Abstract
AIM:
This study was aimed to compare the effects of ondansetron, haloperidol, and dexmedetomidine for reducing postoperative nausea and vomiting (PONV) after laparoscopic cholecystectomy.
METHODS:
This randomised clinical trial study was performed on p.patients who were candidates for abdominal hysterectomy referring to Taleghani hospital in Arak. In this study, 114 patients with abdominal hysterectomy were randomly assigned to three groups (ondansetron, haloperidol, and dexmedetomidine) using the cubull randomisation method.
RESULTS:
The results revealed a significant difference between the three groups of ondansetron, haloperidol and dexmedetomidine in terms of scorpion vomiting in recovery, 2 and 4 hours after surgery, and vomiting score was significantly lower in the ondansetron group compared with the other two groups (P = 0.04; P = 0.02; P = 0.001). There was a significant difference between the three groups of e ondansetron, haloperidol and dexmedetomidine regarding the mean dose of metoclopramide in mg for 24 hours after surgery. Therefore, the dosage of dexmedetomidine in the ondansetron group was less than the other two groups (P = 0.001).
CONCLUSION:
these three drugs are effective in reducing PONV in patients undergoing a hysterectomy. However, the effect of ondansetron was found to be more than the other two drugs in reducing PONV.
Keywords: Dexmedetomidine, Ondansetron, Haloperidol, Nausea, Vomiting, Abdominal hysterectomy
Introduction
Despite the progression of the new and anti-vomiting technique, Postoperative nausea and vomiting (PONV) is still one of the common complications after general anaesthesia and surgery in the immediate 24 postoperative hours, which causes increased unpleasant side effects, prolonged recovery and delayed discharge, as well as increased hospital costs. PONV also leads to loss of appetite, dehydration, and electrolyte imbalance [1] [2]. Vomiting may lead to wound dehiscence and oesophagal rupture, and pneumothorax, etc. The average incidence of PONV is reported to be 53-70% in high-risk patients. Therefore, the prevention of PONV increases patient satisfaction and reduces the cost of treatment. The prevalence of PONV should be monitored effectively, especially in high-risk patients, depending on the female gender, type of surgery, duration of surgery, duration of anaesthesia, and carbon dioxide content, as well as some visitors in Recovery. The chemo trigger zone (CTZ) as a vomiting trigger zone is an area of the medulla oblongata that plays an important role in developing vomiting reflux [2] [3] [4]. Other risk factors for PONV include age over 50, female gender, infection, uremia, motion sickness, migraine, hypercalcemia, anxiety, etc. [3] [4]. Surgeries such as laparoscopic abdominal surgery with peritoneal inflammation, women’s surgery, strabismus, and ear surgery can cause nausea and vomiting [3] [4] [5]. For the prevention of postoperative nausea and vomiting in patients with high-risk to moderate-risk (Scores 1 to 2), prophylactic single-drug administration is recommended, but combining treatment with two or more drugs from different classes of anti-nausea drugs is more effective than single medicine for high-risk patients [3]. It is worth noting that various drugs such as serotonin antagonists, anticholinergics, butyrophenones, Phenothiazines, steroids and histamine H2-receptor antagonists, etc. are used. One of these drugs is endometrin, haloperidol, and doxedetomidine, etc. [3]. Endonestrone is a serotonin receptor antagonist that is very important in preventing nausea and vomiting due to surgery and chemotherapy, and it has an anti-vomiting effect by inhibiting 5HT3 (5-Hydroxytryptamine type 3) receptors in the vomiting centre and chomicosterone [4]. These drugs include ondansetron, haloperidol, and dexmedetomidine, etc. [3]. Ondansetron is a serotonin receptor antagonist, which is very important in preventing nausea and vomiting due to surgery and chemotherapy; it exhibited an anti-vomiting effect by inhibiting 5-Hydroxytryptamine type 3 (5-HT3) receptors in the vomiting centre and the compressor starting area [4]. Haloperidol is a sedative a butyrophenone with an antagonistic effect on dopamine receptor 2, which can cause nausea and vomiting, by inhibiting dopamine receptor 2 in the vomiting centre. Due to its long half-life, its one-time-only injection can provide an appropriate coating within 24 hours [5]. Haloperidol is applied as anti-nausea in relieving nausea and vomiting, as well as in psychiatry and surgery for the management of delusions in people over 40 years of age [6].
Dexmedetomidine is strong, and a highly selective α-2 agonist that attaches to a transmembrane G-protein coupled receptor in the brain and spinal cord, and affects the function of the central nervous system and the central circulatory system to produce its analgesic and sympathetic effects [7]. Dexmedetomidine is potentially used in anaesthesia and intensive care units due to its anxiolytic, analgesic, and sympathetic effects as well as its hemodynamic constants [7] [8]. Regarding available evidence, PONV is one of the main complaints of surgery and anaesthesia and is one of the risk factors for vomiting after abdominal surgery, such as abdominal hysterectomy.
Therefore, the current study was aimed to compare the prophylactic effects of haloperidol, ondansetron and dexmedetomidine in reducing PONV in patients undergoing an abdominal hysterectomy.
Material and Methods
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards, and was approved by Arak University of Medical Sciences Institutional Review Board (Protocol number IR.ARAKMU.REC.2017.111; October 8, 2017).
This randomised clinical trial study was performed on a subset of patients undergoing abdominal hysterectomy referring to Taleghani Hospital of Arak, Iran. In this study, 114 patients with abdominal hysterectomy were randomly divided into three groups (haloperidol, ondansetron and dexmedetomidine) using cubull randomisation. Patients with non-emergency abdominal hysterectomy referred to Taleghani Hospital of Arak were entered into the study after obtaining informed consent. They were entered into the operating room after anesthesiologist’s confirmation. All of the patients referred to complete monitoring including oxygen saturation (SpO2), heart rate (PR), Blood pressure (BP), noninvasive blood pressure (NIBC), and body temperature measurements.
The patients were randomly assigned to 3 groups of 38 patients including the haloperidol group (2 mg IV, 1 cc), ondansetron group (4 mg IV, 1 cc) and dexmedetomidine group (1 µg/kg IV, 1 cc).
The injection of these drugs was performed after the abdomen pulled out of the uterus. Then, all patients received 3-5 cc/kg of crystalloid fluid as Compensatory Volume Expansion (CVE), and patients underwent general anaesthesia. All patients underwent general anesthesia with fentanyl (2 μg/kg), midazolam (0.3-0.5 μg/kg), atracurium (0.5-0.7 μg/kg) and propofol (2-2 mg/kg). Then, the patients were intubated and sub-ventilated. After the endotracheal fixation and hemodynamic stability of the patient, the surgeon was allowed to start surgery.
Patients entered the study after obtaining informed consent, but none of them was aware of the drug received. After the completion of surgery, the patients entered the recovery room, and the questionnaires including questions about the scour of nausea and vomiting, hemodynamic parameters of the patient were completed for the patients. All patients with vomiting scores of 2 and > 5 were treated with metoclopramide. Finally, the obtained data were analyzed by statistical software SPSS 23 and data were presented in the form of statistical tables and charts.
The sample size was determined as reported previously [8]. According to a randomised clinical trial, 114 patients were selected from patients with abdominal hysterectomy.
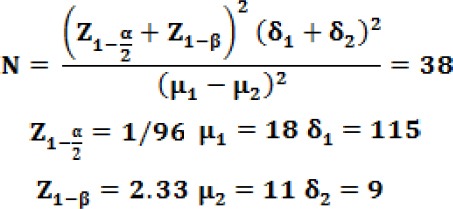
Inclusion criteria
1. All patients suffering from abdominal hysterectomy referred to Taleghani Hospital in Arak who have informed consent. 2. Patients with ASA Class I and II. 3. No history of psychiatric illnesses. 4. No Parkinson’s disease, motion sickness or history of chemotherapy. 5. All patients with general anaesthesia. 6. Patients aged 35-60 years 7. A 150 min maximum duration of surgery
Exclusion criteria
1. ASA ≥ III and IV. 2. Patients outside the age range of 35-60 years. 3. Parkinson’s disease. 4. The presence of patients without informed consent. 5. Psychiatric and psychiatric patients. 6. History of chemotherapy.
Results
Based on data presented in Table 1 and Figure 1, there was no significant difference between the three groups of haloperidol, ondansetron and dexmedetomidine regarding mean age, BMI and mean duration of surgery (P ≥ 0.05). The average age of patients in each group was 46 years, followed by an average BMI (BMI: 30) and an average duration of surgery in three groups (2 hours).
Table 1.
Comparing the mean age, BMI and duration of surgery in patients undergoing an abdominal hysterectomy in the three groups of haloperidol, ondansetron and dexmedetomidine
| Average/group | Ondansetron | Haloperidol | Dexmedetomidine | P-value |
|---|---|---|---|---|
| Mean age | 45/8+/-6/8 | 47/1+/-5/4 | 46/9+/-6/6 | P=0.12 no significant |
| BMI average | 30/9+/-7/6 | 30/6+/-8/8 | 31/1+/-7/8 | P=0.31 no significant |
| Average duration of surgery | 118/4+/-9/6 | 117/6+/-11/1 | 114/5+/-10/4 | P=0.28 no significant |
Figure 1.
Comparison of vomiting and nausea scores in patients undergoing an abdominal hysterectomy in three groups
With regard to the Table 2, there was a significant difference between the three groups in terms of vomiting scores in recovery, 2 and 4 hours after surgery; the scores of vomiting was significantly lower in the ondansetron when comparing with the other two groups (P = 0.04 ; P = 0.02 ; P = 0.001).
Table 2.
Comparison of vomiting and nausea scores in patients undergoing an abdominal hysterectomy in three groups
| Vomiting Scores / Group | Ondansetron | Haloperidol | Dexmedetomidine | P-value |
|---|---|---|---|---|
| Vomiting recovery score | 0/11+/-0/18 | 0/34+/-0/41 | 0/32+/-0/38 | P=0.04 significant |
| Vomiting score 2 hours later | 0/11+/-0/26 | 0/51+/-0/48 | 0/61+/-0/28 | P=0.02 significant |
| Vomiting score 4 hours later | 0/03+/-0/08 | 0/32+/-0/21 | 0/41+/-0/48 | P=0.001 Significant |
| Vomiting score 12 hours later | 0 | 0 | 0 | P≥0.05 no significant |
| Vomiting score 24 hours later | 0 | 0 | 0 | P≥0.05 no significant |
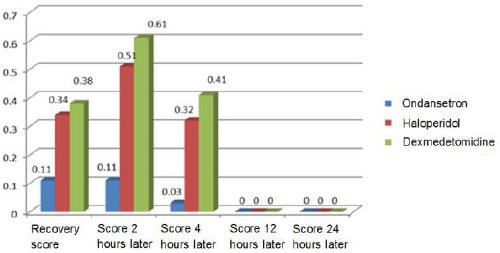
| Frequency Percentage of nausea | %10/1 | %40/6 | %48/3 | P=0.001 Significant |
However, there was no significant difference between the two groups of haloperidol and dexmedetomidine regarding vomiting scores. Furthermore, 12 and 24 hours after surgery, the mean vomiting scores were identically equal in all three groups (P ≥ 0.05); therefore, no significant difference was observed between the three groups.
There was a significant difference in the frequency of nausea among the three groups, and the incidence of nausea exhibited a significant decrease in the ondansetron group as compared to other two groups (P = 0.001).
Our study revealed a significant difference between the three groups of ondansetron, haloperidol, and dexmedetomidine in terms of the mean dose of metoclopramide in mg for 24 hours after surgery; nevertheless, dosage of metoclopramide in the ondansetron group was less than the other two groups (P = 0.001; Table 3; Figure 2).
Table 3.
Comparison of the mean dose of metoclopramide consumed within 24 hours (mg) after operation in patients undergoing an abdominal hysterectomy in the three groups together with Average scoring for recovery
| Average / group | Ondansetron | Haloperidol | Dexmedetomidine | P-value |
|---|---|---|---|---|
| the mean dose of metoclopramide (Mg) | 1/06+/-0/21 | 4/37+/-1/1 | 3/54+/-1/3 | P=0.001 Significant |
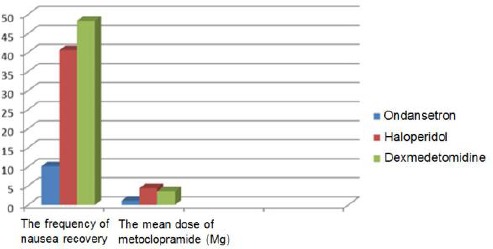
| Average scoring for recovery | 2/7+/-0/85 | 3/37+/-1/1 | 2/9+/-0/91 | P=0.12 no significant |
Figure 2.
Comparison of the mean dose of metoclopramide consumed 24 hours after the operation (mg) and the frequency of nausea in patients undergoing an abdominal hysterectomy in the three groups
Based on Table 3 and Figure 3, no significant difference was found between the three groups regarding the average scoring in recovery, so that the scoring average was determined as three in three groups (P = 0.12).
Figure 3.
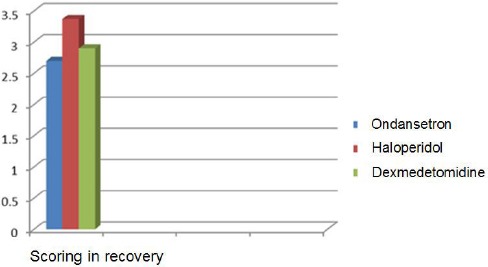
Comparison of average scoring in recovery in patients undergoing an abdominal hysterectomy in three groups
The frequency of hypotension and bradycardia did not exhibit any significant difference between the three groups (P = 0.0001). Furthermore, the incidence of shivering was not revealed to be significant in between the three groups and the incidence of shivering in the three groups was found to be approximately the same (13.5%) (Table 4).
Table 4.
Frequency of hypotension, bradycardia and shivering in patients undergoing an abdominal hysterectomy in three groups
| Average / group | Ondansetron | Haloperidol | Dexmedetomidine | P-value |
|---|---|---|---|---|
| Frequency of hypotension | 0 | %12/9 | %9/6 | P=0.0001 Significant |
| Frequency of bradycardia | 0 | %3/1 | %12/9 | P=0.0001 Significant |
| Frequency of shivering | %12/5 | %15/1 | %12/9 | P=0.11 no significant |
Discussion
Achieving a suitable drug for controlling PONV is one of the important goals of anesthesiologists. Nausea is an unpleasant sensation that is perceived by the person who is aware of the imminent occurrence of vomiting after surgery and is one of the most common complications after surgery in women that occurs during the first 24 hours after surgery and anaesthesia [1]. Despite the advances in the new drugs for PONV, nausea and vomiting remain a common complaint after general anaesthesia, especially in women’s surgeries [1] [2] [3]. This complication can occur between 20% and 30% of patients undergoing general anaesthesia after 24 hours of surgery. Several factors affect the amount of nausea and vomiting after surgery, including the type of surgery (gynaecological surgery, strabismus, Ear surgery), as well as the drugs used and the type of anaesthesia [9] [10] [11] [12] [13]. It is noteworthy that one of the important concerns of anesthesiologists over the years has been the availability of suitable drugs for controlling PONV. These drugs are used as prophylaxis or for the treatment of PONV [9]. The results of previous studies were in agreement with our study. In a study conducted by Haddadi et al., in Iran, the effects of ondansetron and metoclopyramide and dexmedetomidine in the prevention of PONV in children undergoing strabismus surgery have been investigated, where ondansetron was more effective than the other group in controlling PONV [14]. Of course, in our study, ondansetron was compared with haloperidol and dexmedetomidine, while ondansetron has been compared with metoclopramide and dexamethasone in the study above. Another study in 2009, evaluated the effect of ondansetron and the combination of ondansetron and dexamethasone in preventing PONV in patients with laparoscopic cholecystectomy. The results of this study indicated that the combination of ondansetron and dexamethasone was more effective in preventing nausea and vomiting Action [2]. The results of this study were in agreement with our findings, suggesting the effect of endonestrone on controlling PONV.
Pradeep Ret al. assessed the efficacy of haloperidol with ondansetron in preventing PONV after laparoscopic abdominal surgeries. They depicted that ondansetron 4 mg is not having a remarkable benefit over Haloperidol 2 mg for preventing PONV [3]. The results of the mentioned study were not consistent with our findings, because the current study depicted the effect of ondansetron in controlling postoperative nausea and vomiting. The cause of the difference in these two studies may be referred to the type of surgery. Another study demonstrated that oral ondansetron could be more favourable for women undergoing cesarean section with spinal anaesthesia [4]. The results of this study were consistent with our study, which our study has shown the effect of ondansetron on reducing PONV. Dexmedetomidine has been shown to reduce the incidence and severity of PONV, which is similar to dexamethasone. This is superior to dexamethasone in reducing postoperative pain and taking analgesic drugs over the first 24 hours after laparoscopic cholecystectomy [8]. The results of the mentioned study are not the same as our study, because we compared the effects of the three drugs of haloperidol, ondansetron and dexmedetomidine, but mentioned study did not compare the effects of dexamethasone and dexmedetomidine.
Furthermore, haloperidol has been equally demonstrated to be effective, like dexamethasone, for preventing PONV in patients undergoing gynaecological laparoscopic surgery [15]. Concisely, our findings also revealed that haloperidol, ondansetron and dexmedetomidine were effective in controlling postoperative nausea and vomiting that are in agreement with the aforementioned study. A study indicated that ondansetron has favourable effects on decreasing as a cost-effective and easily available medication for prevention of PONV, where endonestrone was nearly 30% more effective than the combination of dexametasone-metocholopromide on PONV [16].
Our study also suggested a significant effect of ondansetron in controlling PONV. Studies have shown that exmedetomidine, haloperidol, and endonestrone drugs have been effective in controlling PONV [17] [18]. However, it should be noted that the effect of ondansetron is more than other drugs in most studies. Considering the negative effect of endonestrone on hemodynamic therapy, it is advised to use this drug [17] [19] [20]. The prophylactic use of this drug is recommended in women’s surgery, especially abdominal hysterectomy.
In conclusion, our data suggested that these drugs are effective in reducing PONV in patients undergoing a hysterectomy. However, the effect of endonestrone on the reduction of postoperative nausea and vomiting is more than the other two drugs.
Footnotes
Funding: This research did not receive any financial support
Competing Interests: The authors have declared that no competing interests exist
References
- 1.Eidi M, Kolahdouzan K, Hosseinzadeh H, Tabaqi R. Comparison of ondansetron vs ondansetron and dexamethasone in the prevention of postoperative nausea and vomiting in patients undergoing surgery with general anesthesia. Iran J Med Sci. 2012;37(3):166–72. PMid:23115448 PMCid:PMC3470085. [PMC free article] [PubMed] [Google Scholar]
- 2.Ahsan K, Abbas N, Naqvi SM, Murtaza G, Tariq S. Comparison of efficacy of ondansetron and dexamethasone combination and ondansetron alone in preventing postoperative nausea and vomiting after laparoscopic cholecystectomy. J Pak Med Assoc. 2014;64(3):242–6. PMid:24864592. [PubMed] [Google Scholar]
- 3.Pradeep R, Amitha S, Srinivasan KV. Comparison of haloperidol with ondansetron in preventing post-operative nausea and vomiting after laparoscopic abdominal surgeries. Indian Journal of Clinical Anaesthesia. 2016;3(4):562–7. [Google Scholar]
- 4.Norouzi A, Jamilian M, Khalili M, Kamali A, Melikof L. Comparison of the effect of oral and intravenous ondansetron on decreasing nausea and vomiting after cesarean section. 2013:101–107. [Google Scholar]
- 5.Aghadavoudi O, Mirkheshti M. Evaluating the effect of intravenous Haoperidol and Midazolam on post-operative nausea and vomiting after strabismus surgery. J Isfahan Med Sch. 2014;32(281):470–6. [Google Scholar]
- 6.Chaparro C, Moreno D, Ramirez V, Fajardo A, Gonzalez D. Haloperidol as prophylactic treatment for postoperative nausea and vomiting. Rev Colomb Anestesiol. 2013;41(1):34–43. https://doi.org/10.1016/j.rca.2012.07.010. [Google Scholar]
- 7.Jin S, Liang DD, Chen C, Zhang M, Wang J. Dexmedetomidine revent post-operative nausea and vomiting on patients during anesthesia. Medicine Baltimore. 2017;96(1):e5770. doi: 10.1097/MD.0000000000005770. https://doi.org/10.1097/MD.0000000000005770 PMid:28072722 PMCid:PMC5228682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohamed H. Bakri, Eman A. Ismail, Ahmed Ibrahim. comparison pf Dexmedetomodine and dexamethasone for preventing of postoperative nausea and vomiting after laparoscopic cholecystectomy. Korean Journal of Anesthesiology. 2015;68(3):254–260. doi: 10.4097/kjae.2015.68.3.254. https://doi.org/10.4097/kjae.2015.68.3.254 PMid:26045928 PMCid:PMC4452669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaiyakunapruk N1, Kitikannakorn N, Nathisuwan S, Leeprakobboon K, Leelasettagool C. The efficacy of ginger for the prevention of postoperative nausea and vomiting:a meta-analysis. Am J Obstet Gynecol. 2006;194(1):95–9. doi: 10.1016/j.ajog.2005.06.046. https://doi.org/10.1016/j.ajog.2005.06.046 PMid:16389016. [DOI] [PubMed] [Google Scholar]
- 10.Rose JB, Watcha MF. Postoperative Nausea and Vomiting in Anesthesia & perioperative complications;second edition. 1999:425–440. PMid:10517365. [Google Scholar]
- 11.Maktabi M, Kamali A, Jelodar HT, Shokrpour M. Comparison of Topical and Subcutaneous Bupivacaine Infiltration with Subcutaneous Ketamine on Postoperative Pain in Total Abdominal Hysterectomy. Transylvanian Review. 2017 doi: 10.5455/medarh.2019.73.15-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleisher LA, Johns RA, Savarese JJ, Wiener-Kronish J, Young Wl. In: Millers Anasthesia. Abtahi A, Kamali F, Mahdavi NS, editors. Vol. 101. Tehran: Andishe Rafi; 2011. [Persian] [Google Scholar]
- 13.Rüsch D, Eberhart LH, Wallenborn J, Kranke P. Nausea and vomiting after surgery under general anesthesia:an evidence-based review concerning risk assessment, prevention, and treatment. Deutsches Ärzteblatt International. 2010;107(42):733. doi: 10.3238/arztebl.2010.0733. PMid:21079721 PMCid:PMC2977990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadadi S, Marzban SH, Khoramnia S, Rahnama O. comparison of the antiemetic effects of Ondansetron and Metoclopramide and Dexamethasone in children undertaken Strabismus Surgery. Journal of Rafsanjan university of medical Science. 2012;11(3):187–96. [Google Scholar]
- 15.Joo J, Park YG, Baek J, Moon YE. Haloperidol dose combined with dexamethasone for PONV prophylaxis in high-risk patients undergoing gynecological laparoscopic surgery:a prospective, randomized, double-blind, dose-response and placebo-controlled study. BMC anesthesiology. 2015;15(1):99. doi: 10.1186/s12871-015-0081-1. https://doi.org/10.1186/s12871-015-0081-1 PMid:26152218 PMCid:PMC4493951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tavasoli A, Khaje dalooi M, Alipour M, Bigdeli N. Comparison of Ondansetron and the Combination Dexamethasone-Metocholopromide on postoperative nausea and vomiting, Ofogh-e-Danesh. GMUHS Journal. 2011;17(2):5–11. [Google Scholar]
- 17.Ronald D. Miller. Miller's Anesthesia. 8th Edition. Chapter 16. PONV; 2015. [Google Scholar]
- 18.Bhattarai B, Shrestha S, Singh J. Comparison of ondansetron and combination of ondansetron and dexamethasone as a prophylaxis for postoperative nausea and vomiting in adults undergoing elective laparoscopic surgery. Journal of Emergencies, Trauma and Shock. 2011;4(2):168. doi: 10.4103/0974-2700.82200. https://doi.org/10.4103/0974-2700.82200 PMid:21769200 PMCid:PMC3132353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brettner F, Janitza S, Prüll K, Weninger E, Mansmann U, Küchenhoff H, Jovanovic A, Pollwein B, Chappell D, Zwissler B, von Dossow V. Gender-specific differences in low-dose haloperidol response for prevention of postoperative nausea and vomiting:a register-based cohort study. PloS one. 2016;11(1):e0146746. doi: 10.1371/journal.pone.0146746. https://doi.org/10.1371/journal.pone.0146746 PMid:26751066 PMCid:PMC4713839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farhat K, Pasha AK, Kazi WA. Comparison of Ondansetron and Metoclopramide for PONV Prophylaxis in Laparoscopic Cholecystectomy. J Anesthe Clinic Res. 2013;4(297):2. https://doi.org/10.4172/2155-6148.1000297. [Google Scholar]


