Abstract
BACKGROUND:
AFP serum levels are considered as diagnostic and specific for hepatocellular carcinoma (HCC) in patients with liver cirrhosis (LC).
AIM:
This study aimed to examine the diagnostic value of AFP in the distinguishing of patients with HCC from patients with LC, and to analyse the potential correlation between AFP levels and liver disease stages.
MATERIAL AND METHODS:
Fifty patients with LC and fifty patients with HCC were included in this study. The majority of the patients were males, while the HBV aetiology was dominant.
RESULTS:
Significant differences between LC and HCC patients were detected for AST, ALT, GGT, bilirubin, AFP and AP. Patients with HCC had higher AFP values compared to LC. There was no significant correlation between the size of the tumour lesion and serum AFP levels. A positive correlation between AFP concentration and GGT activity was determined, as was the negative correlation between AFP and age of the subjects. The AFP value of 23.34 ng/m showed high sensitivity (84%) and specificity (82%).
CONCLUSION:
The size of the surface below the ROC curve (AUC) was 0.877 (0.80-0.95), which makes AFP a good biomarker and this diagnostic test is sufficient to separate patients with HCC and LC.
Keywords: AFP, Hepatocellular carcinoma, Liver cirrhosis
Introduction
Hepatocellular carcinoma (HCC) is a malignant epithelial neoplasm with hepatocellular differentiation, and it is the most common primary malignant liver neoplasm [1]. HCC is ranked sixth in the world among all malignant neoplasms, and due to its rapid progression and poor outcomes it is ranked third regarding mortality from malignant neoplasms: among men, it is the fifth most common malignancy and the third most common the cause of death from malignant neoplasms [1] [2]. Men are three times more likely than women to develop HCC, which is ranked as the eight most common type of cancer [1]. Over the past 20 to 30 years, the occurrence of newly diagnosed HCC has more than doubled in the United Kingdom and the United States, and the mortality rate will rise by 14% among men in the UK for the next 20 years [1] [2] [3].
The cause of higher incidence is an increase in the number of HCV infections as well as migration from regions where HBV is highly represented, such as developing countries, which is also considered to be a biological carcinogen [4] [5]. The risk for HCC disease was additionally increased in patients with higher levels of HBV replication, indicating the presence of hepatitis B antigen (HBeAg) and high levels of HBV DNA [6] [7] [8].
Many studies pointed out serum levels of AFP and GGT as significant prognostic factors in predicting the prognosis of HCC patients [9] [10]. Several studies have shown that younger patients with diagnosed HCC have higher levels of serum AFP compared to older patients with the same pathological condition [11] [12] [13]. Furthermore, recent studies have shown that high serum AFP concentration correlates with poor prognosis in patients with HCC. According to previous studies, elevated serum concentrations of AFP in patients with LC are a risk factor for the development of HCC because increased AFP production in patients with LC is a reflection of large and abnormal hepatic regeneration [14] [15].
AFP serum levels > 400 ng/ml are considered as diagnostic and specific for HCC in patients with liver cirrhosis [16]. According to Gomez Senenta et al., the high specificity of AFP that can be obtained by increasing its diagnostic thresholds and allows the use of this biomarker as a confirmatory test for the diagnosis of HCC [17]. Furthermore, previous studies have shown that AFP values > 200 ng/ml in patients with cirrhosis of HBV and/or HCV etiology are highly predictive for HCC indicating that using higher serum AFP concentration as a limit value of the diagnostic test progressively reduces the number of detected HCC cases, i.e. reduces its sensitivity, but in favor of its specificity [18] [19] [20] [21] [22] [23].
This study aimed to examine the diagnostic value of alpha-fetoprotein in the distinguishing of patients with HCC from patients with liver cirrhosis, i.e. to determine its sensitivity and specificity in the detection of hepatocellular carcinoma in the examined sample. Additionally, the aim was to analyse the potential correlation between AFP levels and liver disease stages.
Material and Methods
In this study 100 patients (57 men and 43 women) were included and categorised into two groups. The first group (n = 50) consisted of patients with diagnosed liver cirrhosis (LC) of HBV and /or HCV viral aetiology, and the average age of patients was 62.70 ± 10.49 years. The second group consisted of patients (n = 50) with proven hepatocellular carcinoma (HCC), and the average age of patients was 63.76 ± 10.92 years. In all patients, total bilirubin and AFP (alpha-fetoprotein) concentrations, AST (aspartate aminotransferase), ALT (alanine aminotransferase), γGT (gamma-glutamyl transferase), AP (alkaline phosphatase) activity were analysed. Biochemical parameters were analysed using the VITROS 5600 Integrated System (Ortho-Clinical Diagnostics, USA) analyser and chemiluminescent microparticle immunoassay ARCHITECT AFP assay (CMIA, Ireland) was used for AFP detection. EHO and computerised tomography (CT) were used to detect and measure the size of this lesion (HCC).
All data were analysed using IBM SPSS Statistics ver. 20 (USA). Variance analysis (ANOVA) was used to test differences. Survival Kaplan-Meier test was used to analyse the coherence between sensitivity and specificity through all possible limit values that determine the positive pathological condition or presence of the disease. The area under the ROC curve (AUC) is the measure of the discriminating power of the diagnostic test.
Results
The percentage of patients diagnosed with LC and HCC, patients’ sex and age, aetiology of disease (HCV, HBV) and significant differences (p values) are presented in Table 1. The group of patients with a diagnosis of liver cirrhosis (LC) had 50 patients (26 males and 24 women), the average age of 62.70 years. In the HCC group (n = 50) consisted of 31 males and 19 women average age was 63.73 years. No significant differences were found between men and women when comparing both diseases; also no significant difference in age and viral aetiology was established. HBV is a dominant viral agent present in 62% of the patients.
Table 1.
Demographic and clinical characteristics of patients with LC and proven HCC
| Characteristics | Patients with LC1 | Patients with HCC2 | Total | p values | |
|---|---|---|---|---|---|
| Gender | Males | 26 or 52 % | 31 or 62 % | 57 | > 0.05 |
| Females | 24 or 48 % | 19 or 38 % | 43 | > 0.05 | |
| Age | Mean | 62.70±10.49 | 63.73±10.92 | 63.29±10.67 | > 0.05 |
| Range | 30-81 | 29-81 | 29-81 | - | |
| Etiology | HBV3 | 28 or 56 % | 34 or 68 % | 62 | > 0.05 |
| HCV4 | 21 or 42 % | 16 or 32 % | 37 | > 0.05 | |
| HBV+HCV | 1 or 2 % | - | 1 | - | |
LC - liver cirrhosis;
HCC - hepatocellular carcinoma;
HBV- hepatitis B virus;
HCV-hepatitis C virus.
Table 2 shows the values of biochemical parameters in LC and HCC patients, and significant differences between them. All patients had high values of biochemical parameters compared to the reference range, while HCC patients had higher values compared to LC patients. Significant differences between these two groups were observed for AST, γGT, AP and AFP values.
Table 2.
Differences in biochemical parameters in LC and HCC patients
| Parameters | Patients with LC1(n = 50) | Patients with HCC2 (n = 50) | P values |
|---|---|---|---|
| Total bilirubin | 43.63 ± 34.17 | 60.82 ± 99.41 | > 0.05 |
| AST | 109.24 ± 145.02 | 160.42 ± 138.48 | < 0.05* |
| ALT | 75.50 ± 91.57 | 78.08 ± 60.77 | > 0.05 |
| GT | 110.98 ± 163.67 | 192.74 ± 188.47 | < 0.05* |
| AP | 123.00 ± 88.03 | 199.56 ± 149.47 | < 0.05* |
| AFP | 256.82 ± 1634.03 | 9129 ± 17547.01 | < 0.05* |
Significant differences at the level of 0.05.
Table 3 shows AFP serum concentrations in LC and HCC patients. Also, AFP values for HCC patients with viral aetiology were presented as well as Spearman correlation between AFP and age of patients. Males in the group of LC and HCC patients had significantly higher values compared to females. Among HCC patients, very high values of AFP (12465.08 ng/l) were determined for patients with HBV aetiology in comparison to patients with HCV aetiology, but these differences were not significant. A negative correlation (-0.053) but not statistically significant was found between the serum levels of AFP concentration and age of the patient (younger patients had more serum AFP values).
Table 3.
Differences in serum concentrations of AFP according to aetiology and gender
| Diagnosis | HBV/HCV | Mean ± SD | p values | Correlation between AFP and age |
|---|---|---|---|---|
| HCC patients (Males/females) | HBV | 12465.08 | < 0.05 | |
| HCV | 2040.92 | -0.053 | ||
| HCC patients | Males | 10790 ± 19757.43 | > 0.05 | |
| Females | 6419 ± 13231.46 | |||
| LC patients | Males | 468.60 ± 2265.50 | > 0.05 | |
| Females | 27.40 ± 62.82 |
Table 4 shows the distribution of serological AFP values between LC and HCC patients. Both groups had a very high percentage of patients with increased serum AFP (> 7 ng/ml). Only 8% of HCC patients had serum AFP values within the reference range.
Table 4.
Distribution of serological AFP values between LC and HCC patients
| AFP concentrations | LC patients | HCC patients | Total |
|---|---|---|---|
| > 7 ng/ml | 32 or 64 % | 46 or 92 % | 78 % |
| < 7 ng/ml | 18 or 36 % | 4 or 8 % | 22 % |
The sensitivity of the medical diagnostic test (true positive rate) gives the ability to detect the presence of the disease; however, the specificity of the medical diagnostic test (true negative rate) gives the ability to determine the absence of the disease. The ROC curves show the relationship between sensitivity and 1-specificity through all possible limit values that determine the positive pathological condition or presence of the disease. The area below the ROC curve (AUC) is the measure of the discriminating power of the diagnostic test [24].
The ROC curve (receiver-operating characteristic curve) is, therefore, a graphical representation of the proportion of successfully identified and falsely identified cases of hepatocellular carcinoma. Figure 1 shows an AUC of 0.877 (0.80-0.95), making this biomarker a good distinguishing parameter for patients with HCC and those with LC.
Figure 1.
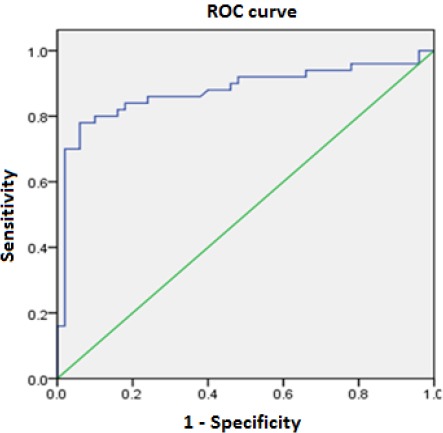
ROC curve of serum alpha-fetoprotein for the diagnosis of hepatocellular carcinoma. The green line corresponds to the 1:1 correlation between two parameters
Table 5 shows AUC values for ROC curve. Also, ROC analysis implies testing all AFP serum values, and for each of the sensitivity and specificity were detected. It has been determined that the optimal limit value of AFP serum concentrations for distinguishing of patients (with and without HCC) within our population was 23.34 ng/ml. At this serum concentration, this test has approximate and high sensitivity and specificity (84% vs 82%) indicating the diagnostic significance of this tumour marker for early diagnosis of hepatocellular carcinoma in LC patients.
Table 5.
Area of measurements under ROC curves (AUC), AFP sensitivity and specificity
| Variable | AUC | STD Error1 | Asympt sig.2 | UCL | LCL | Cut-off | Sens. | Specify. |
|---|---|---|---|---|---|---|---|---|
| AFP | 0.877 | 0.038 | 0.000 | 0.802 | 0.0952 | 23.34 | 84% | 82% |
Under a non-parametric assumption;
Null hypothesis: true area = 0.5; UCL – upper control limit; LCL – lower control limit; Sens. – sensitivity; Spec. – specificity; Optimal cut-off: optimal limit value.
Since our limit value has similar sensitivity and specificity, the limit values with the best performance regarding sensitivity and specificity are shown in Table 6.
Table 6.
Specificity and sensitivity of the different AFP limit values for the diagnosis of hepatocellular carcinoma
| AFP (ng/ml) | Sensitivity % | Specificity % |
|---|---|---|
| 6.27 | 92 % | 34 |
| 12.38 | 88 | 60 |
| 19.14 | 84 | 76 |
| 58.49 | 80 | 90 |
| 81.58 | 78 | 94 |
| 149.77 | 74 | 94 |
| 219.96 | 72 | 98 |
| 341.70 | 70 | 98 |
| 420.67 | 66 | 99 |
Table 7 shows a grouping of patients with HCC in comparison to tumour lesion size and serum AFP level.
Table 7.
Tumor size in HCC patients and correlation of AFP about lesion size
| Tumour size (cm) | N (%) | AFP (ng/ml) | ||
|---|---|---|---|---|
| ≤ 20 | 21-399 | ≥ 400 | ||
| < 3 | 2 or 4 % | 1 | 1 | 0 |
| 3-5 | 11 or 22 % | 1 | 3 | 7 |
| > 5 | 37 or 74 % | 6 | 4 | 27 |
According to the results shown in Table 7, 37 patients with proven HCC (74%) had a tumour> 5 cm, and six patients had normal serum AFP levels, four moderately elevated, and 27 patients with tumour size > 5 cm had significantly elevated values serum AFP. In the group of 11 patients (22%) with proven HCC with tumour size from 3-5 cm, one patient had a normal serum AFP level, in three patients this biomarker was moderately elevated and in 7 patients it was elevated (≥ 400 ng/ml). Only two patients (4%) with proven HCC had tumour size < 3 cm; one of them had a normal serum AFP level, and the other one had moderately elevated AFP concentration.
Discussion
Our research has shown a higher percentage of male patients with LC and HCC diagnosis, while the average age is not different in comparison to women. Most patients had HBV viral aetiology with dominant prevalence in our country [25]. Patients with hepatocellular carcinoma developed after HBV infection have significantly higher mean levels of AFP than patients with proved hepatocellular carcinoma developed in the background of HCV infection. Therefore, a type of viral infection affects serum levels of alpha-fetoprotein in patients with hepatocellular carcinoma.
Due to more progressive disease, HCC patients had higher values of all analysed parameters compared to LC patients. The largest variations were detected for AFP concentrations. The serological values of AFP and γGT correlate, as previously confirmed in the study of Ertle et al., [26]. This correlation shows parallel reexpression of AFP with reexpression of γGT during the development of HCC, otherwise present in embryonic liver and suppressed after birth. Younger patients tend to have higher levels of serum AFP compared to older patients [12]. The results of our study showed a significant reverse correlation between serum AFP levels and age of patients. 84% of our patients with HCC had an elevated level of this biomarker, similar to results in previous studies [27] [28].
This is the first study in Bosnia and Herzegovina that by analysing the ROC curve evaluates the diagnostic performance of serological tests. The AUC value > 0.96 suggests, an excellent discriminatory ability of the diagnostic test [29]. In our study, measurement of AFP has shown a significant ability to discriminate between HCC and LC diagnosis due to its AUC that was very close to the specified value. In previous studies, the ROC curve of the alpha-fetoprotein used as a diagnostic test suggests that the serum AFP level of about 20 ng/ml provides the optimal balance between sensitivity and specificity (optimal cut-off/limit value). According to Trevisiani et al., the best diagnostic cut-off serum AFP level ranged from 16 to 20ng/ml, with a high specificity of 89.4% and a sensitivity of 60% [30]. In our case, this diagnostic test has omitted 40% of HCC cases. If a higher limit value were used, it would progressively reduce the number of detected HCC cases, i.e. would reduce the sensitivity of the diagnostic test. Recent results of Daruzo et al. and El-Hussein et al. indicated a sensitivity of 69% and 68.2% at the limit of 19.2 ng/ml in the first and 25 ng/ml in the second study [31] [32].
Our research has confirmed results from earlier studies where the best sensitivity and specificity balance of the AFP serum level was at the value of 20 ng/ml; in our case, it was 23.34 ng/ml. Optimal AFP serum level for our study had a high sensitivity of 84% and specificity of 82%. This value of sensitivity was higher than values obtained by Arietta et al. where the optimal cut-off value at 21ng/ml had a 76% of sensitivity [33]. However, our sensitivity (84%) and specificity (82%) values were within sensitivity, and specificity ranges from 39% to 97%, and 76% to 95%, respectively, and they are similar to the results from previous studies [19] [20] [21] [22] [23]. However, our results regarding the sensitivity and the specificity of the optimal AFP limit value were more favourable in comparison to results reported by Trevisiana et al., Durazo et al., El-Hussain et al. and Hernandez et al., which were taken as reference in current scientific frameworks in this field [18] [30] [31] [32]. One explanation for this phenomenon would be that in our study only patients with viral aetiology were involved, whereas in the mentioned studies viral aetiology was dominant but not excluded. Accordingly, this biomarker has significantly better performance in hepatocellular carcinoma with viral aetiology compared to other HCC etiologies, as it was demonstrated in the recent study by Ertel et al., [26]. The surface area under the ROC curve (AUC) was 0.877 (0.80-0.95), making this biomarker a good diagnostic test for distinguishing HCC and LC patients.
In the case of our patients, the value of 0.877 is at the very limit so that a diagnostic test could be characterised as excellent, given this value was close to 0.90. Our results were similar to results obtained by Trevisiani et al., where AFP serum concentrations higher than 20 ng/ml had a specificity of 98.4% or less than 2 % of patients without HCC had serological AFP levels higher than 20 ng/ml [30]. Also, the results from our study confirmed previous serum levels of AFP > 400 ng/ml as diagnostic, highly specific for HCC, i.e. that 1% or less of our patients had high AFP concentrations and did not have HCC [34]. It is important to note that high limit value is not useful for detecting early stage of HCC [34]. In these cases, patients with primary liver cancer may have normal levels of serum AFP; however normal or moderately elevated AFP levels do not exclude the HCC diagnosis.
We found that 74% of HCC patients had tumour lesion size more than > 5 cm as it was reported earlier by Arietta et al., (75%) [33]. However, previous studies have found that two-thirds of patients with hepatocellular lesion size < 4 cm have serum AFP levels < 200 ng/ml. Also, it has been reported that up to 20% of HCC patients do not produce elevated levels of AFP [35]. These data are consistent with the results of our study where 16% of HCC patients did not have elevated levels of AFP. Although it may be inferred that smaller tumour size is related with lower serum AFP concentrations in comparison to tumours with larger diameter, our results showed that there was no significant correlation between the size of the tumour lesion and the level of AFP, which is similar to results from previous studies except in case of results obtained by Abbasi et al. who have established the existence of correlation [36] [37]. The absence of correlation between the serum concentration and the size of the hepatocellular lesion could be explained by the fact that tumour differentiation and its AFP secretion ability are more important in determining the level of serum AFP produced by primary liver cancer, than tumour size.
Adequate sensitivity and specificity allow the use of serum AFP values as an extremely useful tool in preclinical HCC detection in liver cirrhosis patients. We believe that the results of our research gave a complete picture of the role of AFP in HCC pathogenesis and suggested the justification of using this parameter in determination and monitoring.
Serum levels of liver biomarkers are significantly higher in HCC patients compared to LC patients. The serum AFP level could not be used as a reliable indicator of tumour lesion size; tumour differentiation and its AFP secretion ability are more important for determining serum AFP levels produced by primary liver cancer than its size alone. The size of the AUC under ROC curve of 0.877 suggests that the determination of AFP serological concentration may be considered as a good diagnostic test for the distinguishing patients with hepatocellular carcinoma and those with cirrhosis (with viral B and C aetiology). Based on the obtained ROC curve, the optimal serum concentration of AFP for discrimination between patients with and without HCC within our population was 23.34 ng/ml. This value achieved a balance of sensitivity and specificity of the serum AFP level, used as a diagnostic test.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Kew MC. Epidemiology of chronic hepatitis B virus infection, hepatocellular carcinoma and hepatitis B virus-induced hepatocellular carcinoma. Pathol Biol. 2010;58:27367. doi: 10.1016/j.patbio.2010.01.005. https://doi.org/10.1016/j.patbio.2010.01.005 PMid:20378277. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2009;55:746108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis. 2010;42:206–214. doi: 10.1016/S1590-8658(10)60507-5. https://doi.org/10.1016/S1590-⇒(10)60507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman M. Hepatocellular carcinoma:epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143–154. doi: 10.1055/s-2005-871194. https://doi.org/10.1055/s-2005-∇94 PMid:15918143. [DOI] [PubMed] [Google Scholar]
- 5.El-Seragh HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36:74–83. doi: 10.1053/jhep.2002.36807. https://doi.org/10.1002/hep.1840360710. [DOI] [PubMed] [Google Scholar]
- 6.McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25:3–8. doi: 10.1055/s-2005-915644. https://doi.org/10.1055/s-2005-915644 PMid:16103976. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Rudolph KL. Hepatocellular carcinoma:epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. https://doi.org/10.1053/j.gastro.2007.04.061 PMid:17570226. [DOI] [PubMed] [Google Scholar]
- 8.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis:incidence and risk factors. Gastroenterology. 2004;127(1):35–50. doi: 10.1053/j.gastro.2004.09.014. https://doi.org/10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JB, Chen Y, Zhang B, et al. Prognostic significance of serum gamma-glutamyl transferase in patients with intermediate hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol. 2011;23(9):787–793. doi: 10.1097/MEG.0b013e32834902dd. https://doi.org/10.1097/MEG.0b013e32834902dd PMid:21730869. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Chen Y, Ge N, et al. Prognostic significance of alpha-fetoprotein status in the outcome of hepatocellular carcinoma after treatment of transarterial chemoembolization. Ann Surg Oncol. 2012;19(11):3540–3546. doi: 10.1245/s10434-012-2368-5. https://doi.org/10.1245/s10434-012-2368-5 PMid:22532305. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki Y, Kakizaki S, Sohara N, et al. Hepatocellular carcinoma in young adults:the clinical characteristics, prognosis, and findings of a patient survival analysis. Dig Dis Sci. 2007;52:1103–1107. doi: 10.1007/s10620-006-9578-2. https://doi.org/10.1007/s10620-006-9578-2 PMid:17380407. [DOI] [PubMed] [Google Scholar]
- 12.Chang PE, Ong WC, Lui HF, Tan CK. Is the prognosis of young patients with hepatocellular carcinoma poorer than the prognosis of older patients?A comparative analysis of clinical characteristics, prognostic features, and survival outcome. J Gastroenterol. 2008;43:881–888. doi: 10.1007/s00535-008-2238-x. https://doi.org/10.1007/s00535-008-2238-x PMid:19012042. [DOI] [PubMed] [Google Scholar]
- 13.Aramaki M, Kawano K, Sasaki A, et al. Hepatocellular carcinoma in young adults. Hepatogastroenterology. 2005;52:1795–1797. PMid:16334779. [PubMed] [Google Scholar]
- 14.Harada T, Shigeta K, Noda K, et al. Clinical implications of alpha-fetoprotein in liver cirrhosis:five-year follow-up study. Hepatogastroenterology. 1980;27:169–75. PMid:6161869. [PubMed] [Google Scholar]
- 15.Arrieta O, Rodríguez-Díaz J, Rosas-Camargo V, et al. Colchicine delays the development of hepatocellular carcinoma in patients with hepatitisvirus related-liver cirrhosis. Cancer. 2006;107:1852–1858. doi: 10.1002/cncr.22198. https://doi.org/10.1002/cncr.22198 PMid:16967451. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma:conclusions of the Barcelona-2000 EASL Conference. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. https://doi.org/10.1016/S0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 17.Gómez Senent S, Gómez Raposo C, Segura Cabral JM. Guía para el diagnóstico, estadificación y tratamiento del hepatocarcinoma. Med Clin. 2007;128:741–748. doi: 10.1157/13106134. https://doi.org/10.1157/13106134. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez JC, Samanda M, Roque A, et al. Diagnostic value of alfa-fetoprotein for hepatocellular carcinoma. Biotechnol Apl. 2011;28(1):34–39. [Google Scholar]
- 19.Soresi M, Magliarisi C, Campagna P, et al. Usefulness of alpha-fetoprotein in the diagnosis of hepatocellular carcinoma. Anticancer Res. 2003;23(2C):1747–1753. PMid:12820452. [PubMed] [Google Scholar]
- 20.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus:incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22(2):432–438. PMid:7543434. [PubMed] [Google Scholar]
- 21.Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19(1):61–66. https://doi.org/10.1002/hep.1840190111 PMid:7506227. [PubMed] [Google Scholar]
- 22.Pateron D, Ganne N, Trinchet JC, et al. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994;20(1):65–71. doi: 10.1016/s0168-8278(05)80468-4. https://doi.org/10.1016/S0168-8278(05)80468-4. [DOI] [PubMed] [Google Scholar]
- 23.McMahon BJ, Bulkow L, Harpster A, et al. Screening for hepatocellular carcinoma in Alaska natives infected with chronic hepatitis B:a 16-year population-based study. Hepatology. 2000;32(4 Pt 1):842–846. doi: 10.1053/jhep.2000.17914. https://doi.org/10.1053/jhep.2000.17914 PMid:11003632. [DOI] [PubMed] [Google Scholar]
- 24.Faraggi D, Reiser B. Estimation of the area under the ROC curve. Stat Med. 2002;21:3093–3106. doi: 10.1002/sim.1228. https://doi.org/10.1002/sim.1228 PMid:12369084. [DOI] [PubMed] [Google Scholar]
- 25.Epidemiološki nadzor nad zaraznim bolestima u Federaciji BiH, 2012. godina. Zavod za javno zdravstvo FBiH, služba za epidemiologiju. Bilten. 2013:31. [Google Scholar]
- 26.Ertle JM, Heider D, Wichert M, et al. A combination of α-fetoprotein and des-γ-carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87:121–131. doi: 10.1159/000346080. https://doi.org/10.1159/000346080 PMid:23406785. [DOI] [PubMed] [Google Scholar]
- 27.Johnson PJ. The role of serum alpha-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin Liver Dis. 2001;5(1):145–159. doi: 10.1016/s1089-3261(05)70158-6. https://doi.org/10.1016/S1089-3261(05)70158-6. [DOI] [PubMed] [Google Scholar]
- 28.Kailapuri GM, Mathews S, Jayanthi V, et al. Alpha-fetoprotein as a tumor marker in hepatocellular carcinoma:investigations in south Indian subjects with hepatotropic virus and aflatoxin etiologies. Int J Infect Dis. 2008;12(6):71–76. doi: 10.1016/j.ijid.2008.04.010. https://doi.org/10.1016/j.ijid.2008.04.010 PMid:18658001. [DOI] [PubMed] [Google Scholar]
- 29.Biao ZA. Semiparametric hypothesis testing procedure for the ROC curve area under a density ratio model. CSDA. 2006;7:1855–1876. [Google Scholar]
- 30.Trevisani F, D'Intino PE, Morselli-Labate AM, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease:influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570–575. doi: 10.1016/s0168-8278(00)00053-2. https://doi.org/10.1016/S0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 31.Durazo FA, Blatt LM, Corey WG, et al. Des-gamma-carboxyprothrombin, alpha-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis and hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23(10):1541–1548. doi: 10.1111/j.1440-1746.2008.05395.x. https://doi.org/10.1111/j.1440-1746.2008.05395.x PMid:18422961. [DOI] [PubMed] [Google Scholar]
- 32.El-Houseini ME, Mohammed MS, Elshemey WM, Hussein TD, Desouky OS, Elsayed AA. Enhanced detection of hepatocellular carcinoma. Cancer Control. 2005;12(4):248–253. doi: 10.1177/107327480501200407. https://doi.org/10.1177/107327480501200407 PMid:16258497. [DOI] [PubMed] [Google Scholar]
- 33.Arrieta O, Cacho B, Morales-Espinosa D, Ruelas-Villavicencio A, Flores-Estrada D, Hernández-Pedro N. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer. 2007;7:28–37. doi: 10.1186/1471-2407-7-28. https://doi.org/10.1186/1471-2407-7-28 PMid:17288606PMCid:PMC1803796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruix J, Sherman M. Management of hepatocellular carcinoma. AASLD Practice Guidelines. Hepatology. 2005;42(5):2208–2236. doi: 10.1002/hep.20933. https://doi.org/10.1002/hep.20933 PMid:16250051. [DOI] [PubMed] [Google Scholar]
- 35.Khan A, Asmaa I, Edward L, Waked I, Taylor-Robinson SD. Diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2009;15(11):1301–1314. doi: 10.3748/wjg.15.1301. https://doi.org/10.3748/wjg.15.1301 PMid:19294759 PMCid:PMC2658831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Darwish A. Assessment of Clinical Significance of Serum Squamous Cell Carcinoma Antigen in Patients with Liver Cirrhosis and Hepatocellular Carcinoma. Thesis submitted for partial fulfillment of the M.Sc. degree in tropical medicine, Faculty of medicine, Cairo University. 2006 [Google Scholar]
- 37.Abbasi A, Bhutto AR, Butt N, Mun SJ. Correlation of serum alpha fetoprotein and tumor size in hepatocellular carcinoma. J Pak Med Assoc. 2012;62(1):33–36. PMid:22352098. [PubMed] [Google Scholar]


