Abstract
Subcutaneous delivery of biotherapeutics has become a valuable alternative to intravenous administration across many disease areas. Although the pharmacokinetic profiles of subcutaneous and intravenous formulations differ, subcutaneous administration has proven effective, safe, well-tolerated, generally preferred by patients and healthcare providers and to result in reduced drug delivery-related healthcare costs and resource use. The aim of this article is to discuss the differences between subcutaneous and intravenous dosing from both health-economic and scientific perspectives. The article covers different indications, treatment settings, administration volumes, and injection devices. We focus on biotherapeutics in rheumatoid arthritis (RA), immunoglobulin-replacement therapy in primary immunodeficiency (PI), beta interferons in multiple sclerosis (MS), and monoclonal antibodies (mAbs) in oncology. While most subcutaneous biotherapeutics in RA, PI, and MS are self-administered at home, mAbs for oncology are still only approved for administration in a healthcare setting. Beside concerns around the safety of biotherapeutics in oncology, a key challenge for self-administration in this area is that doses and dosing volumes can be comparatively large; however, this difficulty has recently been overcome to some extent by the development of high-concentration solutions, the use of infusion pumps, and the coadministration of the dispersion enhancer hyaluronidase. Furthermore, given the increasing number of biotherapeutics being considered for combination therapy and the high dosing complexity associated with these, especially when administered intravenously, subcutaneous delivery of fixed-dose combinations might be an alternative that will diminish these burdens on healthcare systems.
Key Points
| Complex and invasive intravenous administration of biotherapeutics, typically conducted in clinic, contributes to the pressure on healthcare systems. Subcutaneous administration has been shown to be a safe and efficacious dosing alternative that is generally valued by both patients and healthcare professionals. |
| The development of fixed-dose subcutaneous formulations that are delivered independent of patient body weight, technologies that facilitate the injection of dosing volumes > 5 ml, and devices that allow self-administration outside of the hospital setting have all markedly contributed to shifting care to the home setting. |
| Many biotherapeutics are being investigated for combination therapy; subcutaneous administration could further simplify drug delivery with the development of fixed-dose combinations or ready-to-use devices that deliver two or more biotherapeutics via one single subcutaneous injection. |
Introduction
When the first subcutaneous monoclonal antibodies (mAbs) in oncology were developed as an alternative to intravenous infusions [1], this delivery approach for biotherapeutics was already established in several disease areas, including diabetes, rheumatoid arthritis (RA), multiple sclerosis (MS), and primary immunodeficiency (PI). The access required for intravenous infusions, the standard administration route for biologic drugs in oncology, involves invasive procedures that can be inconvenient and painful and a drain on the time and resources of patients, healthcare professionals (HCPs), and the healthcare system in general [2–4].
After subcutaneous formulations of trastuzumab and rituximab were introduced in Europe in 2013 and 2014, respectively, it became evident that a change from intravenous infusions to subcutaneous bolus injections in oncology not only reduced the drug administration burden on the healthcare system but was also generally preferred by patients and HCPs [5–8]. This positive impact led to intensified research aiming to understand the processes behind the absorption of biotherapeutics via interstitial tissue and the impact of changes in the pharmacokinetic profile on efficacy, safety, immunogenicity, and tolerability [9–12].
Subcutaneous medications for diabetes, RA, MS, or PI can be administered in the home by patients or caregivers, but biotherapeutics for cancer treatment are yet to be approved for administration outside a designated healthcare setting. However, given the beneficial impact of home administration on costs and resources [13, 14], efforts are ongoing to assess the feasibility of HCPs administering trastuzumab subcutaneously in the home environment. This change is expected to improve patient quality of life (QoL) and provide support to patients who live far from a hospital or have difficulty traveling. As initial data have been promising, with the safety profile of home-administration consistent with that in a hospital setting [15], it has been proposed that, with appropriate training, patients may administer doses at home in the future [16].
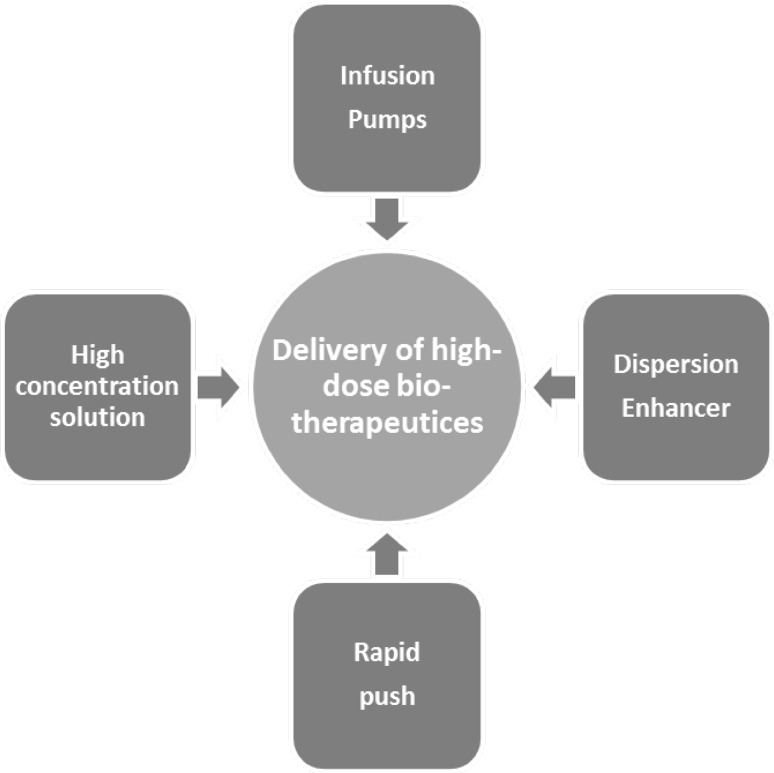
A number of formulation challenges remain to be overcome before home-administration of subcutaneous biotherapeutics can become a reality. Even if a biotherapeutic has been shown to be safe and well-tolerated, home-administration in a life-threatening disease carries the risk of under- or over-dosing. This is particularly true for high-volume dosing solutions, which can be challenging for patients and caregivers. Therefore, scientists have been focusing on ways to further optimize subcutaneous applications of high-dose biotherapeutics (Fig. 1). Significant progress has been made towards the development of high-concentration formulations [17] to reduce the overall volume of a subcutaneous medication. Other work has aimed at improvements in self-injection; it has been shown that coadministration of the dispersion enhancer hyaluronidase facilitates spreading of an injected volume in the subcutaneous interstitial space and, therefore, enables injection of a larger volume at an individualized rate at the patient’s preferred injection site (e.g. thigh, abdomen, or upper arm) [18]. Moreover, infusion pumps can complement these efforts by helping overcome the back pressure generated by subcutaneous tissue during injection [19].
Fig. 1.
Overview of methodologies that can facilitate subcutaneous administration of high-dose biotherapeutics. These different techniques can be used alone or in combination
Another important aspect in the development of subcutaneous administration is the integration of a fixed dosing regimen, i.e. patients receive the same dose independent of body size. This type of dosing regimen has a number of advantages over body weight- or body surface area-adjusted dosing; a fixed dose is less complicated as dose calculations are not necessary, which reduces the potential for dosing errors. Furthermore, population pharmacokinetic (PopPK) modeling using data from clinical trials of various biotherapeutics has confirmed that fixed dosing of mAbs could be feasible for the majority of molecules assessed, without impairing the safety and efficacy of the treatment [20–23].
The aim of this review is to provide an overview of the current status and recent advances in subcutaneous dosing of biotherapeutics compared with intravenous administration in key disease areas. We discuss the impact of subcutaneous biotherapeutic administration on the healthcare system, describe the subcutaneous formulations and administration approaches to overcome issues with high-volume formulations, and describe pharmacokinetic considerations when switching from intravenous to subcutaneous formulations. We also discuss the potential of subcutaneous administration in the context of the increasing number of biotherapeutics being considered for combination therapy [24].
Recent Advances in Subcutaneous Biotherapeutic Administration
This section provides an overview of the major subcutaneous biotherapeutic originator formulations deployed in RA, PI, and MS, and the mAbs in oncology approved by the European Medicines Agency (EMA) and/or the US FDA. Indications have been selected to cover different administration settings, dosing volumes, and injection devices.
RA is a chronic inflammatory autoimmune disease influenced by both genetic and environmental factors that causes inflammation and deformity of the joints [25]. Other autoimmune diseases, such as juvenile RA, psoriatic arthritis, ankylosing spondylitis, plaque psoriasis, Crohn’s disease, or ulcerative colitis, are treated by a range of similar and overlapping drug classes. Most common treatments include nonsteroidal anti-inflammatory drugs, oral immunosuppressants, disease-modifying antirheumatic drugs, and biologics [26]. Most biotherapeutics approved for autoimmune diseases are available in a subcutaneous formulation that can be self-administered at home with a prefilled syringe, a pen injector, or an autoinjector. Currently, the majority of biologic treatments in RA are tumor necrosis factor (TNF)-α inhibitors. Among these, etanercept (Enbrel), adalimumab (Humira), and certolizumab pegol (Cimzia) are available only as subcutaneous formulations. Golimumab (Simponi) offers both intravenous and subcutaneous dosing alternatives, and infliximab (Remicade) is the sole anti-TNFα with only an intravenous formulation on the market. Approved non-anti-TNFα biologics include the B-cell-depleting therapy rituximab (Rituxan/MabThera), with an intravenous formulation, and the selective co-stimulating modulator abatacept (Orencia), with both subcutaneous and intravenous formulations. The interleukin (IL)-6 receptor antagonist (anti-IL-6R) tocilizumab (Actemra) is available for intravenous and subcutaneous administration, and the anti-IL-6R sarilumab (Kevzara) and the IL-1 receptor antagonist (anti-IL-1) anakinra (Kineret) can only be administered subcutaneously (Table 1).
Table 1.
| Molecule | Brand name (originator) | Dosing frequency | Injection volume | Device |
|---|---|---|---|---|
| Anti-TNFα | ||||
| Etanercept | Enbrel (Amgen)a | q1w or twice weekly | 0.5–1 ml | Prefilled syringe, vial, prefilled pen/autoinjector, prefilled cartridge for reusable autoinjector |
| Adalimumab | Humira (AbbVie)a | q2w | 0.4–0.8 ml | Prefilled syringe, vial, prefilled pen |
| Certolizumab pegol | Cimzia (UCB - Euronext and BEL20)a | q2w and q4w | 1 ml | Prefilled syringe, vial, prefilled pen |
| Golimumab | Simponi (Janssen)b | q1m | 0.5–1 ml | Prefilled syringe, prefilled pen/autoinjector |
| Anti-IL-6 | ||||
| Tocilizumab | Actemra (Roche)b | q1w and q2w | 0.9 ml | Prefilled syringe, prefilled pen |
| Sarilumab | Kevzara (Sanofi-Aventis)a | q2w | 1.14 ml | Prefilled syringe, prefilled pen |
| Anti-IL-1 | ||||
| Anakinra | Kineret (Swedish Orphan Biovitrum GmbH)a | q1d or q2d | 0.67 ml | Prefilled syringe |
| Selective co-stimulating modulator | ||||
| Abatacept | Orencia (Bristol-Myers Squibb)b | q1w | 1 ml | Prefilled syringe, prefilled pen/autoinjector |
TNF tumor necrosis factor, IL interleukin, qXw once every X weeks, qXd once every X days, qXm once every X months
aOnly a subcutaneous formulation marketed
bBoth subcutaneous and intravenous formulations marketed
PI is a collection of disorders in which part of the immune system is either missing or does not function normally; treatment includes immunoglobulin-replacement therapy delivered either intravenously or subcutaneously [27]. Unlike in RA, subcutaneous dosing volumes in PI treatment are typically > 10 ml. The first subcutaneous immunoglobulin (Vivaglobin) was licensed for self-administration in the USA in 2006 [28]. Since then, many more products have been approved by the EMA or the FDA for subcutaneous self-administration, typically using an infusion pump or syringe driver pump. Hizentra, Vivaglobin, and HyQvia are only available for subcutaneous delivery, but other PI products can be purchased for intravenous or intramuscular use. The dosing frequency of the subcutaneous immunoglobulin formulations typically ranges between once weekly and once monthly and varies depending on the treatment cycle. The dose may need to be individualized according to pharmacokinetic and clinical response. Maximum volumes per injection site are either not specified in the label or are between 15 (Subcuvia) and 50 ml (Hizentra). Hyqvia is the only immunoglobulin administered following subcutaneous pre-injection of the dispersion enhancer recombinant human hyaluronidase (rHuPH20, Sect. 4), and the maximum volume is 600 ml per site for patients weighing ≥ 40 kg and up to 300 ml per site for patients weighing < 40 kg [64, 65]. In other disease conditions, approved treatments with immunoglobulins are typically for intravenous application and not approved for self-administration. These indications include chronic lymphocytic leukemia, chronic demyelinating polyradiculoneuropathy, idiopathic thrombocytopenic purpura, or multifocal motor neuropathy formulations [29].
MS is a demyelinating disease that damages the nerve cells, resulting in a wide range of symptoms [30]. Currently, five beta interferon disease-modifying biotherapeutics are on the market for relapsing-remitting MS. The first approved beta interferon (Avonex) was made available as an intramuscular formulation for weekly administration. However, the newer formulations are approved for subcutaneous administration in the home setting, with dosing frequencies ranging from once every other day (Betaseron) to once every 2 weeks (Plegridy). The immunomodulator glatiramer acetate (Copaxone) is also available as a subcutaneous formulation, with administration once daily or three times weekly. Given the low injection volume of 0.5–1 ml, all these treatments can be self-administered using a prefilled syringe, an injection pen, or an autoinjector (Table 2).
Table 2.
Beta interferons and glatiramer acetate for subcutaneous administration in multiple sclerosis [64, 65]
| Molecule | Brand name (originator) | Dosing frequency | Injection volume | Device |
|---|---|---|---|---|
| Interferon | ||||
| Interferon beta-1b | Betaseron/Betaferon (Bayer) | q2d | 0.25–1 ml | Prefilled syringea, vial, autoinjector |
| Extavia (Novartis) | q2d | 0.25–1 ml | Prefilled syringea, vial, autoinjector | |
| Peg-interferon beta-1a | Plegridy (Biogen) | q2w | 0.5 ml | Prefilled syringe, prefilled pen/autoinjector |
| Interferon beta-1a | Rebif (EMD Serono/Pfizer) | Three times per week | 0.2–0.5 ml | Prefilled syringe, prefilled pen/autoinjector, electronic injection system |
| Glatiramer acetate | ||||
| Glatiramer acetate | Copaxone (Teva) | q1d or three times per week | 1 ml | Prefilled syringe, pen/autoinjector |
qXd once every X days, qXw once every X weeks
aPrefilled diluent syringe with vial
The mode of action for most mAbs in the treatment of cancer is either to attach and/or block antigens on cancer cells or to interfere with antigen action on other non-cancerous cells or free-floating proteins. Other mAbs can boost the immune response by targeting so-called immune system checkpoints [31]. While most mAbs on the market for cancer treatment are administered intravenously, subcutaneous dosing alternatives have recently been developed, for example, trastuzumab (Herceptin) for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer and rituximab (MabThera/Rituxan Hycela) for non-Hodgkin’s lymphoma (NHL) [32, 33]. These two subcutaneous fixed-dose formulations have the same dosing frequency as the approved bodyweight- or body surface area-adjusted intravenous preparations (Table 3). For trastuzumab, a subcutaneous dose is given every 3 weeks, and the rituximab dosing frequency depends on the type of follicular lymphoma and treatment setting and, therefore, ranges between every 3 weeks and every 3 months. Subcutaneous trastuzumab and rituximab are not approved for home- or self-administration and are dosed manually by an HCP using a vial and handheld syringe in a hospital, physician’s office, or infusion center [32, 33].
Table 3.
| Molecule | Brand name (originator) | Dosing frequency | Injection volume | Device |
|---|---|---|---|---|
| Anti-HER2 mAb | ||||
| Trastuzumab | Herceptin (Roche)a,b | q3w | 5 ml | Vial and syringe |
| Anti-CD20 mAb | ||||
| Rituximab | MabThera/Rituxan Hycela (Roche)a,b | q3w–q3mc | 11.7–13.4 ml | Vial and syringe |
CD cluster of differentiation, HER human epidermal growth factor receptor, mAb monoclonal antibody, qXw once every X weeks, qXm once every X months
aSubcutaneous and intravenous formulation marketed
bSubcutaneous formulation contains recombinant human hyaluronidase (rHuPH20)
cDepending on the type of follicular lymphoma
Daratumumab (Darzalex) is another mAb being developed for subcutaneous administration. Daratumumab binds to cluster of differentiation (CD)-38 overexpressed on multiple myeloma cells and is already marketed for the treatment of multiple myeloma with an intravenous formulation and a weight-adjusted dosing regimen [34]. The novel subcutaneous formulation with a fixed-dose regimen has reached phase III clinical development [35, 36].
The Health-Economic Impact of High-Volume Subcutaneous Biotherapeutic Administration
Of the approved subcutaneous biotherapeutics described, immunoglobulins for replacement therapy in PI and mAbs for the treatment of cancer are deployed as high-volume injections (usually > 5 ml). In this section, we discuss the impact of high-volume subcutaneous administration on patients and HCP preferences and on healthcare costs and resources. Observations from an examination of the available data for trastuzumab and rituximab, which are both approved only for administration in a designated healthcare setting, as well as for immunoglobulins that can be self-administered in the home setting, are highlighted. Overall, data support that subcutaneous administration is simpler than intravenous infusions, can reduce drug delivery-related healthcare costs and resources, and is largely preferred by both patients and HCPs.
Changing the trastuzumab administration route from intravenous to subcutaneous led to several modifications to the treatment regimen, including a move from a bodyweight-adjusted treatment (including the removal of the loading dose) to a fixed-dose regimen. A fixed-dose treatment regimen refers to a single consistent dose given to all patients in all treatment cycles. The subcutaneous dosing alternative presents a number of advantages over intravenous dosing, including hospital and clinical cost savings, reduced time and resource use, increased flexibility in appointment scheduling, and reduced capacity bottlenecks and nursing overtime [5, 37, 38].
Evidence that subcutaneous dosing reduced overall administration time was initially gained from the pivotal registration study, HannaH, which investigated subcutaneous compared with intravenous trastuzumab administration [39]. This was a phase III, randomized, open-label study in patients with HER2-positive breast cancer in the (neo)adjuvant setting. Patients were randomized to receive a loading dose of intravenous trastuzumab 8 mg/kg followed every 3 weeks by a maintenance dose of 6 mg/kg (n = 299) or a fixed dose of subcutaneous trastuzumab 600 mg every 3 weeks (n = 297). HannaH demonstrated that the duration of subcutaneous injections was generally between 1 and 5 min (average 3.3 min) compared with 30–90 min for intravenous administration [40].
In the subsequent PrefHer study, patients with HER2-positive early breast cancer were randomly allocated to either receive four cycles of subcutaneous trastuzumab followed by four cycles of intravenous trastuzumab (n = 245) or vice versa (n = 243) [5]. The respective doses and dosing regimens were the same as in the HannaH study. A time and motion assessment demonstrated that, per session, subcutaneous administration using a handheld syringe saved a mean of 55 min (range 40–81; p < 0.0001) of patient chair time (time between entry and exit of infusion chair) versus intravenous dosing. Moreover, the active HCP time, defined as time actively dedicated by any staff member to prespecified tasks, was reduced by a mean 17 min (range 5–28; p < 0.0001) [41].
These reductions in administration time were reflected in reduced costs, as reported by a PrefHer TaM substudy conducted in the UK from February 2012 to February 2013. This study was a noninterventional, prospective, multicentre, descriptive research study that assessed the cost of HCP time and consumables used in trastuzumab subcutaneous versus intravenous administration. A total of 24 patient episodes were recorded (12 subcutaneous, 12 intravenous). The mean cost of HCP time, including drug preparation and administration, reduced from approximately £132.00 for intravenous to £32.00 for subcutaneous dosing. The cost of consumables was also lower for subcutaneous administration, averaging approximately £1.20 versus £12.90 for intravenous. Total cost savings with SC administration (versus IV) were £111.81 per patient episode (95% confidence interval (CI) of this difference 100.44–123.56; p < 0.001) [42]. The authors concluded that, over a full course of treatment (18 cycles), subcutaneous administration of trastuzumab represented savings of approximately £2012.00 per patient compared with intravenous administration [42].
An independent observational, non-interventional, prospective, monocentric time, motion and cost assessment study in Belgium reported similar outcomes. A total of 130 patient episodes were recorded (65 intravenous, 65 subcutaneous) from October 2015 until November 2016. The doses and dosing regimens were the same as in the HannaH study, and 18 administrations were considered one treatment cycle (1 year). The study demonstrated that patients receiving intravenous trastuzumab spent more time in the daycare oncology unit [mean 172.7 min (min 84, max 320, SD 53.6)] than those receiving subcutaneous treatment [mean 50.2 min (min 5, max 166, SD 33.3)]. Compared with intravenous dosing, subcutaneous administration resulted in a reduction in mean active HCP time: 13.9 min (95% CI 12.6–15.1) compared with 67.6 min (95% CI 64.6–70.7) for intravenous administration. In addition, intravenous administration required additional HCP time for treatment preparation in the hospital pharmacy [9.8 min (min 5.2, max 16.4, SD 2.6)]. The mean cost of HCP time per patient episode was €7.9 (95% CI 7.1–8.6) and €37.4 (95% CI 34.8–39.9) for subcutaneous and intravenous trastuzumab, respectively. The mean overall cost was €10.6 (95% CI 9.8–11.3) for subcutaneous administration and €224.48 (95% CI 221.93–227.03) for intravenous administration. The authors concluded that, in the base-case scenario, subcutaneous administration of trastuzumab led to a total cost saving of €212.93 per administration or €3832.74 per treatment cycle [43]. Significant cost savings were also seen in various other countries [7, 8, 44–47].
In the international PrefHer study, 415/467 patients preferred subcutaneous over intravenous dosing (88.9%; 95% CI 85.7–91.6; p < 0.0001; two-sided test against null hypothesis of 65% subcutaneous preference); 45/467 preferred intravenous (9.6%; 95% CI 7–13); and 7/467 indicated no preference (1.5%; 95% CI 1–3) [5]. The most common reasons for the preference were time saved and reduced pain/discomfort. The perceived qualitative impact of replacing intravenous with subcutaneous trastuzumab was assessed in a survey with HCPs from 17 centers participating in the PrefHer study. Respondents were asked to indicate their level of agreement with several statements on a scale of 1–7 (where 1 was strongly disagree and 7 was strongly agree). The strongest agreements were observed for the following statements: “Due to the ready-to-use, fixed dose of subcutaneous trastuzumab formulations, potential dosing errors would be avoided” (6.2); “Due to the ready-to-use, fixed dose of subcutaneous trastuzumab formulations, there would be less drug wastage” (6.1); and “Staff would have increased availability for other tasks in the pharmacy” (5.9). In total, 235 HCPs participated in the preference questionnaire: 181 (77.0%; 95% CI 71.1–82.2) were more satisfied with subcutaneous administration, 7 (3.0%; 95% CI 1.2–6.0) were more satisfied with intravenous; and 47 (20.0%; 95% CI 15.1–25.7) indicated no preference for either route [48].
The increasing use of the mAb rituximab in the treatment of hematological malignancies in both induction and maintenance settings has placed a major burden on infusion services, resulting in long waiting times for eligible patients [49]. Development of subcutaneous rituximab has reduced infusion time from at least 90 min with intravenous rituximab to just 6–8 min [50]. This has helped alleviate the burden on overall healthcare resources due to the high number of patients treated with rituximab. Similar to trastuzumab, the conversion from intravenous to subcutaneous administration included a change from body surface area-adjusted dosing to a subcutaneous fixed dose. In cycle 1 of both dosing regimens, rituximab is still administered intravenously in a body surface area-adjusted manner.
The multinational, multicentre, non-interventional TaM study [8] was conducted to assess the time actively spent by HCPs and patients during drug administration (chair time). For subcutaneous rituximab, data were collected as part of the MabCute study (actual enrolment 694 patients) both for induction and subsequent maintenance treatment in relapsed–refractory indolent NHL (iNHL). Patients received induction therapy consisting of rituximab every 3–4 weeks for 8 cycles (intravenous 375 mg/m2 in cycle 1 then subcutaneous 1400 mg in cycles 2–8) and 6–8 cycles of standard chemotherapy. Patients who experienced a complete or partial response continued into standard maintenance treatment (subcutaneous rituximab 1400 mg every 8 weeks for 24 months). The MabCute data for rituximab intravenous dosing were collected at the same time in the same centers in the real-world setting in patients with either relapsed/refractory iNHL or previously untreated iNHL. The data revealed a reduction in active HCP time and mean patient chair time of 32% (35.0 min for intravenous versus 23.7 min for subcutaneous; p < 0.0001) and 74% (262.1 min for intravenous, including 180.9 min infusion versus 67.3 min for subcutaneous, including 8.3 min for subcutaneous injection administration), respectively. It was concluded that these time savings could increase the efficiency of the day oncology units, and the time freed with the use of subcutaneous dosing could be deployed on other activities, increasing the number of available appointments, and/or reducing waiting lists. Similar conclusions were also drawn from other time and motion and cost-effectiveness studies [51–54].
Patient preferences were assessed in the PrefMab study. In this randomized, open-label, crossover, phase IIIb study, patients with diffuse large B-cell lymphoma or follicular lymphoma received 8 cycles of rituximab according to two schedules. In arm A, 372 patients received 1 cycle of intravenous rituximab 375 mg/m2 and 3 cycles of subcutaneous rituximab 1400 mg, then 4 cycles of intravenous rituximab 375 mg/m2; in arm B, 371 patients received 4 cycles of intravenous rituximab 375 mg/m2, then 4 cycles of subcutaneous rituximab 1400 mg. Alongside rituximab, both arms received 6–8 cycles of chemotherapy. A patient preference questionnaire was completed post-rituximab administration by 620 and 591 patients at cycle 6 and cycle 8 of therapy, respectively. The majority of patients preferred subcutaneous over intravenous rituximab therapy, regardless of whether they received subcutaneous rituximab during the first or second part of the study; 79–81% expressed an overall preference for subcutaneous administration at cycle 6 and 77–84% at cycle 8. The most common reasons for the preference were less time required in the clinic and increased comfort with subcutaneous administration [55].
For patients with immunodeficiency who receive lifelong immunoglobulin-replacement therapy, improved QoL through home-administration appears to be the most significant driver for moving from an intravenous to a subcutaneous dosing alternative, as subcutaneous immunoglobulin (SCIG) facilitates home-administration [56]. A variety of intravenous immunoglobulin (IVIG) and SCIG treatments are on the market (Sect. 2). Subcutaneous formulations typically have a higher dosing frequency than intravenous formulations, mainly due to challenges inherent to the high-volume doses required. A systematic review of studies comparing the efficacy and safety of IVIG and SCIG was conducted based on data from 1028 eligible patients receiving immunoglobulin replacement (67.7% were adults, 56.3% had a diagnosis of common variable immunodeficiency). The meta-analysis was performed using a random-effects method for immunoglobulin trough levels (odds ratio (OR) 1.00, range 0.84–1.15; p < 0.01), infection rates (OR 0.59, range 0.36–0.97; p = 0.04), and adverse events (OR 0.09, range 0.07–0.11; p < 0.001), which showed a significant preference for SCIG over IVIG. Moreover, SCIG resulted in better health-related QoL and faster functional recovery with less time off work [57].
A cost-minimization analysis of SCIG (IgPro20, Hizentra) compared with IVIG was conducted from September 2010 to November 2011 as part of a prospective, multicentre, open-label, single-arm study. After a screening period, 24 Japanese patients with PI entered an IVIG treatment period with three planned infusions every 3 or 4 weeks, followed by a 12-week SCIG wash-in and wash-out period, and a 12-week SCIG efficacy period. The difference in medical costs and productivity loss from changes in hospital frequency, as well as the impact on QoL between the two routes of administration, was evaluated. Subcutaneous dosing markedly reduced hospital-related absenteeism, with the number of patients and caregivers not absent from work or housework duties and with no reduction in working time increasing from four (17.4%) at week 1 to nine (39.1%) at week 24. Productivity loss was reduced by 60% from baseline at weeks 12 and 24, which resulted in a reduction in costs of ¥10,875 per patient per month at weeks 12 and 24. The Life Quality Index scores included areas such as treatment interferences with daily activities, treatment-related problems (such as convenience or painfulness of infusions), convenience of the setting in which the treatment was conducted, and costs of therapy and transportation to the location of therapy. Index scores for all domains were higher with SCIG than with IVIG [58].
An evidence-based review of 25 randomized and non-randomized trials as well as health-economic assessments comparing SCIG versus IVIG substitution therapy in patients with immunodeficiency showed that improvements in QoL with SCIG were mainly related to home therapy. It was found that the main advantage from an economical point of view lies in fewer days of school or work absence [59].
The Complexities and Improvements for Subcutaneous Administration of High-Volume Biologics
Hyaluronan (hyaluronic acid) and collagen fibers are the main components in the extracellular matrix in subcutaneous connective tissue. Hyaluronan provides a barrier against the spreading of injected fluids [18]. Therefore, the subcutaneous administration of larger volumes at a single injection site could cause difficulties for the administering HCP, patient, and/or caregiver. As splitting dosing volumes that exceed 20 ml for administration at different injection sites can result in reduced patient convenience and less reproducible serum levels, the focus of this section is on efforts made to optimize products to facilitate subcutaneous administration of high-volume biotherapeutics (Fig. 1). These efforts aim to further reduce dosing complexity in the hospital setting but also potentially provide a platform for home- or self-administration. Specifically, the development of high-concentration formulations, the use of infusion pumps, as well co-dosing of the dispersion enhancer hyaluronidase have all been shown to largely facilitate the subcutaneous administration of biotherapeutics.
The preparation of biologics solutions at a concentration of > 100 mg/ml is generally feasible today [60], which is a major step towards reducing the dosing volume. However, the development of such high-concentration dosing solutions remains a challenge for many reasons, including the high viscosities involved, limitations in protein solubility, protein degradation and aggregation, protein stability in long-term storage, and the difficulty of achieving mAb concentrations > 500 mg/ml [17].
Attempts have been made to further overcome the obstacles to large-volume subcutaneous injections, and significant experience is available from work in the field of immunoglobulin-replacement therapy. Subcutaneous infusion of a high-volume solution of immune human serum globulin was first reported in 1952, when a subcutaneous infusion of 20 ml was administered to an 8-year-old patient with agammaglobulinemia [61].
These days, infusion pump-facilitated subcutaneous administration of high-volume immunoglobulins is an established dosing alternative to intravenous infusions, offering the possibility of home- and self-administration, and is generally preferred by patients and caregivers. However, as some patients report local pain, itching, erythema, and induration at the injection site with subcutaneous infusions, such doses are typically given more frequently in smaller individual doses than intravenous infusions (weekly or every 2 weeks subcutaneously versus monthly intravenously).
While infusion pump-facilitated high-volume subcutaneous infusions (3–20 ml, typically administered over 5–20 min) are a safe, effective, and convenient alternative to invasive and lengthy intravenous infusions, some patients still prefer the convenience of the so-called rapid push subcutaneous administration. Here, a syringe and butterfly needle are used to deliver the dosing solution over short intervals (e.g. in < 5 min) according to an individual patient’s comfort level [62].
Subcutaneous infusion of larger volumes can be further facilitated using rHuPH20. This enzyme supports dispersion of a subcutaneously injected volume by temporarily depolymerizing the polysaccharide hyaluronic acid in the intercellular ground substance of connective tissue [63]. rHuPH20 (Hylenex) is FDA approved as an adjuvant to increase the absorption and dispersion of other injected drugs by hypodermoclysis (interstitial infusion), and as an adjunct in subcutaneous urography for improving resorption of radiopaque agents [64]. The safety profile of rHuPH20 supporting the registration of Hylenex has been studied in rodents and non-human primates. Intravenous doses from 38 to 12,000 units/kg in rhesus primates and 10,500 units/kg in rodents have been generally well-tolerated. In primates, rHuPH20 has been shown to be well-tolerated at 45,000 units per subcutaneous injection and 4500 units per periocular injection. A comprehensive overview on the nonclinical safety and mode of action of rHuPH20 has been published [18].
rHuPH20 is commercially available in HyQvia, an SCIG approved by both the EMA and the FDA for the treatment of PI and hypogammaglobulinemia [65]. HyQvia comprises one vial containing hyaluronidase and a second vial containing human immunoglobulin. To ensure optimal depolymerization of hyaluronic acid in the interstitial space, the two components are administered sequentially through the same needle, with rHuPH20 introduced first, followed by the immunoglobulin. In a recent study in patients with PI, rHuPH20-facilitated immunoglobulin (IGHy) administered subcutaneously was largely preferred over SCIG without pre-injection of hyaluronidase. Of the 69 patients who completed a satisfaction survey at the finish of immunoglobulin treatment, 28 received IVIG then SCIG before receiving IGHy, and 41 received IVIG before receiving IGHy; 65.9 and 75.0%, respectively, preferred the IGHy [66]. Subcutaneous co-formulations with rHuPH20 were approved for the mAbs trastuzumab and rituximab for use in the treatment of HER2-positive breast cancer and lymphoma, respectively [12]. Contrasting with HyQvia, rHuPH20 is co-formulated with the subcutaneous active biotherapeutic, omitting the need for pre-injection of the enzyme (sequential administration) [32, 33].
Today, particularly in the area of oncology treatment, a large number of intravenous biologics are reaching the market. In parallel, the already high pressure on healthcare budgets and resources is expected to continue to increase. Intravenous infusion represents a key drug-delivery challenge because of its invasive nature, the need for administration in a controlled healthcare setting, and expanding costs. It is therefore expected that companies will continue to consider developing subcutaneous dosing alternatives for intravenous biotherapeutics. Inspired by the benefits of subcutaneous dosing to patients and the overall healthcare system, significant research is being conducted with a focus on further facilitating subcutaneous injections, such as enhancements in infusion-rate-dependent tolerability, improvements in infusion pump technology, and novel drug-delivery devices [67–69].
Pharmacokinetic Differences Between Intravenous and Subcutaneous Administration and Potential Impact on Safety and Efficacy
This section is primarily based on available clinical data. Nonclinical data are described only where human data on a specific topic are lacking. A review article detailing the nonclinical evidence for subcutaneous administration of biotherapeutics has been published [10].
General Differences in the Intravenous Versus Subcutaneous Pharmacokinetic Profiles of Biotherapeutics
Potential differences in the safety, immunogenicity, and efficacy of subcutaneous versus intravenous administration are best visualized by comparing the pharmacokinetic profiles of a therapeutic protein that can be administered via either route. A biotherapeutic intravenous infusion or injection directly into the bloodstream usually results in immediate maximum serum concentrations (Cmax), whereas the pharmacokinetic profile of subcutaneously injected therapeutic proteins is typically characterized by a slow absorption rate from the subcutaneous extracellular matrix with Cmax levels below those achieved with intravenous dosing. This absorption pattern of macromolecules into the blood is a consequence of their limited permeability across the vascular endothelia; therefore, the lymphatics provide an alternative absorption pathway into the circulation system [70, 71]. However, absorption via the lymphatics has also been described as a barrier for complete uptake of subcutaneously injected molecules. Other factors that can contribute to incomplete bioavailability of subcutaneously injected molecules can include the composition, volume, pH, and viscosity of the dosing formulation; interactions with interstitial glycosaminoglycans and proteins; and enzymatic degradation [70, 72, 73].
Pharmacokinetic Considerations for the Development of Subcutaneous Formulations for which Intravenous Treatments are Already Approved
Traditionally, mAb treatments in oncology were developed for intravenous infusion based on a bodyweight- or body surface area-adjusted dosing regimen that aimed to correct potential interpatient variability and drug distribution and elimination issues [23].
Generally, when developing a subcutaneous dosing alternative for a molecule that is already on the market or in late-stage development with an intravenous bodyweight- or body surface area-adjusted regimen, a change to a fixed dose or dose bands for predefined weight categories could be considered. A fixed dose would require less complex handling, avoid the need for dose calculations, and reduce the potential for dosing errors. A regimen that needs to consider a patient’s bodyweight or size can be challenging, especially when the subcutaneous formulation is considered to facilitate home- or patient self-administration and requires a ready-to-use injection device.
Today, based on emerging knowledge about the pharmacokinetics of mAbs, considerable pharmacokinetic/pharmacodynamic data from clinical studies, and extensive PopPK modeling, there is evidence that fixed dosing of mAbs could generally be justified without impairing the safety and efficacy of the treatment [20, 23]. Wang et al. [20] used data from mAb studies published in peer-reviewed journals to evaluate body size-based dosing and fixed dosing for 12 mAbs in terms of their population and individual performance in adult patients. They found that the different dosing approaches performed similarly across the mAbs investigated. Body size-adjusted administration resulted in lower pharmacokinetic variability for five mAbs, whereas the pharmacokinetic variability of the other seven mAbs was lower with a fixed dosing approach. The average variability in area under the plasma concentration–time curve (AUC) and Cmax of all 12 mAbs was similar following the two dosing approaches (AUC 42.4 versus 44.2%, Cmax 30.1 and 30.3% by fixed dosing and bodyweight/body surface area-based dosing, respectively). The authors recommended that fixed dosing should be the preferred approach in adult first-in-human studies because of the advantages in ease of dose preparation, reduced costs, and reduced chances of dosing errors [20].
Moreover, as described by Hendrikx et al. [23], mAbs typically distribute within the blood plasma and extracellular fluids only, which increase less than proportionally with an increase in bodyweight. Depending on the mAb, intracellular degradation after binding to the target can be the primary route of elimination and is not affected by body size. Therefore, administering mAbs with a fixed dose represents a valuable treatment alternative to weight- and body surface area-adjusted administration.
Generally, whether a dosing approach is acceptable depends on the width of the targeted range of exposure, and the therapeutic index must be large enough to accommodate the exposure differences introduced by flat dosing across the body weight ranges.
When developing the subcutaneous formulation for trastuzumab and rituximab, the change from intravenous to subcutaneous administration included a switch from weight- and body surface area-adjusted dosing to a fixed dose [21, 39, 74]. The selection of a subcutaneous fixed dose was based on a pharmacokinetic bridging approach assuming that a subcutaneous dose achieving serum trough concentrations (Ctrough) non-inferior to those with intravenous dosing would achieve comparable saturation of target receptors and result in noninferior efficacy [32, 33]. Previous comparisons of different dosing regimens [75, 76] revealed that trastuzumab or rituximab Cmax did not correlate with clinical efficacy. Therefore, despite the lower Cmax after subcutaneous administration, the risk of underexposing patients with the novel subcutaneous dosing regimen was considered low.
The intravenous and subcutaneous dosing regimens for trastuzumab and rituximab have been described previously [21, 39, 74]. Subcutaneous trastuzumab is administered with a fixed subcutaneous dose from cycle 1 onwards, omitting the need for a loading dose. Subcutaneous rituximab still requires a first body surface area-adjusted intravenous dose. For both mAbs, the dosing frequency is the same as with the respective intravenous dosing schedules. The pivotal phase III studies used a noninferiority design to compare the pharmacokinetics, efficacy, and safety of the fixed subcutaneous versus intravenous regimens. The trastuzumab HannaH study was a randomized, open-label study in the (neo)adjuvant setting in patients with HER2-positive, operable, locally advanced, or inflammatory breast cancer (see Sect. 3 for more details on trial design). The coprimary endpoints were Ctrough at pre-dose cycle 8 before surgery and pathologic complete response (pCR) [39]. The rituximab Sabrina study was a randomized, open-label study in patients with previously untreated grade 1–3a, CD20-positive follicular lymphoma. The primary endpoint for stage I was Ctrough at pre-dose cycle 8, and the primary endpoint for stage II was overall response at the end of induction [21, 74]. Patients were randomly assigned (205:205) to intravenous rituximab 375 mg/m2 or subcutaneous rituximab 1400 mg plus chemotherapy.
In both the HannaH and the Sabrina studies, subcutaneous administration of trastuzumab and rituximab resulted in noninferior Ctrough and efficacy to intravenous dosing and a similar safety profile [21, 39, 74]. In HannaH, the geometric mean ratio of Ctrough subcutaneous to Ctrough intravenous was 1.33 (90% CI 1.24–1.44), and 40.7% (95% CI 34.7–46.9) of the intravenous group and 45.4% (95% CI 39.2–51.7) of the subcutaneous group achieved a pCR. The difference between groups in pCR was 4.7% (95% CI − 4.0 to 13.4). In Sabrina, the geometric mean ratio of Ctrough subcutaneous to Ctrough intravenous was 1.5 (90% CI 1.3–1.7). Overall response at the end of induction was 84.9% (95% CI 79.2–89.5) in the intravenous group and 84.4% (78.7–89.1) in the subcutaneous group. The fixed-dose regimens for trastuzumab and rituximab were further supported by exposure–response analyses of response and grade ≥ 3 adverse events, which did not identify a statistically meaningful impact of bodyweight or body surface area, respectively, nor of exposure or route of administration of the respective mAbs [21, 74].
It is of note that while the pharmacokinetic-non-inferiority clinical bridging approach was successful for rituximab and trastuzumab, the situation can be different for other mAbs in other indications, especially when Cmax is expected to contribute to efficacy.
Impact of Subcutaneous Administration of Biotherapeutics on Hypersensitivity and Infusion-Related Reactions
While the abovementioned studies demonstrated that Cmax did not contribute to the efficacy of trastuzumab and rituximab, the question remains as to whether the lower Cmax with subcutaneous administration results in fewer hypersensitivity and infusion-related reactions (IRRs) than intravenous administration, where higher maximum serum levels are generally achieved.
The underlying mechanism of immune reactions to mAb infusions remains unclear. These reaction symptoms can be characterized by flushing, rash, fever, rigors, chills, dyspnea, mild, and/or severe hypotension with(out) bronchospasms, cardiac dysfunction, and/or anaphylaxis. For mAbs, such reactions are typically mild to moderate and occur predominantly during the first infusion [77]. In clinical practice, one way to reduce the extent and rate of hypersensitivity and IRRs is to continue dosing at a slower intravenous infusion rate [77, 78]. From a pharmacokinetic perspective, this slowing of the intravenous infusion rate results in a slower increase of mAb serum levels, similar to what is achieved with subcutaneous dosing. Importantly, although serum drug levels will still be lower with subcutaneous administration than those achieved with slower intravenous infusion, the principle of lowering mAb serum concentrations in both dosing strategies is the same. It has, therefore, been proposed that when Cmax-related adverse reactions are observed during intravenous infusion, a change to subcutaneous dosing may be advantageous for the patient [79].
Alemtuzumab (Campath-H1) is an example of an mAb with marked IRRs during the first intravenous infusion. The biotherapeutic is directed against CD52 cells and is approved for the intravenous treatment of chronic lymphocytic leukemia. Small pilot studies using subcutaneous infusion reported that immediate flu-like toxicities associated with intravenous treatment were less severe when using the subcutaneous route of administration [80]. Furthermore, acute subcutaneous IRRs, such as rigor, rash or urticaria, nausea, hypotension, or bronchospasm, were rare or absent in contrast to the observations previously made for intravenous infusions [81].
Recently, published data on the tolerability of first-dose subcutaneous administration for daratumumab have become available. Daratumumab is an mAb approved for the intravenous treatment of multiple myeloma, and in clinical trials (monotherapy and combination treatments; n = 820) intravenous dosing was associated with IRRs in 46% of patients with the first infusion, 2% with the second infusion, and 3% with subsequent infusions. With the first intravenous infusion, 4–9% of patients had grade 3 events, and with second or subsequent infusions < 1% of patients had grade 3 events. Median time to onset of a reaction was 1.4 h [65]. An open-label, multicentre, dose-escalation phase Ib study assessing the safety, pharmacokinetics, and efficacy of subcutaneous daratumumab 1200 mg (n = 8) and 1800 mg (n = 33) found lower rates of IRRs with daratumumab. IRRs were reported in 9 of 41 patients (22%) and were mostly grade 1/2 in severity; these included chills, fever, rigors, vomiting, itching, edema of the tongue, noncardiac chest pain, and wheezing. All IRRs developed during or within 6 h of the first subcutaneous infusion. No IRRs were reported with subsequent infusions [82].
Overall, while there is a trend towards a reduction in rate and severity of IRRs with the subcutaneous route for the above examples, data are currently still limited, and head-to-head clinical studies with different mAbs are required to further investigate the topic.
Impact of Subcutaneous Administration of Biotherapeutics on Antidrug Antibodies
Following subcutaneous injection, molecules reach the systemic circulation via either the blood capillaries or the lymphatic system. Unlike small molecules, biotherapeutics with molecular weights of > 20 kDa exhibit limited transport into the blood capillaries and cross into the circulation system predominantly via the lymphatics [10, 71]. This increased exposure to the lymphatic system has led to the suggestion that subcutaneous administration of biotherapeutics could be more immunogenic than intravenous dosing, but this hypothesis may not be universally valid [83].
In this context, not only the formation of antidrug antibodies (ADAs) with the different administration routes per se but also their impact on the exposure, efficacy, and safety of the biotherapeutic needs to be considered. With the pivotal noninferiority trials for trastuzumab and rituximab, the HannaH and Sabrina studies, respectively, two larger head-to-head studies comparing the immunogenicity of intravenous versus subcutaneous administration with the same dosing regimen have become available [21, 74]. In the HannaH study, 8.1% of patients (30/296) treated with intravenous trastuzumab and 15.9% of patients (47/295) receiving subcutaneous trastuzumab developed antibodies against trastuzumab [65]. Trastuzumab pharmacokinetics and pCR rates within each treatment arm were similar regardless of trastuzumab–ADA status or treatment cycle. Likewise, the incidence of administration-related reactions was not statistically different independent of the ADA status [84]. In the Sabrina study, which was performed in a CD20-positive follicular lymphoma treatment setting, the incidence of treatment-induced/enhanced anti-rituximab ADAs in the subcutaneous group was low and similar to that observed in the intravenous group (2% (4/205) versus 1% (2/205), respectively) [65]. In patients testing positive for rituximab ADAs, no impact on B-cell depletion, efficacy, pharmacokinetics, or safety was found [74]. A lack of impact of ADAs on efficacy, safety, or pharmacokinetics was also described for abatacept or tocilizumab in RA [85, 86]. However, these data cannot be directly compared with the HannaH and Sabrina studies, as the intravenous and subcutaneous treatments were given at different dosing frequencies.
A recent review looking into the subcutaneous administration route and its impact on immunogenicity for therapeutic proteins highlighted the importance of further clinical research in the field, with the aim to understand key factors that are likely to contribute to the immunogenicity in addition to the route of administration. Namely, the composition of the drug delivery formulation, the indication and disease state of the patient population, the mode of action of the molecule, differences in the dose and dosing frequency, or concomitant medications that could modulate the immune system should all be considered [11].
In addition, as many of the biologic therapeutics given subcutaneously today are immunosuppressive drugs, such as rituximab, tocilizumab, abatacept, or golimumab, a key question that needs to be answered in future trials is whether therapeutic proteins intended to stimulate the immune response can be administered subcutaneously without triggering significant ADA formation, and whether these ADAs would, in turn, impact the pharmacokinetics, efficacy, or safety compared with intravenous administration.
Impact of Subcutaneous Administration of Biotherapeutics on Bioavailability
A drawback of subcutaneous administration of biotherapeutics is the incomplete bioavailability of the injected molecule, which can range widely from 50 to 80% for mAbs and even more for other biotherapeutics. The underlying presystemic catabolism at the subcutaneous administration site or in the lymphatic system is still poorly understood with respect to both the involved enzymes and their translation across species. For mAbs, subcutaneous bioavailability appears to be inversely correlated with clearance after intravenous dosing, so that mAbs with a lower intravenous clearance exhibit higher subcutaneous bioavailability [87]. This correlation may be due to the involvement of hematopoietic cells (e.g. macrophages or dendritic cells) in both subcutaneous first-pass clearance and systemic clearance after intravenous dosing [88]. Richter and Jacobsen [10] have only recently reviewed the current understanding and knowledge of this area, so we do not discuss it further.
As incomplete bioavailability typically results in the need for a higher dose for subcutaneous infusions than for intravenous infusions, costs of goods can be higher for subcutaneous formulations. In an attempt to increase the subcutaneous bioavailability of a biotherapeutic, subcutaneous infusions can be coadministered (or produced as coformulations) with the dispersion-enhancer hyaluronidase (discussed in detail in Sect. 4), an enzyme that facilitates spreading of an injected fluid in the subcutaneous tissue [18]. This increased dispersion in the interstitial tissue can result in a higher bioavailability of a co-injected molecule. Initial evidence supporting this hypothesis was found in a rat study on the absolute intradermal bioavailability for peg-interferon alfa-2b and the therapeutic antibody infliximab. Bioavailability was calculated to increase from 61 to 108% and from 59 to 94%, with and without co-injecting with hyaluronidase, respectively. In addition, the time to reach Cmax (Tmax) was lower for both molecules in the presence of hyaluronidase, indicating a faster absorption into the circulation system in the presence of the hyaluronidase (Tmax of infliximab decreased from 48 to 18.7 h) [63]. The impact of hyaluronidase on the fraction absorbed is likely to depend on the molecule coadministered. In a study with trastuzumab with and without hyaluronidase using the mini-pig model, the fraction absorbed from compartmental pharmacokinetic analysis was similar across formulations and estimated to be 85%. Comparable to what was observed for intradermal peg-interferon alfa-2b and infliximab in the rat, subcutaneous trastuzumab absorption was more rapid from the hyaluronidase-containing formulation than from the control without hyaluronidase (average first-order absorption rate constants were 0.828 and 0.166/day, respectively) [32].
Limited clinical data on the impact of hyaluronidase on bioavailability are available for SCIG in the treatment of PI. Hyaluronidase-facilitated subcutaneous administration did improve the bioavailability by approximately 20% as compared with subcutaneous administration in the absence of the enzyme [89]. The authors reported that, when investigating the AUC, normalized by dose per kilogram, for IGHy compared with IGSC alone from a prior study, hyaluronidase improved the bioavailability of the immunoglobulin by approximately 20%. The ratio of AUC per dose per kilogram for IGHy/IGSC was 120.4% (90% CI 115.5–125.5). A low subcutaneous bioavailability in PI can increase the treatment burden for patients who require lifelong replacement therapy, as the resulting higher dose and larger dosing volume may necessitate administration at multiple injection sites, more frequent dosing, and dose adjustment to achieve immunoglobulin serum concentrations comparable to those with intravenous administration.
In a study of tocilizumab in healthy volunteers, coadministration of hyaluronidase resulted in a slightly increased tocilizumab exposure, a trend towards lower pharmacokinetic variability, and earlier Tmax compared with administration without hyaluronidase [90]. The earlier Tmax and increased absorption rate following subcutaneous coadministration with hyaluronidase has been shown to increase the absorption rate of insulin, resulting in superior glycemic control compared with insulin alone, meaning lower insulin requirements and reduced hypoglycemic excursions [91].
As understanding of the impact of the various anatomic injection sites on subcutaneous absorption remains limited [10], the injection sites for the subcutaneous formulations of trastuzumab and rituximab are restricted to those studied in clinical trials (thigh for trastuzumab and abdomen for rituximab) [64, 65]. Xu et al. [92] investigated the absolute bioavailability of golimumab, an anti-TNFα human IgG1κ, among three different injection sites. Healthy male volunteers (n = 78) were randomly assigned to either a single dose of golimumab 100 mg via a 30-min intravenous infusion (n = 23) or subcutaneous administration at the upper arm (n = 18), the abdomen (n = 18), or the thigh (n = 19). The absorption of golimumab was found to be similar independent of the injection site. The overall mean bioavailability of subcutaneous golimumab was 51% (upper arm, mean 52.0%, coefficient of variation (CV) 23; abdomen, mean 47.0%, CV 30; thigh, mean 54.1%, CV 34; p = 0.364). Similar findings were reported by Ortega et al. [93]. In a randomized, open-label, parallel-design, phase I study, 60 healthy volunteers were randomly assigned (1:1:1:1 ratio) to receive a single dose of either mepolizumab 250 mg by intravenous injection, subcutaneous injection (upper arm, abdomen, or thigh), or intramuscular injection. The mean bioavailability for each injection site was 64% (90% CI 55–73), 75% (90% CI 66–86), 71% (90% CI 62–82), and 81% (90% CI 71–94) for subcutaneous abdomen, arm, thigh, or intramuscular, respectively. While the above studies show comparable bioavailability independent of the anatomic site of injection, results may differ depending on the individual biotherapeutic. Therefore, additional clinical evidence is required to allow a more general conclusion on whether different injection sites can be used for a biotherapeutic without impairing the established efficacy or safety profile.
Conclusion and Future Perspectives
In diseases such as diabetes, RA, or MS, subcutaneous administration of biologics, including home- or self-administration, is already the standard of care. Treatments have been shown to be safe and well-tolerated, the injection volume does typically not exceed 1 ml, and dosing is facilitated with prefilled syringes, auto-injectors, or injection pens. Even in immunoglobulin-replacement therapy requiring large subcutaneous infusions, patient self-administration or caregiver-supported subcutaneous dosing in the home setting represents an established treatment modality, facilitated using infusion pumps, the rapid-push methodology, or the dispersion enhancer hyaluronidase (Fig. 1).
Similarly, in oncology, with the introduction of subcutaneous versions of trastuzumab and rituximab and a number of other molecules currently in development, subcutaneous administration is becoming an attractive alternative to invasive and time-consuming intravenous infusions. The key question remains, to what extent will subcutaneous dosing further complement or replace intravenous dosing in this area? The clear health-economic benefit of a change from intravenous to subcutaneous dosing due to a reduction in drug administration-related healthcare resources and costs will likely foster future research on subcutaneous administration in oncology. While subcutaneous dosing would be expected to reduce Cmax-related side effects of a biotherapeutic compared with intravenous dosing, it still remains to be elucidated whether this administration route is feasible for all molecules. A molecule’s overall safety and tolerability profile and especially the impact of absorption via the lymphatic system with subcutaneous administration on the formation of ADAs, and their impact on efficacy and safety, needs to be the subject of research for each individual mAb.
When looking at the pharmacoeconomic benefit of switching intravenous hospital-based to subcutaneous home-based administration for immunoglobulins while at the same time maintaining the clinical outcomes, a similar change could also be considered in oncology. Patients receiving a subcutaneous biologic as monotherapy in the maintenance/adjuvant setting or in combination with oral chemotherapy are particularly expected to benefit from a subcutaneous dosing alternative, as the change to this administration route reduces the time required for frequent hospital visits. To enable convenient self-administration, the products should preferentially be delivered as fixed doses, ideally in a ready-to-use injection device.
An additional area in which subcutaneous administration could be beneficial is in combination therapy, as more biologic treatments are developed for co-dosing, requiring relatively time-consuming sequential parenteral administration. In oncology, where patients receive multiple treatments intravenously, the availability of a subcutaneous alternative for one of the intravenous regimens could itself reduce treatment complexity and costs. Complex dosing regimens could be further simplified using subcutaneous fixed-dose combinations that contain two or more active molecules co-formulated within the same formulation. An alternative would be the development of multiple-chamber devices that either inject the products sequentially or pre-mix them directly before subcutaneous injection. This type of device would allow flexibility in combining different biologics without extensive formulation development and provide the option to use available long-term stability data of the individual biologics in the event that the same primary container is used.
Whether a biotherapeutic should be available in only a subcutaneous formulation or whether having both the subcutaneous and intravenous formulations on the market would be preferred should be carefully evaluated. While preference studies reveal that the majority of patients prefer subcutaneous over intravenous dosing, some patients do continue to favor intravenous infusions. In addition, if a biotherapeutic is dosed in combination with an intravenously administered molecule, the convenience advantage of the subcutaneous route is not as strong as with monotherapy or in combination with an orally or subcutaneously administered molecule. Moreover, especially for treatments that require immediate absorption and high Cmax values to be efficacious, intravenous administration will continue to play a major role.
Funding
Support for third party medical writing assistance for this manuscript was provided by F. Hoffmann La Roche Ltd.
Conflict of interest
Beate Bittner, Wolfgang Richter, and Johannes Schmidt are employees of F. Hoffmann La Roche and own stock in Roche.
References
- 1.Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53:192–201. doi: 10.1177/0091270012436560. [DOI] [PubMed] [Google Scholar]
- 2.Kruse GB, Amonkar MM, Smith G, Skonieczny DC, Stavrakas S. Analysis of costs associated with administration of intravenous single-drug therapies in metastatic breast cancer in a U.S. population. J Manag Care Pharm. 2008;14:844–857. doi: 10.18553/jmcp.2008.14.9.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vescia S, Baumgartner AK, Jacobs VR, Kiechle-Bahat M, Rody A, Loibl S, et al. Management of venous port systems in oncology: a review of current evidence. Ann Oncol. 2008;19:9–15. doi: 10.1093/annonc/mdm272. [DOI] [PubMed] [Google Scholar]
- 4.Tetteh EK, Morris S. Evaluating the administration costs of biologic drugs: development of a cost algorithm. Health Econ Rev. 2014;4:26. doi: 10.1186/s13561-014-0026-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pivot X, Gligorov J, Muller V, Curigliano G, Knoop A, Verma S, et al. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol. 2014;25:1979–1987. doi: 10.1093/annonc/mdu364. [DOI] [PubMed] [Google Scholar]
- 6.De Cock E, Kritikou P, Tao S, Waterboer T, Carella AM. Time savings with rituximab subcutaneous (SC) injection vs rituximab intravenous (IV) infusion: final analysis from a time-and-motion study in 8 countries. Am Soc Hematology. In: 55th Annual meeting and exposition, New Orleans; 2012.
- 7.De Cock E, Pan YI, Tao S, Baidin P. Time savings with trastuzumab subcutaneous (SC) injection verse trastuzumab intravenous (IV) infusion: a time and motion study in 3 russian centers. Value Health. 2014;17:A653. doi: 10.1016/j.jval.2014.08.2380. [DOI] [PubMed] [Google Scholar]
- 8.De Cock E, Kritikou P, Sandoval M, Tao S, Wiesner C, Carella AM, et al. Time savings with rituximab subcutaneous injection versus rituximab intravenous infusion: a time and motion study in eight countries. PLoS One. 2016;11:e0157957. doi: 10.1371/journal.pone.0157957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richter WF, Bhansali SG, Morris ME. Mechanistic determinants of biotherapeutics absorption following SC administration. AAPS J. 2012;14:559–570. doi: 10.1208/s12248-012-9367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter WF, Jacobsen B. Subcutaneous absorption of biotherapeutics: knowns and unknowns. Drug Metab Dispos. 2014;42:1881–1889. doi: 10.1124/dmd.114.059238. [DOI] [PubMed] [Google Scholar]
- 11.Hamuro L, Kijanka G, Kinderman F, Kropshofer H, Bu DX, Zepeda M, et al. Perspectives on subcutaneous route of administration as an immunogenicity risk factor for therapeutic proteins. J Pharm Sci. 2017;106:2946–2954. doi: 10.1016/j.xphs.2017.05.030. [DOI] [PubMed] [Google Scholar]
- 12.Shpilberg O, Jackisch C. Subcutaneous administration of rituximab (MabThera) and trastuzumab (Herceptin) using hyaluronidase. Br J Cancer. 2013;109:1556–1561. doi: 10.1038/bjc.2013.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardulf A. Immunoglobulin treatment for primary antibody deficiencies: advantages of the subcutaneous route. BioDrugs. 2007;21:105–116. doi: 10.2165/00063030-200721020-00005. [DOI] [PubMed] [Google Scholar]
- 14.Beaute J, Levy P, Millet V, Debre M, Dudoit Y, Le Mignot L, et al. Economic evaluation of immunoglobulin replacement in patients with primary antibody deficiencies. Clin Exp Immunol. 2010;160:240–245. doi: 10.1111/j.1365-2249.2009.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett C. At-home subcutaneous injection of trastuzumab meets safety endpoint. Oncol Times. 2017;39:26–27. doi: 10.1097/01.COT.0000524502.41911.56. [DOI] [Google Scholar]
- 16.Tjalma W, Huizing M, Papadimitriou K. The smooth and bumpy road of trastuzumab administration: from intravenous (IV) in a hospital to subcutaneous (SC) at home. Facts Views Vis ObGyn. 2017;9:51–55. [PMC free article] [PubMed] [Google Scholar]
- 17.Garidel P, Kuhn AB, Schafer LV, Karow-Zwick AR, Blech M. High-concentration protein formulations: how high is high? Eur J Pharm Biopharm. 2017;119:353–360. doi: 10.1016/j.ejpb.2017.06.029. [DOI] [PubMed] [Google Scholar]
- 18.Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4:427–440. doi: 10.1517/17425247.4.4.427. [DOI] [PubMed] [Google Scholar]
- 19.Wasserman RL. Recombinant human hyaluronidase-facilitated subcutaneous immunoglobulin infusion in primary immunodeficiency diseases. Immunotherapy. 2017;9:1035–1050. doi: 10.2217/imt-2017-0092. [DOI] [PubMed] [Google Scholar]
- 20.Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size-based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49:1012–1024. doi: 10.1177/0091270009337512. [DOI] [PubMed] [Google Scholar]
- 21.Davies A, Merli F, Mihaljevic B, Siritanaratkul N, Solal-Celigny P, Barrett M, et al. Pharmacokinetics and safety of subcutaneous rituximab in follicular lymphoma (SABRINA): stage 1 analysis of a randomised phase 3 study. Lancet Oncol. 2014;15:343–352. doi: 10.1016/S1470-2045(14)70005-1. [DOI] [PubMed] [Google Scholar]
- 22.Hourcade-Potelleret F, Lemenuel-Diot A, McIntyre C, Brewster M, Lum B, Bittner B. Use of a population pharmacokinetic approach for the clinical development of a fixed-dose subcutaneous formulation of trastuzumab. CPT Pharmacomet Syst Pharmacol. 2014;3:e87. doi: 10.1038/psp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendrikx J, Haanen J, Voest EE, Schellens JHM, Huitema ADR, Beijnen JH. Fixed dosing of monoclonal antibodies in oncology. Oncologist. 2017;22:1212–1221. doi: 10.1634/theoncologist.2017-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henricks LM, Schellens JH, Huitema AD, Beijnen JH. The use of combinations of monoclonal antibodies in clinical oncology. Cancer Treat Rev. 2015;41:859–867. doi: 10.1016/j.ctrv.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Angelotti F, Parma A, Cafaro G, Capecchi R, Alunno A, Puxeddu I. One year in review 2017: pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol. 2017;35:368–378. [PubMed] [Google Scholar]
- 26.Wang L, Wang FS, Gershwin ME. Human autoimmune diseases: a comprehensive update. J Intern Med. 2015;278:369–395. doi: 10.1111/joim.12395. [DOI] [PubMed] [Google Scholar]
- 27.Shabaninejad H, Asgharzadeh A, Rezaei N, Rezapoor A. A comparative study of intravenous immunoglobulin and subcutaneous immunoglobulin in adult patients with primary immunodeficiency diseases: a systematic review and meta-analysis. Expert Rev Clin Immunol. 2016;12:595–602. doi: 10.1586/1744666X.2016.1155452. [DOI] [PubMed] [Google Scholar]
- 28.Kobrynski L. Subcutaneous immunoglobulin therapy: a new option for patients with primary immunodeficiency diseases. Biologics. 2012;6:277–287. doi: 10.2147/BTT.S25188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, et al. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139:S1–S46. doi: 10.1016/j.jaci.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Auricchio F, Scavone C, Cimmaruta D, Di Mauro G, Capuano A, Sportiello L, et al. Drugs approved for the treatment of multiple sclerosis: review of their safety profile. Expert Opin Drug Saf. 2017;16:1359–1371. doi: 10.1080/14740338.2017.1388371. [DOI] [PubMed] [Google Scholar]
- 31.Rotte A, Jin J, Lemaire V. Mechanistic overview of immune checkpoints to support the rational design of their combinations in cancer immunotherapy. Ann Oncol. 2018;29(1):71–83. doi: 10.1093/annonc/mdx686. [DOI] [PubMed] [Google Scholar]
- 32.Bittner B, Richter WF, Hourcade-Potelleret F, McIntyre C, Herting F, Zepeda ML, et al. Development of a subcutaneous formulation for trastuzumab - nonclinical and clinical bridging approach to the approved intravenous dosing regimen. Drug Res (Stuttg) 2012;62:401–409. doi: 10.1055/s-0032-1321831. [DOI] [PubMed] [Google Scholar]
- 33.Bittner B, Richter WF, Hourcade-Potelleret F, Herting F, Schmidt J. Non-clinical pharmacokinetic/pharmacodynamic and early clinical studies supporting development of a novel subcutaneous formulation for the monoclonal antibody rituximab. Drug Res (Stuttg) 2014;64:569–575. doi: 10.1055/s-0033-1363993. [DOI] [PubMed] [Google Scholar]
- 34.Dimopoulos MA, Oriol A, Nahi H, San-Miguel J, Bahlis NJ, Usmani SZ, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 35.Janssen Research & Development L. A study of subcutaneous versus (vs.) intravenous administration of daratumumab in participants with relapsed or refractory multiple myeloma; 2017. https://clinicaltrials.gov/ct2/show/NCT03277105. Accessed 31 Mar 2018.
- 36.Janssen Research & Development L. A study of subcutaneous daratumumab versus active monitoring in participants with high-risk smoldering multiple myeloma. 2017. https://clinicaltrials.gov/ct2/show/NCT03301220. Accessed 31 Mar 2018.
- 37.Jackisch C, Muller V, Dall P, Neumeister R, Park-Simon TW, Ruf-Dordelmann A, et al. Subcutaneous trastuzumab for her2-positive breast cancer—evidence and practical experience in 7 german centers. Geburtshilfe Frauenheilkd. 2015;75:566–573. doi: 10.1055/s-0035-1546172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pivot X, Gligorov J, Muller V, Barrett-Lee P, Verma S, Knoop A, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14:962–970. doi: 10.1016/S1470-2045(13)70383-8. [DOI] [PubMed] [Google Scholar]
- 39.Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim SB, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13:869–878. doi: 10.1016/S1470-2045(12)70329-7. [DOI] [PubMed] [Google Scholar]
- 40.Pivot X, Semiglazov V, Chen S, Moodley S, Manihkas A, Coccia-Portugal M et al. Subcutaneous injection of trastuzumab—analysis of administration time and injection site reactions. ESMO 2012.
- 41.De Cock E, Pivot X, Hauser N, Verma S, Kritikou P, Millar D, et al. A time and motion study of subcutaneous versus intravenous trastuzumab in patients with HER2-positive early breast cancer. Cancer Med. 2016;5:389–397. doi: 10.1002/cam4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burcombe R, Chan S, Simcock R, Samanta K, Percival F, Barrett-Lee P. Subcutaneous trastuzumab (Herceptin®): a UK time and motion study in comparison with intravenous formulation for the treatment of patients with HER2-positive early breast cancer. Adv Breast Cancer Res. 2013;2:133–140. doi: 10.4236/abcr.2013.24022. [DOI] [Google Scholar]
- 43.Tjalma W, Van den Mooter T, Mertens T, Bastiaens V, Huizing M. PapadimitriounK. Subcutaneous trastuzumab (Herceptin) versus intravenous trastuzumab for the treatment of patients with HER2-positive breast cancer: A time, motion and cost assessment study in a lean operating day care oncology unit. Eur J Obstet Gynecol Reprod Biol. 2018;221:46–51. doi: 10.1016/j.ejogrb.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Andrade S, Santos A. Hospital resources consumption associated with trastuzumab treatment in breast cancer in portugal. Value Health. 2013;16:A418. doi: 10.1016/j.jval.2013.08.546. [DOI] [Google Scholar]
- 45.Lieutenant V, Toulza E, Pommier M. Lortal-Canguilhem B [Is Herceptin(®) (trastuzumab) by subcutaneous a mini revolution? Pharmaco-economic study] Bull Cancer. 2015;102:270–276. doi: 10.1016/j.bulcan.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Mylonas C, Kourlaba G, Fountzilas G, Skroumpelos A, Maniadakis N. Cost-minimization analysis of trastuzumab intravenous versus trastuzumab subcutaneous for the treatment of patients with HER2+ early breast cancer and metastatic breast cancer in Greece. Value Health. 2014;17:A640–A641. doi: 10.1016/j.jval.2014.08.2310. [DOI] [PubMed] [Google Scholar]
- 47.De Cock E, Semiglazov V, Lopez Vivanco G, Verma S, Pivot X, Gligorov J et al. Time savings with trastuzumab subcutaneous vs intravenous administration: a time and motion study. St. Gallen 2013, Abstr. P209
- 48.De Cock E, Pan I, Sandoval M, Millar D, Knoop A. Healthcare professionals’ perceptions of the impact on clinical management of switching from the intravenous to the subcutaneous formulation of trastuzumab. In: European breast cancer conference 2014, Glasgow, Abstr. 42
- 49.Sehn LH, Donaldson J, Filewich A, Fitzgerald C, Gill KK, Runzer N, et al. Rapid infusion rituximab in combination with corticosteroid-containing chemotherapy or as maintenance therapy is well tolerated and can safely be delivered in the community setting. Blood. 2007;109:4171–4173. doi: 10.1182/blood-2006-11-059469. [DOI] [PubMed] [Google Scholar]
- 50.Solal-Celigny P. Rituximab by subcutaneous route. Expert Rev Hematol. 2015;8:147–153. doi: 10.1586/17474086.2015.1024651. [DOI] [PubMed] [Google Scholar]
- 51.Rule S, Collins GP, Samanta K. Subcutaneous vs intravenous rituximab in patients with non-Hodgkin lymphoma: a time and motion study in the United Kingdom. J Med Econ. 2014;17:459–468. doi: 10.3111/13696998.2014.914033. [DOI] [PubMed] [Google Scholar]
- 52.Ariza SS. Time and motion study for rituximab SC vs IV in Colombian patients with non-Hodgkin lymphoma. Value Health. 2015;18:A207. doi: 10.1016/j.jval.2015.03.1200. [DOI] [Google Scholar]
- 53.Bax P, Postma M. Cost-minimization of mabthera intravenous versus subcutaneous administration. Value Health. 2013;16:A390–A391. doi: 10.1016/j.jval.2013.08.392. [DOI] [Google Scholar]
- 54.Pereira C, Santos A. Resource consumption evaluation associated with rituximab administration in Portugal. Value Health. 2013;16:A386. doi: 10.1016/j.jval.2013.08.366. [DOI] [Google Scholar]
- 55.Rummel M, Kim TM, Aversa F, Brugger W, Capochiani E, Plenteda C, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomized, open-label, crossover study (PrefMab) Ann Oncol. 2017;28:836–842. doi: 10.1093/annonc/mdw685. [DOI] [PubMed] [Google Scholar]
- 56.Vultaggio A, Azzari C, Milito C, Finocchi A, Toppino C, Spadaro G, et al. Subcutaneous immunoglobulin replacement therapy in patients with primary immunodeficiency in routine clinical practice: the VISPO prospective multicenter study. Clin Drug Investig. 2015;35:179–185. doi: 10.1007/s40261-015-0270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Abolhassani H, Sadaghiani MS, Aghamohammadi A, Ochs HD, Rezaei N. Home-based subcutaneous immunoglobulin versus hospital-based intravenous immunoglobulin in treatment of primary antibody deficiencies: systematic review and meta analysis. J Clin Immunol. 2012;32:1180–1192. doi: 10.1007/s10875-012-9720-1. [DOI] [PubMed] [Google Scholar]
- 58.Igarashi A, Kanegane H, Kobayashi M, Miyawaki T, Tsutani K. Cost-minimization analysis of IgPro20, a subcutaneous immunoglobulin, in Japanese patients with primary immunodeficiency. Clin Ther. 2014;36:1616–1624. doi: 10.1016/j.clinthera.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Lingman-Framme J, Fasth A. Subcutaneous immunoglobulin for primary and secondary immunodeficiencies: an evidence-based review. Drugs. 2013;73:1307–1319. doi: 10.1007/s40265-013-0094-3. [DOI] [PubMed] [Google Scholar]
- 60.Gikanga B, Turok R, Hui A, Bowen M, Stauch OB, Maa YF. Manufacturing of high-concentration monoclonal antibody formulations via spray drying-the road to manufacturing scale. PDA J Pharm Sci Technol. 2015;69:59–73. doi: 10.5731/pdajpst.2015.01003. [DOI] [PubMed] [Google Scholar]
- 61.Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9:722–728. [Google Scholar]
- 62.Shapiro R. Subcutaneous immunoglobulin therapy by rapid push is preferred to infusion by pump: a retrospective analysis. J Clin Immunol. 2010;30:301–307. doi: 10.1007/s10875-009-9352-2. [DOI] [PubMed] [Google Scholar]
- 63.Bookbinder LH, Hofer A, Haller MF, Zepeda ML, Keller GA, Lim JE, et al. A recombinant human enzyme for enhanced interstitial transport of therapeutics. J Control Release. 2006;114:230–241. doi: 10.1016/j.jconrel.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 64.U.S. Food and Drug Administration; 2018. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed 31 Mar 2018.
- 65.The Electronic Medicines Compendium; 2018. https://www.medicines.org.uk/emc/about-the-emc. Accessed 31 Mar 2018.
- 66.Wasserman RL, Melamed I, Stein MR, Engl W, Sharkhawy M, Leibl H, et al. Long-term tolerability, safety, and efficacy of recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulin for primary immunodeficiency. J Clin Immunol. 2016;36:571–582. doi: 10.1007/s10875-016-0298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dias C, Abosaleem B, Crispino C, Gao B, Shaywitz A. Tolerability of high-volume subcutaneous injections of a viscous placebo buffer: a randomized, crossover study in healthy subjects. AAPS PharmSciTech. 2015;16:1101–1107. doi: 10.1208/s12249-015-0288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Praestmark KA, Stallknecht B, Jensen ML, Sparre T, Madsen NB, Kildegaard J. Injection technique and pen needle design affect leakage from skin after subcutaneous injections. J Diabetes Sci Technol. 2016;10:914–922. doi: 10.1177/1932296815626723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doughty DV, Clawson CZ, Lambert W, Subramony JA. Understanding subcutaneous tissue pressure for engineering injection devices for large-volume protein delivery. J Pharm Sci. 2016;105:2105–2113. doi: 10.1016/j.xphs.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 70.McLennan DN, Porter CJ, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Technol. 2005;2:89–96. doi: 10.1016/j.ddtec.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 71.Supersaxo A, Hein WR, Steffen H. Effect of molecular weight on the lymphatic absorption of water-soluble compounds following subcutaneous administration. Pharm Res. 1990;7:167–169. doi: 10.1023/A:1015880819328. [DOI] [PubMed] [Google Scholar]
- 72.Zuidema J, Kadir F, Titulaer H, Oussoren C. Release and absorption rates of intramuscularly and subcutaneously injected pharmaceuticals (II) Int J Pharm. 1994;105:189–207. doi: 10.1016/0378-5173(94)90103-1. [DOI] [Google Scholar]
- 73.Hawley A, Davis S, Illum L. Targeting of colloids to lymph nodes: influence of lymphatic physiology and colloidal characteristics. Adv Drug Deliv Rev. 1995;17:129–148. doi: 10.1016/0169-409X(95)00045-9. [DOI] [Google Scholar]
- 74.Davies A, Merli F, Mihaljevic B, Mercadal S, Siritanaratkul N, Solal-Celigny P, et al. Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): a randomised, open-label, phase 3 trial. Lancet Haematol. 2017;4:e272–e282. doi: 10.1016/S2352-3026(17)30078-9. [DOI] [PubMed] [Google Scholar]
- 75.Tobinai K, Igarashi T, Itoh K, Kobayashi Y, Taniwaki M, Ogura M, et al. Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Ann Oncol. 2004;15:821–830. doi: 10.1093/annonc/mdh176. [DOI] [PubMed] [Google Scholar]
- 76.Vogel CL, Cobleigh MA, Tripathy D, Gutheil JC, Harris LN, Fehrenbacher L, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 77.Lenz HJ. Management and preparedness for infusion and hypersensitivity reactions. Oncologist. 2007;12:601–609. doi: 10.1634/theoncologist.12-5-601. [DOI] [PubMed] [Google Scholar]
- 78.Chung CH. Managing premedications and the risk for reactions to infusional monoclonal antibody therapy. Oncologist. 2008;13:725–732. doi: 10.1634/theoncologist.2008-0012. [DOI] [PubMed] [Google Scholar]
- 79.Mao CP, Brovarney MR, Dabbagh K, Birnbock HF, Richter WF, Del Nagro CJ. Subcutaneous versus intravenous administration of rituximab: pharmacokinetics, CD20 target coverage and B-cell depletion in cynomolgus monkeys. PLoS One. 2013;8:e80533. doi: 10.1371/journal.pone.0080533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Warner JL, Arnason JE. Alemtuzumab use in relapsed and refractory chronic lymphocytic leukemia: a history and discussion of future rational use. Ther Adv Hematol. 2012;3:375–389. doi: 10.1177/2040620712458949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lundin J, Kimby E, Bjorkholm M, Broliden PA, Celsing F, Hjalmar V, et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL) Blood. 2002;100:768–773. doi: 10.1182/blood-2002-01-0159. [DOI] [PubMed] [Google Scholar]
- 82.Usmani SZ, Nahi H, Mateos M-V, Lokhorst HM, Chari A, Kaufman JL, et al. Open-label, multicenter, dose escalation phase 1b study to assess the subcutaneous delivery of daratumumab in patients (pts) with relapsed or refractory multiple myeloma (PAVO) Blood. 2016;128:1149. doi: 10.1182/blood-2016-03-705210. [DOI] [Google Scholar]
- 83.Fathallah AM, Bankert RB, Balu-Iyer SV. Immunogenicity of subcutaneously administered therapeutic proteins-a mechanistic perspective. AAPS J. 2013;15:897–900. doi: 10.1208/s12248-013-9510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hegg R, Pienkowski T, Chen S, Staroslawska E, Falcon S, Kovalenko N, et al. Immunogenicity of trastuzumab intravenous and subcutaneous formulations in the Phase III HannaH study. Ann Oncol. 2012;23:103. [Google Scholar]
- 85.Genovese MC, Covarrubias A, Leon G, Mysler E, Keiserman M, Valente R, et al. Subcutaneous abatacept versus intravenous abatacept: a phase IIIb noninferiority study in patients with an inadequate response to methotrexate. Arthritis Rheum. 2011;63:2854–2864. doi: 10.1002/art.30463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ogata A, Tanimura K, Sugimoto T, Inoue H, Urata Y, Matsubara T, et al. Phase III study of the efficacy and safety of subcutaneous versus intravenous tocilizumab monotherapy in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66:344–354. doi: 10.1002/acr.22110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haraya K, Tachibana T, Nezu J. Quantitative prediction of therapeutic antibody pharmacokinetics after intravenous and subcutaneous injection in human. Drug Metab Pharmacokinet. 2017;32(4):208–217. doi: 10.1016/j.dmpk.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 88.Richter WF, Christianson GJ, Frances N, Grimm HP, Proetzel G, Roopenian DC. Hematopoietic cells as site of first-pass catabolism after subcutaneous dosing and contributors to systemic clearance of a monoclonal antibody in mice. MAbs. 2018 doi: 10.1080/19420862.2018.1458808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wasserman RL, Melamed I, Stein MR, Gupta S, Puck J, Engl W, et al. Recombinant human hyaluronidase-facilitated subcutaneous infusion of human immunoglobulins for primary immunodeficiency. J Allergy Clin Immunol. 2012;130(951–7):e11. doi: 10.1016/j.jaci.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 90.Morcos PN, Zhang X, McIntyre C, Bittner B, Rowell L, Hussain Z. Pharmacokinetics and pharmacodynamics of single subcutaneous doses of tocilizumab administered with or without rHuPH20. Int J Clin Pharmacol Ther. 2013;51:537–548. doi: 10.5414/CP201847. [DOI] [PubMed] [Google Scholar]
- 91.Hompesch M, Muchmore DB, Morrow L, Ludington E, Vaughn DE. Improved postprandial glycemic control in patients with type 2 diabetes from subcutaneous injection of insulin lispro with hyaluronidase. Diabetes Technol Ther. 2012;14:218–224. doi: 10.1089/dia.2011.0117. [DOI] [PubMed] [Google Scholar]
- 92.Xu Z, Wang Q, Zhuang Y, Frederick B, Yan H, Bouman-Thio E, et al. Subcutaneous bioavailability of golimumab at 3 different injection sites in healthy subjects. J Clin Pharmacol. 2010;50:276–284. doi: 10.1177/0091270009340782. [DOI] [PubMed] [Google Scholar]
- 93.Ortega H, Yancey S, Cozens S. Pharmacokinetics and absolute bioavailability of mepolizumab following administration at subcutaneous and intramuscular sites. Clin Pharmacol Drug Dev. 2013;3:57–62. doi: 10.1002/cpdd.60. [DOI] [PubMed] [Google Scholar]