Abstract
BACKGROUND:
Corneal blindness resulting from various medical conditions affects millions worldwide. The rapid developing tissue engineering field offers design of a scaffold with mechanical properties and transparency similar to that of the natural cornea.
AIM:
The present study aimed at to prepare and investigate the properties of PVA/chitosan blended scaffold by further cross-linking with 1-Ethyl-3-(3-dimethyl aminopropyl)-carbodiimide (EDC) and 2 N-Hydroxysuccinimide (NHS) as potential in vitro carrier for human limbal stem cells delivery.
MATERIAL AND METHODS:
Acetic acid dissolved chitosan was added to PVA solution, uniformly mixed with a homogenizer until the mixture was in a colloidal state, followed by H2SO4 and formaldehyde added and the sample was allowed to cool, subsequently it was poured into a tube and heated in an oven at 60°C for 50 minutes. Finally, samples were soaked in a cross-linking bath with EDC, NHS and NaOH in H2O/EtOH for 24 h consecutively stirred to cross-link the polymeric chains, reduce degradation. After soaking in the bath, the samples were carefully washed with 2% glycine aqueous solution several times to remove the remaining amount of cross-linkers, followed by washed with water to remove residual agents. Later the cross-linked scaffold subjected for various characterization and biological experiments.
RESULTS:
After viscosity measurement, the scaffold was observed by Fourier transform infrared (FT-IR). The water absorbency of PVA/Chitosan was increased 361% by swelling. Compression testing demonstrated that by increasing the amount of chitosan, the strength of the scaffold could be increased to 16×10−1 MPa. Our degradation results revealed by mass loss using equation shows that scaffold degraded gradually imply slow degradation. In vitro tests showed good cell proliferation and growth in the scaffold. Our assay results confirmed that the membrane could increase the cells adhesion and growth on the substrate.
CONCLUSION:
Hence, we strongly believe the use of this improved PVA/chitosan scaffold has potential to cut down the disadvantages of the human amniotic membrane (HAM) for corneal epithelium in ocular surface surgery and greater mechanical strength in future after successful experimentation with clinical trials.
Keywords: Tissue engineering, Biocompatibility, Chitosan, Poly (vinyl alcohol), PVA, Corneal cells
Introduction
The cornea is a clear, avascular, multi-laminar structure plays an important role in vision [1]. The World Health Organization (WHO), an agency of the United Nations has recognised corneal diseases as a major cause of blindness in the world, next to cataract, which affects more than 10 million people [2] [3]. At present, the corneal transplantation is the only existing therapy of choice [4] [5]. Besides, a severe scarcity of fresh donor corneas [6] and an unknown threat of immune rejection had seen with routine heterograft; hence, it is very imperative and crucial to construct a corneal equivalent to replace pathologic corneal tissue.
Corneal tissue engineering has appeared as a viable approach to developing corneal tissue alternates [7] [9]. The design of a scaffold with mechanical properties and transparency similar to that of the natural cornea is significant for the regeneration of corneal tissues but also able to resist the culture conditions, flexible to the shape of the cornea and quite strong for surgical manipulation including the suturing [10]. Currently, the substratum commonly used is the human amniotic membrane (HAM) [11], which includes denuded HAM over an intact membrane [12]. However, many inherent problems still exist like the thinness, wrinkling nature, sterile storage and early degradation, its possible danger for the spread of pathogens and the risk of immune-mediated graft rejection etc. [13]. Since HAM has many disadvantages, there has been a substantial amount of research to find a good alternative source for replacement.
The key challenge in tissue engineering is the designing of an artificial extracellular matrix (ECM) component because it can support cell growth and allow deposition of the natural ECM proteins over it during the initial stages [14]. Although various biomaterial scaffolds are available for many applications like sutures, bone plates, heart valves and screws [15] [16], recent years had also witnessed tremendous research attention towards the improvement of few other naturally derived biopolymers like silk [17] and purified ECM based molecules like collagen, elastin and glycosaminoglycan (GAGs) [18] [19]. Besides the above, polylactic acid (PLA), polycaprolactone (PCL) [20] [21], and the PVA membranes as well as their blends, have been widely used in the production of scaffolds for various biomedical applications [20] [21].
The development of chitosan-based biomaterial attracted much attention [22] recently for various applications because of its novel potentials like minimal foreign body reactions and intrinsic antibacterial property [23]. Also, the biocompatibility, biodegradability and chitosan’s ability to mould into various forms and geometries make it to suitable for cell ingrowth and conduction [24] [25]. Since chitosan alone is not sufficient to support cell growth, enhancing its mechanical strength needs another partner like polyvinyl alcohol (PVA), a biodegradable polymer/or its blends often used in tissue engineering applications. The addition of chitosan to the PVA solution has an effect of thickener, increasing the viscosity and giving rise to uniform nanofibers, even for low PVA concentration [26]. These favourable intermolecular interactions between PVA and chitosan influence the culture of corneal epithelial stem cells. Chitosan containing hydroxyl and amine groups has, therefore, the potential to miscible with PVA due to the ability to form hydrogen bonds.
On the other hand, stem cells provide a potentially boundless source of cells for treating a plethora of human diseases [27] [28]. The corneal limbus, located at the corneoscleral junction, believed to harbour the cornea stem cells in the basal layer of the epithelium [29] [30]. These limbal epithelial stem cells (LESCs) possess all of the properties of an adult stem cell population [31] and are responsible for maintaining and regenerating the corneal epithelium throughout the life. Also, limbal stem cells also act as a “barrier” to conjunctival epithelial cells and normally prevent them from migrating on to the corneal surface [32]. Extensive studies performed to investigate the feasibility of explant culture method of cultivating corneal epithelial cells and their characteristics in comparison to the limbal explant culture [33]. Hence, the present study employed cultured corneal epithelial cells (HCEC), as an ideal substitute to test the ability of PVA cross-linked chitosan together with amine coupling through EDC-NHS scaffold to facilitate their growth.
The present study objectives were as follows: (i) to develop a biodegradable and non-toxic PVA cross-linked chitosan scaffold by further cross-link with EDC and NHS; (ii) to characterize its physiochemical properties to support the growth of HCEC, so that it had the ability to facilitate enhanced adhesion, expansion and proliferation of HCEC; while maintaining its mechanical properties (iii) to investigate the corneal epithelial marker and antimicrobial peptide expression in the HCEC. With consideration of the ultimate goal to use the methods in clinical applications, we were mindful of the potential risks of using culture media containing defined or undefined animal derivatives. Such components have the potential to transmit communicable diseases and provoke immunological problems during transplantation. To reduce the potential harmful complications and to minimise any risk for future patients, we used a culture medium that was free of supplements containing non-human animal derivatives.
Subjects and Methods
Polyvinyl alcohol (87-89% hydrolysed), the average molecular weight of 72000 gmol−1), acetic acid (AA 35% pure), and glutaraldehyde (GA) (25% aqueous solution) were purchased from Merck (Merck Specialities Pvt Limited Mumbai, India). Chitosan [poly (β-(1-4)-2-amino-2-deoxy-D-glucopyranose)] (75% degree of deacetylation) (medium molecular weight of 190,000–310,000) was purchased from Himedia, Mumbai, India.
The chitosan powder was separately dissolved in 1% acetic acid (20 mL) at room temperature. The PVA (4 g) was dissolved completely in Milli-Q water (20 mL) by heating. The chitosan was added to the PVA solution and mixed uniformly with a homogeniser at 300 rpm and 90°C for 30 minutes until the mixture was in a colloidal state. After adding H2SO4 (10 mL) and formaldehyde (5 mL) and stirring, the sample was cooled to room temperature. Finally, the sample was poured into a tube and heated in an oven at 60°C for 50 minutes. Finally, samples were soaked in a cross-linking bath with EDC, NHS and 0.1 M NaOH (4 mg/mL) in H2O/EtOH 2:1 for 24 h consecutively stirred to cross-link the polymeric chains, reduce degradation, and enhance the biomechanical properties of the scaffolds for delivery or tissue repair. After soaking in the bath, the samples were carefully washed with 2% aqueous glycine solution several times to remove the remaining amount of cross-linkers, followed by washing with water to remove residual agents. The present study experimented on trial base with few scaffolds sterilised with either ethylene oxide gas or alcohol by complete immersion in 75%, 50%, 25%, 5% and 1% alcohol solution with an incubation time of 10min. Eventually, the scaffold was washed twice with water and incubated for 10 min. Each, followed by dried, separated and used for plating of cells. The scaffold preparation and related experiments were carried out at the Polymer Nanotechnology Center of B.S Abdur Rahman Crescent University (BSA), Vandalur, Chennai, India.
The viscosity of solutions was measured by Brookfield Model DV-III viscometer (Brookfield Engineering Laboratories Inc, Stoughton, MA) before the cross-linking process was begun.
The samples were examined by FT-IR analysis with a Perkin Elmer, model 2000 spectroscopy. For IR analysis, 2-6 mg of the scraped samples (about 10 µm thick) were carefully mixed with 500mg of KBr (infrared grade) and pelletized under vacuum. Then, pellets between 4000-400 cm−1 were analyzed with 120 scans averaging 4- cm−1 resolution in attenuated total reflection (ATR) mode. The FT-IR analysis was used to characterise the presence of specific chemical groups of PVA and chitosan, chemical interactions and the crosslinking effect in the polymeric scaffolds and to identify the effects of the above process on functional groups.
The optical clarity of the scaffolds is a major pre-requisite for the scaffold platform as they serve the purpose of an artificial extracellular matrix for the cornea, whose primary role to participate in the visual activity [34]. Hence, the scaffold samples were examined for optical clarity by using a Beckman DU-800 spectrophotometer and scanning was done within the visible range of wavelengths (400-800 nm).
Three dumbbell-shaped specimens of 4mm wide and 10mm length were punched out from each scaffold using a dying instrument. Mechanical properties such as tensile strength (MPa) and percentage of elongation at break (percentage) were measured using a universal testing machine (INSTRON model 1405) at an extension rate of 5 mm/min.
The quantity of water imbibed by a material is an important property, as it greatly contributes to the biocompatibility of the end material and decides if the material may be useful for biomedical purposes. To access the water sorption potential of the prepared scaffold, the PVA/Chitosan nanofibrous scaffolds were oven dried at 50°C and placed in a 24-well plate. Each well-contained 1mL of a phosphate buffered solution (PBS; pH 7.4). The scaffolds were incubated in vitro at 37°C for different periods (1, 3, 7, and 10 days) [35]. After immersion of the scaffolds in PBS solution for these different periods, excess PBS was wiped from the swollen saturated PVA/Chitosan scaffold, the amount of fluid uptake was determined by careful removal of samples from the medium after wiping off excess fluid with filter paper. The swelling ratio value (S) was calculated using the following formula 1:
S = (Ww−Wd)/Wd×100 (1)
For this test, the samples were weighed for determination of the wet weight (Ww) as a function of immersion time and dried weight (Wd) of the samples.
The degradation study of the scaffolds was carried out in vitro by incubating the samples in PBS at pH 7.4, 37°C for different periods. After each degradation period, the samples were washed and subsequently dried in a vacuum oven at room temperature for 24 hours. To find out the degradation index (Di), the weight of the samples (Wt) and the degradation index was calculated before and after the degradation test using the mass loss using equation 2:
Di = (W0−Wt)/W0×100 (2)
Human corneal epithelial cells (HCEC) were obtained from the commercially available source as primary corneal epithelial cells (Normal, Human (ATCC® PCS-700-010). On every passage, cells obtained by trypsinisation using 0.5% trypsin™ were cryopreserved as secondary cells. The in vitro cytotoxicity of the prepared scaffolds was tested using both NIH3T3 fibroblasts cell line and HCEC. Cells (105) were seeded into each well of 24 wells plate. The culture liquid contained DMEM (Dulbecco’s modified Eagle’s medium), 10% fortified bovine calf serum (FBS), and 1% penicillin-streptomycin solution. The cell culture of PVA/Chitosan scaffold cycles lasted for three days. After 72 hours of incubation, 3-(4,5-dimethylthiazol-2-yl) - 2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/mL) (Sigma, Munich, Germany) was added into each well and incubated for 90-120 minutes. Then, all the media was discarded and 600 μL DMSO was added to each well. An ELISA reader at 590 nm measured the optical density (OD) values after 30 minutes with a reference filter of 620 nm.
Cells were formalin fixed and paraffin embedded for routine histological processing and stained with hematoxylin and eosin (H&E) to visualise the cell attachment and proliferation on the scaffold. The same procedure was followed for cells plated in the Petri plate, which was used as a control. The processed samples were observed by using a light microscope with specific image analysis software from Zeiss [36].
The scaffold washed thrice with PBS, followed by washed with Dulbecco’s Modified Eagle’s Medium (DMEM) twice and incubated in a CO2 incubator. After thorough checking HCEC cells viability, they were seeded onto the scaffold with 4 ml of Epilife medium. The same procedure adopted for control.
After the plated cells reached confluency, they were trypsinised (0.02%), and RNA isolation was done (using a QIAGEN kit method) for further expression studies. With a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an an internal control, the mRNA expression of different molecular markers for corneal epithelial stem cells and antimicrobial peptides were analyzed by semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) as described by previous reports [37] [38]. PCR amplification of the first-strand cDNAs was performed with specific primer pairs, designed from published human gene sequences (Table 1) for different markers in a GeneAmp PCR System 9700 (Applied Biosystems) and resultant product of amplification and documented in BioRad gel documentation system; Bio-Rad Laboratories, UK.
Table 1.
Human primer sequences used for semi-quantitative RT-PCR
| Gene Name | Primer sequence - 3’-5’ | Annealing temperature (°C) | Base pair size (bp) |
|---|---|---|---|
| Corneal Epithelial Stem Markers | |||
| ABCG2 | FP: 5’ AGTTCCATGGCACTGGCCATA 3’ RP: 5’ TCAGGTAGGCAATTGTGAAGG 3’ |
62 | 379 |
| Cytokeratin 3 | FP: 5’ GGCAGAGATCGAGGGTCTC 3’ RP: 5’GTCATCCTTCGCCTGCTGTAG 3’ |
64 | 145 |
| Connexin 43 | FP:5´ CCTTCTTGCTGATCCAGTGC 3´ RP 5´ ACCAAGGACACCACCAGCAT 3´ |
63 | 150 |
| Antimicrobial peptide – AMP | |||
| hBD-1 | FP: GCCTCCAAAGGAGCCAGCGT RP: CTTCTGGTCACTCCCAGCTCA |
54 | 287 |
| hBD-2 | FP: CAGCCATCAGCCATGAGG RP: TGGCTTTTTGCAGCATTTT |
55 | 204 |
| hBD-3 | FP: AGCCTAGCAGCTATGAGGATC RP: CTTCGGCAGCATTTTCGGCCA |
61 | 205 |
All experiments were performed in triplicate. Summary of data was reported as-as mean ± standard deviation (SD). For statistical analysis, SPSS version 12.0 was used. To compare the different groups, the statistical Student’s t-test was used, due to the small sample size, considering a significance level at p < 0.05.
Results
The viscosities of the solutions were measured by Brookfield Model DV-III viscometer. The viscosity of the PVA solution was 557 centipoise, and that of the PVA/chitosan solution (with the weight ratio of 90/10) was 1726 centipoise. This is in line with the results by Paipitak et al., [39], who reported a linear increase in the viscosity of the PVA solution after blending with increasing amounts of chitosan. The high viscosity increases the interaction of two polymers, mainly through hydrogen bonding, and decreases the effects of surface tension. This will result in the formation of fibres with uniform morphology [40].
The optical clarity of the scaffold was done with the wavelength in the visible range of 400-800 nm. Our results showed that PVA/chitosan scaffold was found to be highly transparent with 88% of optical transparency compared with standard cornea as a positive control that showed a range of 72-82% of transparency, whereas, the human amniotic membrane (HAM) has an optical transmission of 78% only.
FT-IR spectroscopy was used to assess the chemical groups of the polymers. Figure 1a and 1b show the FT-IR spectra of PVA/chitosan and HAM. Typical FT-IR spectra of PVA/chitosan blended films having various absorption bands compared to IR spectra of denuded HAM. The specific intensity of absorption bands of chitosan/PVA blend and HAM are indicating the similar presence of protein. In Figure 1a, for the chitosan sample, the major characteristic peaks around 611 and 1152 cm−1 related to the saccharide structure (as the repeating unit of chitosan) are observable [41] [42]. Also, the strong absorption peaks at 1653, 1553 and 1346 cm−1 are shown, which are characteristic of chitosan and have been reported as amide I, II, and III peaks, respectively. The sharp peaks at 1335 and 1452 cm−1 could be assigned to the CH3 symmetrical deformation mode. Also, the broad peaks at 1079 and 1152 cm−1 indicate the C–O stretching vibration in chitosan, and another broad peak at 3333.60 cm−1 is caused by amine N–H symmetrical vibration. The peak observed at around 2936 cm−1 is due to the typical C–H stretch vibrations [43]. Besides the above, all major peaks related to hydroxyl and acetate groups are shown in the FT-IR spectrum of PVA. More specifically, the broadband observed at 3753.62 cm−1 is associated with the O–H stretch from the intermolecular and intramolecular hydrogen bonds.
Figure 1.
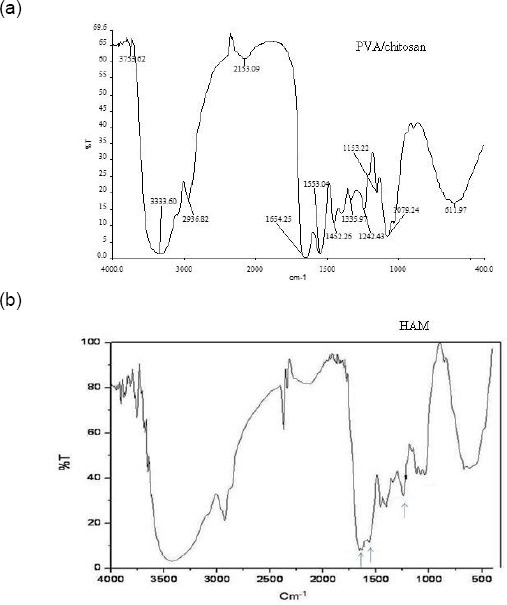
FTIR spectra of the PVA/chitosan cross-linked (a) and HAM scaffolds (b)
The vibrational band observed between 2936.82 and 2153.09 cm−1 is the result of the C–H stretch from alkyl groups and the peaks between 1653 and 11553 cm−1 are due to the C=O and C–O stretches from the remaining acetate groups in PVA (saponification reaction of polyvinyl acetate) [44] [45] [46]. These observations indicate the existence of good miscibility between chitosan and PVA and this is most likely due to the formation of intermolecular hydrogen bonds between the amino and hydroxyl groups in chitosan and the hydroxyl groups in PVA.
Figure 1b shows the FT-IR spectra of HAM. The absorption band around 1600–1640 cm−1 corresponds to amide-I protein absorption band and is mainly attributed to C=O stretching mode, and the other absorption band around 1510–1560 cm−1 corresponds to amide-II protein absorption band which attributed to N–H bending mode and C–N stretching mode [47]. The peaks at around 1210–1300 and 1070–1080 cm−1 attributed to protein (amide III) and also to the phosphodiester group of nucleic acids, glyco- and phospholipids. The amide III bands resulted from an in-phase combination of C–N stretching and N–H in-plane bending, with some contribution from C–C stretching and C=O bending vibrations [48]. Compared with FT-IR spectra of HAM, the peak intensity of the PVA/chitosan blend related to the amide groups tends to decrease, suggesting the formation of intermolecular hydrogen bonds between the polymer chains [23]. Such a result may explain the high stability of the cross-linked PVA with chitosan, during at least 1-2 weeks of immersion.
Fluid uptake is an important parameter, which influences the chemical and physical characteristics of the scaffolds after and before cell seeding. Herein, swelling experiments were performed after cross-linking of PVA and PVA/chitosan and immersed in phosphate buffered saline (PBS) for a defined period, taken out and gently pressed in between the filter papers and weighed. A representative result of fluid uptake behaviour is shown in Figure 2 for PVA and PVA/Chitosan cross-linked scaffolds. Our results revealed that chitosan strongly influences the swelling volume of the scaffold and increases it from 440% to 1590% over the period (1-24 h). The increased swelling volume could be attributed to a more flexible or relaxed network formed by the inter- and intra-polymer reactions and also to the more of hydrophilic groups in PVA/Chitosan blend. The results may be attributed to the fact that chitosan is a cationic biopolymer, and its content in the scaffold results in loosening of the network chains due to existing repulsion between the cationic chains of chitosan. This observation is in agreement with previous studies which reported that chitosan increases the swelling rate when blended with PVA; however the degree of swelling rate increase or reduction depends on factors such as weight ratio of the components, pH, temperature, and so on [35] [43] [44] [45] [46].
Figure 2.
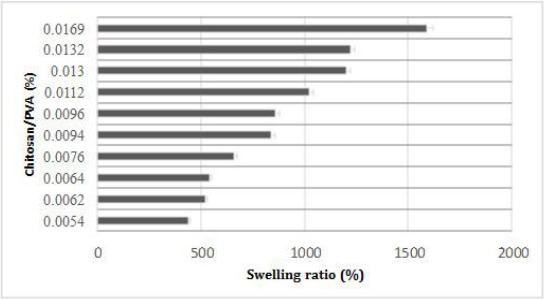
Swelling as a function of PVA and chitosan scaffold at various composition percentages (%) over the period (1-24 h). Graphical data are presented as mean (n = 3) ± standard deviation of three independent experiments
Degradation is the process through which useful physicochemical properties of the polymers are lost. This can include loss of polymer mass through mechanisms such as solvation and depolymerisation. Degradation behaviour of PVA and PVA/Chitosan scaffold using the PBS immersion method was shown in Figure 3. It was observed that the degradation rate of PVA/Chitosan scaffold was much slower than of PVA samples. PVA/Chitosan scaffolds started to degrade from 6th day onwards, and this slow degradation was continued until day 16. This could be due to the higher density of chemical cross-linking between cross-linkers and amine groups of chitosan and leads to slower depolymerisation [49] [50].
Figure 3.
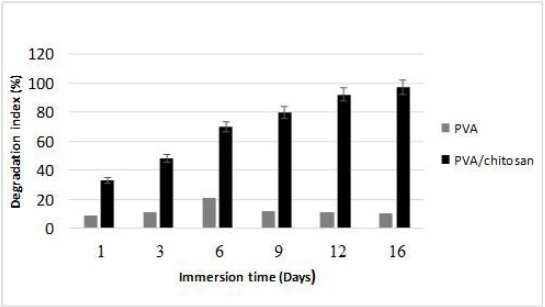
Measurement rate of breakdown of PVA/chitosan scaffolds. Dry weight ratio or degradation behaviour (%) of PVA and chitosan/PVA hydrogels was measured using PBS at different time points. Graphical data are presented as mean (n = 3) ± standard deviation of three independent experiments
The mechanical properties (Young’s modulus, tensile strength and elongation at break percentage (%) of PVA/Chitosan blend scaffold was investigated in dry and wet states, and the observation was shown in Table 2. The mechanical properties of a scaffold used for tissue engineering are very important due to the need for the structural stability to oppose the various stresses incurred during culture in vitro or implantation in vivo while the surgeon is handling the membrane.
Table 2.
Mechanical properties of PVA/Chitosan blend
| S. No | Scaffold | Tensile strength MPa | Elongation at Break (%) | Tearing maximum strength load (N) |
|---|---|---|---|---|
| 1. | PVA/Chitosan | 4.38 ± 0.09 | 20.58 ± 0.36 | 2.87 ± 0.02 |
| 2. | Positive control HAM | 1.68 ± 0.08 | 10.09 ± 0.8 | Nil |
The cytotoxicity of the pure PVA, Chitosan, PVA/chitosan blend scaffolds have been evaluated by MTT assay. This assay is based on the conversion of MTT to blue formazan by mitochondria in living cells. The amount of formazan formed indicates the level of cell metabolism. However, it does not accurately represent the number of living cells.
Each experiment was repeated three times, and a low standard deviation of assay results was found. The optical density of formazan at 570 nm was measured for 24h, 48h and 72 h of incubation, respectively. The MTT assay indicated that both NIH3T3 and the cultured human corneal epithelial cells (HCECs) viability, was highest from days 3 to 5, and was not affected by the concentration of PVA used to prepare membranes. The viability of NIH 3T3 (90%) and HCECs cultured on PVA/chitosan (91%) was higher compared with either NIH3T3 or HCECs cultured on PVA alone (78% and 80%). However, it was less when compared with non-treated control cells (98%). H & E stained cells further confirmed the cell viability (Figure 4).
Figure 4.
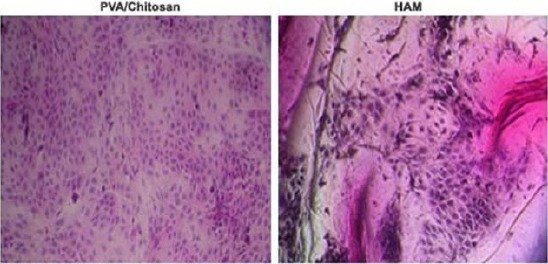
H&E staining of cultured HCEC in HAM and PVA/Chitosan scaffolds
Therefore, these results clearly showed that the PVA/Chitosan blend scaffolds are not deleterious for cell activity and may be safe for their use as a delivery substrate, wound dressing or soft tissue repair [51] [52] [53].
Besides the above, the present study have also investigated the expression of different molecular markers for corneal epithelial stem cells (ABCG2, connexin43 and cytokeratin 3) (Figure 5, a, b, and c) and antimicrobial peptides (AMP) such as, hBD 1, 2, 3 and LL37 in cultured corneal epithelial cells (Figure 6, a, b, c, and d). Our results show that culture corneal epithelial cells were expressed hBD 1, 2, 3 and LL37 and the stem cell markers confirming the corneal epithelial nature of the cells.
Figure 5.
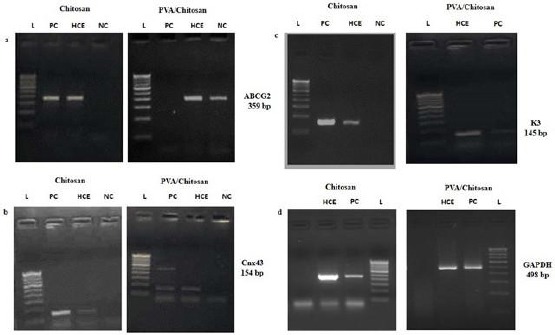
Semi-quantitative RT-PCR for SC-associated markers ABCG2 (379 bp), differentiation-associated markers, K3 (145 bp), and connexin 43 (154 bp) expressed by corneal epithelial cells (a, b, and c); A 100 bp DNA ladder is shown in the first left lane. GAPDH, a housekeeping gene, was used as an internal control (d)
Figure 6.
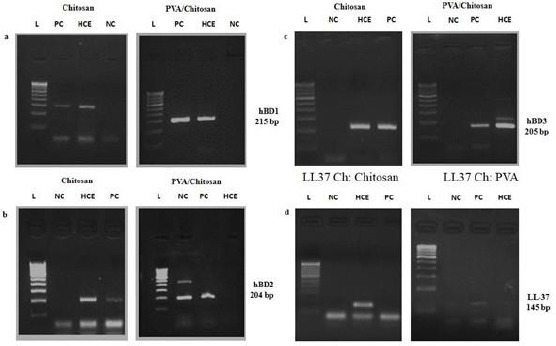
PCR amplification for AMPs expression by human corneal epithelial cells. Semi-quantitative RT-PCR for AMP-associated markers hBD1 (215 bp), hBD2 (204 bp) and hBD3 (205 bp) expressed by corneal epithelial cells (a, b, and c); A 100 bp DNA ladder is shown in the first left lane. GAPDH, a housekeeping gene, was used as an internal control (d)
Discussion
Tissue engineering in the cornea has often maintained the use of a carrier system for delivery of corneal stromal stem cells and corneal endothelial cell progenitors [54] [55]. Natural materials, such as collagen, silk and gelatin, which have excellent biocompatibility, biodegradability and low immunogenicity, have been extensively utilised for corneal tissue engineering [18] [19]. Although, scaffolds are preferentially a biodegradable one while providing a favourable microenvironment for cell adherence and proliferation but also expected to degrade gradually, allowing surrounding tissues to replace and sustain the scaffold function [56] [57]. In this regard, cross-linking reagents commonly used to modify scaffolds for enhancing both physical and chemical stability. For instance, in recent years, fibrin [58], human amniotic membrane (HAM) [59], and cellular feeder layers such as 3T3 fibroblasts [58] [60] have facilitated the expansion of corneal epithelial cells. However, each of these agents has their own merits and some drawbacks.
In this study, we have modified polyvinyl alcohol (PVA) fibres by blending with chitosan to fabricate a Nanofibrous scaffold by cross-linking with EDC and NHS. The PVA/chitosan scaffolds have been found to exhibit physicochemical and biological properties, which we compared with HAM scaffolds to better meet the requirements of cultured corneal epithelial cells. The FT-IR spectra provide information about the functional groups of constituent polymers present in the scaffold. The IR spectra shown in Figure 1a indicates the presence of poly (vinyl) alcohol and chitosan, as evident from the peaks observed. It is clear from the IR results on the peaks that constituent polymers PVA and chitosan are present in the cross-linked scaffold. Whereas in the spectrum of denuded HAM (Figure 1b), nine characteristic absorption bands at the frequencies of 3306, 2954, 1651, 1548, 1451, 1394, 1241, 1077, and 645 cm−1 were observed.
The water absorption capacity of scaffold results clearly shows that water intake capacity constantly increases when the wt. Fraction increases from 1 to 4.0 and after that the equilibrium swelling constantly decreases (data not shown). Thus, an optimum swelling is reached at a PVA/chitosan wt. The fraction of 4.0. The results may be explained as follows, since both the constituent polymers, that is PVA and chitosan are hydrophilic, their increasing wt. Fraction results in enhanced hydrophilicity of the matrix, which results in an increased water sorption capacity. However, beyond 4.0 wt. Fraction of PVA, the water sorption capacity falls, which may be explained by the fact that when the PVA content is high, and the resulting scaffold is enriched in crystalline region of PVA, this accounts for lower water sorption tendency of the PVA. A similar observation was observed with chitosan. The results may be attributed to the fact that chitosan is a cationic biopolymer, and its increasing content in the scaffold results in loosening of the network chains due to existing repulsion between the cationic chains of chitosan.
It is well established that chitosan is a potential scaffold for in vitro bovine corneal epithelial cell culture with the ability to preserve the corneal epithelial cell phenotype to maintain biological function to a certain extent [61] [62] [63]. Hence, the cellular behaviour of a biomaterial is an important factor determining the biocompatibility of a biomaterial [64]. After cells contact biomaterials, cells will undergo their morphological changes to stabilise the cell and material interface. In our study, we monitored cell viability on PVA cross-linked chitosan using an MTT assay and observed cell morphology periodically to assess any differences in cell morphology. No obvious difference noticed with HCEC and NIH3T3 morphology in cultured cells using light microscopy. Our result implies that HCEC could favourably attach and proliferate on the PVA/chitosan surface, and cells were able to infiltrate the scaffolds and successfully form a 3D corneal epithelium [65] with appropriate pre-clinical and clinical experimentation in future.
Also, the cultured epithelium displayed a phenotype similar to human corneal epithelium as stem cells have certain unique characteristics, which include longevity, high capacity of self-renewal with a long cell cycle time and a short S-phase duration, increased the potential for error-free proliferation, and poor differentiation [66]. Semi-quantitative reverse transcriptase polymerase chain reaction (RT-PCR) was done on the cultured cells at varying intervals of time for expression of ABCG2, connexin43 (Cnx43), and keratin 3 (K3). The cells cultured over PVA/chitosan were able to maintain the expression of putative stem cell markers ABCG2, Cnx43 and K3.
Previous studies have shown that the connexins are gap junction proteins involved in cell-cell communication, and are important cell differentiating factors. To date, Cx-43 and Cx-50 are the only two gap junction proteins that have been identified in the corneal epithelium. Cx-43 is abundant on the basal corneal epithelium but is absent from limbal stem cells; thus, Cx-43 is proposed as a negative corneal stem cell marker [67]. Therefore, according to the phenotype of the HCECs cultured on the PVA/chitosan, they were HCE, but not limbal stem cells.
Antimicrobial peptides (AMPs) form an integral part of the innate immune system and provide defence against a range of pathogens as well as modulating immune responses [68]. This help provides a baseline defence against invading pathogens, and several are up-regulated in response to infection and inflammatory stimuli [69] [70] [71] and play a critical role as a microbial barrier [72] (Alison et al., 2004). The human β-defensins (hBD) and the cathelicidin LL37 [73] are peptides expressed by epithelia throughout the body including epithelia of the oral cavity. There are now 28 known β-defensin genes found in human. However; expression of hBD1, 2, and 3 have been most investigated [74]. Despite the constant threat from pathogenic microbes in the air and foreign objects around in the laboratory, the incidence of infection in the culture condition is expected amazingly low [75]. However, in spite of the presence of antibiotics in the culture medium or having an intact sterile surface condition, our results found the expression of all three defensin group AMPs in oral epithelial cells. This model can serve as a useful basic tool for the study of tissue innate immune responses as a purely epithelial model.
In summary, we modified chitosan by cross-linking its polymers with the naturally occurring cross-linker PVA in a safer and faster way and characterised the phenotypes of HCECs cultured on PVA/chitosan. We demonstrated that the improved development of PVA/chitosan showed good biocompatibility for cell adhesion, expansion, and proliferation. Besides the above, this polymer scaffold will be promising scaffold alternative to AM for clinical use in the future for the transplantation of cultivated limbal stem cells onto the ocular surface with successful clinical trial and experimentation. Therefore, future applications of safe and rapid development of PVA/chitosan membranes can be considered for reconstruction of the cornea and other tissue engineering applications. About biocompatibility, although PVA/Chitosan scaffold produced no or low toxic tissue response, it is yet to be determined whether they produce any inflammatory response, as they are clinically significant. Therefore, further studies are necessary to investigate to rule out the possibility of any possible concerns in their use.
Acknowledgements
The authors are thankful to Deanship for Graduate Studies and Academic Research, University of Tabuk (UT), Kingdom of Saudi Arabia supported to buy some chemicals and reagents for this research (0121-1438-S).
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJC, Richter E, Sakurai E, Newcomb MT. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–97. doi: 10.1038/nature05249. https://doi.org/10.1038/nature05249 PMid:17051153 PMCid:PMC2656128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Universal Eye Health. A Global Action Plan 2014-2019. Geneva: WHO; 2013. [Google Scholar]
- 3.VISION 2020. The Right to Sight. [[Last cited on 2017 Jan 19]]. Available from: http://www.iapb.org/vision-2020/
- 4.McLaughlin CR, Tsai RJF, Latorre MA, Griffith M. Bioengineered corneas for transplantation and in vitro toxicology. Front Biosci. 2009;14:3326–37. doi: 10.2741/3455. https://doi.org/10.2741/3455. [DOI] [PubMed] [Google Scholar]
- 5.Ruberti JW, Zieske JD. Prelude to corneal tissue engineering-Gaining control of collagen organisation. Prog Retin Eye Res. 2008;27:549–77. doi: 10.1016/j.preteyeres.2008.08.001. https://doi.org/10.1016/j.preteyeres.2008.08.001 PMid:18775789 PMCid:PMC3712123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niethammer D, Kümmerle-Deschner J, Dannecker GE. Side-effects of long-term immunosuppression versus morbidity in autologous stem cell rescue:striking a balance. Rheumatology (Oxford) 1999;38:747–50. doi: 10.1093/rheumatology/38.8.747. https://doi.org/10.1093/rheumatology/38.8.747. [DOI] [PubMed] [Google Scholar]
- 7.Wilson SL, Wimpenny I, Ahearne M, Rauz S, El Haj AJ, Yang Y. Chemical and Topographical Effects on Cell Differentiation and Matrix Elasticity in a Corneal Stromal Layer Model. Adv Funct Mater. 2012;22:3641–49. https://doi.org/10.1002/adfm.201200655. [Google Scholar]
- 8.Ghezzi CE, Rnjak-Kovacina J, Kaplan DL. Corneal tissue engineering:recent advances and future perspectives. Tissue Eng Part B Rev. 2015;21:278–87. doi: 10.1089/ten.teb.2014.0397. https://doi.org/10.1089/ten.teb.2014.0397 PMid:25434371 PMCid:PMC4442593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Z, You J, Liu X, Cooper S, Hodge C, Sutton G, Crook JM, Wallace GG. Biomaterials for corneal bioengineering. Biomedical Materials. 2018;13(3):032002. doi: 10.1088/1748-605X/aa92d2. https://doi.org/10.1088/1748-605X/aa92d2 PMid:29021411. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–93. doi: 10.1016/S0140-6736(96)11188-0. https://doi.org/10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura T, Sotozono C, Bentley AJ, Mano S, Inatomi T, Koizumi N, Fullwood NJ, Kinoshita S. Long-Term Phenotypic Study after Allogeneic Cultivated Corneal Limbal Epithelial Transplantation for Severe Ocular Surface Diseases. Ophthalmol. 2010;117:2247–54. doi: 10.1016/j.ophtha.2010.04.003. https://doi.org/10.1016/j.ophtha.2010.04.003 PMid:20673588. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi N, Fullwood NJ, Bairaktaris G, Inatomi T, Kinoshita S, Quantock AJ. Cultivation of Corneal Epithelial Cells on Intact and Denuded Human Amniotic Membrane. Invest Ophthalmol Visual Sci. 2000;41:2506–2513. PMid:10937561. [PubMed] [Google Scholar]
- 13.Kreft ME, Dragin U. Amniotic membrane in tissue engineering and regenerative medicine. Zdrav Vestn. 2010;79:707–15. [Google Scholar]
- 14.Liu C, Xia Z, Czernuszka J. Design and development of three-dimensional scaffolds for tissue engineering. Chem Engineer Res and Des. 2007;85:1051–64. https://doi.org/10.1205/cherd06196. [Google Scholar]
- 15.Agrawal C, Ray RB. Biodegradable polymeric scaffolds for musculoskeletal tissue engineering. J Biomed Mat Res. 2001;55:141–50. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. https://doi.org/10.1002/1097-4636(200105)55:2<141::AID-JBM1000>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 16.Shrivats R, McDermott MC, Hollinger JO. Bone tissue engineering:state of the union. Drug discovery today. 2014;19:781–86. doi: 10.1016/j.drudis.2014.04.010. https://doi.org/10.1016/j.drudis.2014.04.010 PMid:24768619. [DOI] [PubMed] [Google Scholar]
- 17.Kang KB, Lawrence BD, Gao XR, Luo Y, Zhou Q, Liu A, Guaiquil VH, Rosenblatt MI. Micro- and Nanoscale Topographies on Silk Regulate Gene Expression of Human Corneal Epithelial Cells. Invest Ophthalmol Vis Sci. 2017;58:6388–98. doi: 10.1167/iovs.17-22213. https://doi.org/10.1167/iovs.17-22213 PMid:29260198 PMCid:PMC5736325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chong EJ, Phan TT, Lim IJ, Zhang YZ, Bay BH, Ramakrishna S, Lim CT. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007;3:321–30. doi: 10.1016/j.actbio.2007.01.002. https://doi.org/10.1016/j.actbio.2007.01.002 PMid:17321811. [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez MC, García-Carvajal ZY, Jobbágy M, Rubio F, Yuste L, Rojo F, Ferrer ML, Del Monte F. Poly (vinyl alcohol) scaffolds with tailored morphologies for drug delivery and controlled release. Adv Funct Mater. 2007;17:3505–13. https://doi.org/10.1002/adfm.200700093. [Google Scholar]
- 20.Keane TJ, Badylak SF. Biomaterials for tissue engineering applications. Semin Pediatr Surg. 2014;23:112–18. doi: 10.1053/j.sempedsurg.2014.06.010. https://doi.org/10.1053/j.sempedsurg.2014.06.010 PMid:24994524. [DOI] [PubMed] [Google Scholar]
- 21.Kong B, Mi S. Electrospun Scaffolds for Corneal Tissue Engineering:A Review. Materials. 2016;9:614. doi: 10.3390/ma9080614. https://doi.org/10.3390/ma9080614 PMid:28773745 PMCid:PMC5509008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang Q, Luo C, Lu B, Fu Q, Yin H, Qin Z, Lyu D, Zhang L, Fang Z, Zhu Y, Yao K. Thermosensitive chitosan-based hydrogels releasing stromal cell derived factor-1 alpha recruit MSC for corneal epithelium regeneration. Acta Biomater. 2017;61:101–13. doi: 10.1016/j.actbio.2017.08.001. https://doi.org/10.1016/j.actbio.2017.08.001 PMid:28780431. [DOI] [PubMed] [Google Scholar]
- 23.Bonilla J, Fortunati E, Atarés L, Chiralt A, Kenny JM. Physical, structural and antimicrobial properties of poly vinyl alcohol-chitosan biodegradable films. Food Hydrocoll. 2014;35:463–70. https://doi.org/10.1016/j.foodhyd.2013.07.002. [Google Scholar]
- 24.Rinaudo M. Chitin and chitosan:properties and applications. Prog Polym Sci. 2006;31:603–32. https://doi.org/10.1016/j.progpolymsci.2006.06.001. [Google Scholar]
- 25.Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS. Chitosan and its derivatives for tissue engineering applications. Biotechnol Adv. 2008;26:1–21. doi: 10.1016/j.biotechadv.2007.07.009. https://doi.org/10.1016/j.biotechadv.2007.07.009 PMid:17884325. [DOI] [PubMed] [Google Scholar]
- 26.Lin T, Fang J, Wang H, Cheng T, Wang X. Using chitosan as a thickener for electrospinning dilute PVA solutions to improve fibre uniformity. Nanotechnol. 2006;17:3718–23. https://doi.org/10.1088/0957-4484/17/15/017. [Google Scholar]
- 27.Potten CS, Loeffler M. Stem cells attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Develop. 1990;110:1001–20. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 28.Dąbrowska AM, Skopiński P. Stem cells in regenerative medicine - from laboratory to clinical application - the eye. Cent Eur J Immunol. 2017;42:173–80. doi: 10.5114/ceji.2017.69360. https://doi.org/10.5114/ceji.2017.69360 PMid:28860936 PMCid:PMC5573891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun TT, Tseng SC, Lavker RM. Location of corneal epithelial stem cells. Nature. 2010;463:10–11. doi: 10.1038/nature08805. https://doi.org/10.1038/nature08805 PMid:20182462. [DOI] [PubMed] [Google Scholar]
- 30.Perry KJ, Thomas AG, Henry JJ. Expression of pluripotency factors in larval epithelia of the frog Xenopus:evidence for the presence of cornea epithelial stem cells. Dev Biol. 2013;374:281–94. doi: 10.1016/j.ydbio.2012.12.005. https://doi.org/10.1016/j.ydbio.2012.12.005 PMid:23274420 PMCid:PMC3558918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad S, Figueiredo F, Lako M. Corneal epithelial stem cells:characterization, culture and transplantation. Regen Med. 2006;1:29–44. doi: 10.2217/17460751.1.1.29. https://doi.org/10.2217/17460751.1.1.29 PMid:17465818. [DOI] [PubMed] [Google Scholar]
- 32.Dua HS, Saini JS, Azuara-Blanco A, Gupta P. Limbal stem cell deficiency:concept, aetiology, clinical presentation, diagnosis and management. Indian J Ophthalmol. 2006;48:83–92. [PubMed] [Google Scholar]
- 33.Nakamura T1, Inatomi T, Sotozono C, Koizumi N, Kinoshita S. Successful primary culture and autologous transplantation of corneal limbal epithelial cells from minimal biopsy for unilateral severe ocular surface disease. Acta Ophthalmol Scand. 2004;82:468–71. doi: 10.1111/j.1395-3907.2004.00285.x. https://doi.org/10.1111/j.1395-3907.2004.00285.x PMid:15291944. [DOI] [PubMed] [Google Scholar]
- 34.Wang HY, Wei RH, Zhao SZ. Evaluation of corneal cell growth on tissue engineering materials as artificial cornea scaffolds. Int J Ophthalmol. 2013;6:873–78. doi: 10.3980/j.issn.2222-3959.2013.06.23. PMid:24392340 PMCid:PMC3874531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Souza Costa-Júnior E, Pereira MM, Mansur HS. Properties and biocompatibility of chitosan films modified by blending with PVA and chemically crosslinked. J Mater Sci Mater Med. 2009;20:553–61. doi: 10.1007/s10856-008-3627-7. https://doi.org/10.1007/s10856-008-3627-7 PMid:18987949. [DOI] [PubMed] [Google Scholar]
- 36.Zonari E, Giacomo D, Carolina P, Boccalatte FE, Lidonnici MR, Anna KR, Alessandro A, Giuliana F, Luigi N, Bernhard G. Efficient Ex Vivo Engineering and Expansion of Highly Purified Human Hematopoietic Stem and Progenitor Cell Populations for Gene Therapy. Stem Cell Reports. 2017;8:977–90. doi: 10.1016/j.stemcr.2017.02.010. https://doi.org/10.1016/j.stemcr.2017.02.010 PMid:28330619 PMCid:PMC5390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li DQ, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001;73:449–59. doi: 10.1006/exer.2001.1054. https://doi.org/10.1006/exer.2001.1054 PMid:11825017. [DOI] [PubMed] [Google Scholar]
- 38.Sasirekha K, Geetha K, Krishnakumar S. Culture and characterisation of limbal epithelial cells and oral mucosal cells. Indian J Med Res. 2010;131:422–28. [PubMed] [Google Scholar]
- 39.Paipitak K, Pornpra T, Mongkontalang P, Techitdheer W, Pecharapa W. Characterization of PVA-chitosan nanofibers prepared by electrospinning. Procedia Eng. 2011;8:101–05. https://doi.org/10.1016/j.proeng.2011.03.019. [Google Scholar]
- 40.Bhattarai N, Edmondson D, Veiseh O, Matsen FA, Zhang M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials. 2005;26:6176–84. doi: 10.1016/j.biomaterials.2005.03.027. https://doi.org/10.1016/j.biomaterials.2005.03.027 PMid:15885770. [DOI] [PubMed] [Google Scholar]
- 41.Mansur HS, Costa HS. Nanostructured poly(vinyl alcohol)/bioactive glass and poly(vinyl alcohol)/chitosan/bioactive glass hybrid scaffolds for biomedical applications. Chem Eng J. 2008;137:72–83. https://doi.org/10.1016/j.cej.2007.09.036. [Google Scholar]
- 42.Costa EDS, Jr, Mansur HS. Preparation and characterization of chitosan/poly(vinylalcohol)blend chemically crosslinked by glutaraldehyde for tissue engineering application. Química Nova. 2008;31:1460–66. [Google Scholar]
- 43.Don TM, King CF, Chiu WY, Peng CA. Preparation and characterization of chitosan-g-poly(vinyl alcohol)/poly(vinyl alcohol) blends used for the evaluation of blood-contacting compatibility. Carb Polym. 2006;63:331–39. https://doi.org/10.1016/j.carbpol.2005.08.023. [Google Scholar]
- 44.Shigemasa Y, Matsuura H, Sashiwa H, Saimoto H. Evaluation of different absorbance ratios from infrared spectroscopy for analyzing the degree of deacetylation in chitin. Int J Bio Macromol. 1996;18:237–42. doi: 10.1016/0141-8130(95)01079-3. https://doi.org/10.1016/0141-8130(95)01079-3. [DOI] [PubMed] [Google Scholar]
- 45.Mansur HS, Sadahira CM, Souza AN, Mansur AAP. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater Sci Eng C. 2008;28:539–48. https://doi.org/10.1016/j.msec.2007.10.088. [Google Scholar]
- 46.Wang C, Shen M, Zhang N, Wang S, Xu Y, Chen S, Chen F, Yang K, He T, Wang A, Su Y, Cheng T, Zhao J, Wang J. Reduction Impairs the Antibacterial Activity but Benefits the LPS Neutralization Ability of Human Enteric Defensin 5. Sci Rep. 2016;6:22875–82. doi: 10.1038/srep22875. https://doi.org/10.1038/srep22875 PMid:26960718 PMCid:PMC4785407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Socrates G. Infrared and Raman characteristic group frequencies:tables and charts. John Wiley & Sons; 2004. PMid:15340682. [Google Scholar]
- 48.Grdadolnik J. Conformation of bovine serum albumin as a function of hydration monitored by infrared Spectroscopy. Int J Vibr Spec. 2002;6:1–5. [Google Scholar]
- 49.Suh JK, Matthew HW. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering:a review. Biomaterials. 2000;21:2589–98. doi: 10.1016/s0142-9612(00)00126-5. https://doi.org/10.1016/S0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 50.Berger J, Reist M, Mayer JM, Felt O, Peppas NA, Gurny R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 2004;57:19–34. doi: 10.1016/s0939-6411(03)00161-9. https://doi.org/10.1016/S0939-6411(03)00161-9. [DOI] [PubMed] [Google Scholar]
- 51.Duan B, Yuan X, Zhu Y, Zhang Y, Li X, Zhang Y, Yao K. A nanofibrous composite membrane of PLGA-chitosan/PVA prepared by electrospinning. Eur Polym J. 2006;42:2013–22. https://doi.org/10.1016/j.eurpolymj.2006.04.021. [Google Scholar]
- 52.Huang HL, Yao ZH, Yang ZH, Wang Y, Shi DA, Yin JH. Preparation and characterization of chitosan/poly(vinyl alcohol) blend fibers. J Appl Poly Sci. 2001;80:2558–65. https://doi.org/10.1002/app.1365. [Google Scholar]
- 53.Mansur HS, de E, Mansur AAP, Barbosa-Stancioli EF. Cytocompatibility evaluation in cell-culture systems of chemically cross-linked chitosan/PVA hydrogels. Mater Sci Eng C. 2009;29:1574–83. https://doi.org/10.1016/j.msec.2008.12.012. [Google Scholar]
- 54.Yuan S, Fan G. Stem cell-based therapy of corneal epithelial and endothelial diseases. Regen Med. 2015;10:495–04. doi: 10.2217/rme.15.3. https://doi.org/10.2217/rme.15.3 PMid:26022766. [DOI] [PubMed] [Google Scholar]
- 55.Kim KH, Mian S. Diagnosis of corneal limbal stem cell deficiency. Curr Opin Ophthalmol. 2017;28:355–62. doi: 10.1097/ICU.0000000000000387. https://doi.org/10.1097/ICU.0000000000000387 PMid:28426441. [DOI] [PubMed] [Google Scholar]
- 56.Deshpande P, Notara M, Bullett N, Daniels JT, Haddow DB, MacNeil S. Development of a surface-modified contact lens for the transfer of cultured limbal epithelial cells to the cornea for ocular surface diseases. Tissue Eng Part A. 2009;15:2889–02. doi: 10.1089/ten.tea.2008.0528. https://doi.org/10.1089/ten.tea.2008.0528 PMid:19265461. [DOI] [PubMed] [Google Scholar]
- 57.Levis HJ, Brown RA, Daniels JT. Plastic compressed collagen as a biomimetic substrate for human limbal epithelial cell culture. Biomaterials. 2010;31:7726–37. doi: 10.1016/j.biomaterials.2010.07.012. https://doi.org/10.1016/j.biomaterials.2010.07.012 PMid:20674002. [DOI] [PubMed] [Google Scholar]
- 58.Rama P, Bonini S, Lambiase A, Golisano O, Paterna P, De Luca M, Pellegrini Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72:1478–85. doi: 10.1097/00007890-200111150-00002. https://doi.org/10.1097/00007890-200111150-00002 PMid:11707733. [DOI] [PubMed] [Google Scholar]
- 59.Sabater AL, Perez VL. Amniotic membrane use for management of corneal limbal stem cell deficiency. Curr Opin Ophthalmol. 2017;28:363–69. doi: 10.1097/ICU.0000000000000386. https://doi.org/10.1097/ICU.0000000000000386 PMid:28426442. [DOI] [PubMed] [Google Scholar]
- 60.Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002;295:1009–14. doi: 10.1126/science.1069210. https://doi.org/10.1126/science.1069210 PMid:11834815. [DOI] [PubMed] [Google Scholar]
- 61.Yeh LK, Chen YH, Chiu CS, Hu FS, Young TH, Wang IJ. The phenotype of bovine corneal epithelial cells on chitosan membrane. J Biomed Mater Res A. 2009;90:18–26. doi: 10.1002/jbm.a.32077. https://doi.org/10.1002/jbm.a.32077 PMid:18481792. [DOI] [PubMed] [Google Scholar]
- 62.Vázquez N, Chacón M, Meana Á, Menéndez-Menéndez Y, Ferrero-Gutierrez A, Cereijo-Martín D, Naveiras M, Merayo-Lloves J. Keratin-chitosan membranes as scaffold for tissue engineering of human cornea. Histol Histopathol. 2015;30:813–21. doi: 10.14670/HH-11-585. PMid:25587895. [DOI] [PubMed] [Google Scholar]
- 63.Li YH, Cheng CY, Wang NK, Tan HY, Tsai YJ, Hsiao CH, Ma DH, Yeh LK. Characterization of the modified chitosan membrane cross-linked with genipin for the cultured corneal epithelial cells. Colloids Surf B Biointerfaces. 2015;126:237–44. doi: 10.1016/j.colsurfb.2014.12.029. https://doi.org/10.1016/j.colsurfb.2014.12.029 PMid:25576808. [DOI] [PubMed] [Google Scholar]
- 64.Lin T, Fang J, Wang H, Cheng T, Wang X. Using chitosan as a thickener for electrospinning dilute PVA solutions to improve fibre uniformity. Nanotechnol. 2006;17:3718–23. https://doi.org/10.1088/0957-4484/17/15/017. [Google Scholar]
- 65.Biazar E, Baradaran-Rafii A, Heidari-keshel S, Tavakolifard S. Oriented nanofibrous silk as a natural scaffold for ocular epithelial regeneration. J Biomat Sci Polym E. 2015;26:1139–51. doi: 10.1080/09205063.2015.1078930. https://doi.org/10.1080/09205063.2015.1078930 PMid:26324020. [DOI] [PubMed] [Google Scholar]
- 66.Hong H, Yiu SC. Stem cell-based therapy for treating limbal stem cells deficiency:A review of different strategies. Saudi J Ophthalmol. 2014;28:188–94. doi: 10.1016/j.sjopt.2014.06.003. https://doi.org/10.1016/j.sjopt.2014.06.003 PMid:25278795 PMCid:PMC4181755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Z, Evans WH, Pflugfelder SC, Li DQ. Gap junction protein connexin 43 serves as a negative marker for a stem cell-containing population of human limbal epithelial cells. Stem cells. 2006;24:1265–73. doi: 10.1634/stemcells.2005-0363. https://doi.org/10.1634/stemcells.2005-0363 PMid:16424398 PMCid:PMC2906383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.da Costa JP, Cova M, Ferreira R, Vitorino R. Antimicrobial peptides:an alternative for innovative medicines? Appl Microbiol Biotechnol. 2015;99:2023–40. doi: 10.1007/s00253-015-6375-x. https://doi.org/10.1007/s00253-015-6375-x PMid:25586583. [DOI] [PubMed] [Google Scholar]
- 69.McDermott AM, Redfern RL, Zhang B, Pei Y, Huang L, Proske RJ. Defensin expression by the cornea:multiple signalling pathways mediate IL-1 beta stimulation of hBD-2 expression by human corneal epithelial cells. Invest Ophth Vis Sci. 2003;44:1859–65. doi: 10.1167/iovs.02-0787. https://doi.org/10.1167/iovs.02-0787 PMCid:PMC1497872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Narayanan S, Manning J, Proske R, McDermott AM. Effect of hyperosmolality on beta-defensin gene expression by human corneal epithelial cells. Cornea. 2006;25:1063–68. doi: 10.1097/01.ico.0000228785.84581.35. https://doi.org/10.1097/01.ico.0000228785.84581.35 PMid:17133055 PMCid:PMC2430508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang LC, Proske RJ, McDermott AM. Expression of the peptide antibiotic LL-37/hCAP18 (Cathelicidin) by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:U319–U319. doi: 10.1167/iovs.02-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alison MM. Defensins and other antimicrobial peptides at the ocular surface. Ocul Surf. 2004;2:229–47. doi: 10.1016/s1542-0124(12)70111-8. https://doi.org/10.1016/S1542-0124(12)70111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kolar SS, McDermott AM. Role of host-defense peptides in eye diseases. Cell Mol Life Sci. 2011;68:2201–13. doi: 10.1007/s00018-011-0713-7. https://doi.org/10.1007/s00018-011-0713-7 PMid:21584809 PMCid:PMC3637883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maltsevai A, Fleiszig SM, Evans DJ, Kerr S, Sidhu SS, McNamaran A. Exposure of human corneal epithelial cells to contact lenses in vitro suppresses the upregulation of human β-defensin-2 in response to antigens of Pseudomonas aeruginosa. Exp Eye Res. 2007;85:142–53. doi: 10.1016/j.exer.2007.04.001. https://doi.org/10.1016/j.exer.2007.04.001 PMid:17531223. [DOI] [PubMed] [Google Scholar]
- 75.Jin X, Qin Q, Lin Z, Chen W, Qu J. Expression of toll-like receptors in the Fusarium solani infected cornea. Curr Eye Res. 2008;33:319–24. doi: 10.1080/02713680802008238. https://doi.org/10.1080/02713680802008238 PMid:18398706. [DOI] [PubMed] [Google Scholar]


