi: 10.1016/j.jhin.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Yu H, Baser O, Wang L. Burden of Clostridium difficile-associated disease among patients residing in nursing homes: a population-based cohort study. BMC Geriatr. 2016;16:193. doi: 10.1186/s12877-016-0367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European CDC [ECDC]. Clostridium difficile basic facts. http://www.ecdc.europa.eu/EN/HEALTHTOPICS/CLOSTRIDIUM_DIFFICILE_INFECTION/BASIC_FACTS/Pages/basic_facts.aspx. Accessed 20 May 2013.
- 23.Garey KW, Aitken SL, Gschwind L, et al. Development and validation of a Clostridium difficile health-related quality-of-life questionnaire. J Clin Gastroenterol. 2016;50:631–637. doi: 10.1097/MCG.0000000000000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peery AF, Dellon ES, Lund J, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilcox MH, Ahir H, Coia JE, et al. Impact of recurrent Clostridium difficile infection: hospitalization and patient quality of life. J Antimicrob Chemother. 2017;72:2647–2656. doi: 10.1093/jac/dkx174. [DOI] [PubMed] [Google Scholar]
- 26.Organisation for Economic Co-operation and Development (OECD) Education at a glance 2017: OECD indicators. Paris: OECD Publishing; 2017. [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Maruish ME, editor. User’s manual for the SF-36V2®v2 health survey. 3. Lincoln, RI: Quality Metric Inc; 2011. [Google Scholar]
- 29.Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4:353–365. doi: 10.2165/00019053-199304050-00006. [DOI] [PubMed] [Google Scholar]
- 30.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14:1523–1532. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 31.Buono JL, Carson RT, Flores NM. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes. 2017;15:35. doi: 10.1186/s12955-017-0611-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gbito KE. The impact of antibiotic associated diarrhea on health-related quality of life in hospitalized patients (Unpublished masters thesis). The University of Texas School of Public Health, Houston, Texas; 2009. https://search.proquest.com/openview/2c025c7da2ca63ef25aaac0ea0939017/1.pdf?pq-origsite=gscholar&cbl=18750&diss=y. Accessed 21 Mar 2018.
- 33.Kelly CP, Lamont JT. Patient education: antibiotic-associated diarrhea caused by Clostridium difficile. In: EL Baron (ed) Uptodate. https://www.uptodate.com/contents/antibiotic-associated-diarrhea-caused-by-clostridium-difficile-beyond-the-basics. Accessed 1 Nov 2017.
- 34.Ekberg H, Kyllonen L, Madsen S, Grave G, Solbu D, Holdaas H. Increased prevalence of gastrointestinal symptoms associated with impaired quality of life in renal transplant recipients. Transplantation. 2007;83:282–289. doi: 10.1097/01.tp.0000251923.14697.f5. [DOI] [PubMed] [Google Scholar]
- 35.Siddiqui U, Bini EJ, Chandarana K, et al. Prevalence and impact of diarrhea on health-related quality of life in HIV-infected patients in the era of highly active antiretroviral therapy. J Clin Gastroenterol. 2007;41:484–490. doi: 10.1097/01.mcg.0000225694.46874.fc. [DOI] [PubMed] [Google Scholar]
- 36.Bartsch SM, Curry SR, Harrison LH, Lee BY. The potential economic value of screening hospital admissions for Clostridium difficile. Eur J Clin Microbiol Infect Dis. 2012;31:3163–3171. doi: 10.1007/s10096-012-1681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konijeti GG, Sauk J, Shrime MG, Gupta M, Ananthakrishnan AN. Cost-effectiveness of competing strategies for management of recurrent Clostridium difficile infection: a decision analysis. Clin Infect Dis. 2014;58:1507–1514. doi: 10.1093/cid/ciu128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee BY, Popovich MJ, Tian Y, et al. The potential value of Clostridium difficile vaccine: an economic computer simulation model. Vaccine. 2010;28:5245–5253. doi: 10.1016/j.vaccine.2010.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stranges PM, Hutton DW, Collins CD. Cost-effectiveness analysis evaluating fidaxomicin versus oral vancomycin for the treatment of Clostridium difficile infection in the United States. Value Health. 2013;16:297–304. doi: 10.1016/j.jval.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Talbot GH, Kleinman L, Davies EW, Hunsche E, Roberts L, Nord CE. The Clostridium difficile infection—daily symptoms (CDI-DaySyms™) patient-reported outcome (PRO) questionnaire: final validation and responder thresholds. Open Forum Infectious Diseases. 2017;4:S394. doi: 10.1093/ofid/ofx163.980. [DOI] [Google Scholar]