Abstract
Periodontitis is one of the most prevalent inflammatory diseases, characterized by gingival inflammation and alveolar bone loss. MicroRNAs (MiRNAs) are important regulators of inflammation and involved in periodontitis pathogenesis. In this work, we studied the roles of microRNA-21 (miR-21) in periodontitis. MiR-21 is up-regulated in both periodontitis patients and the mice that induced with periodontitis. We tested the roles of miR-21 in the macrophages challenged by periodontitis pathogen Porphyromonas gingivalis (P. gingivalis) lipopolysaccharide (LPS). MiR-21 expression is up-regulated in P. gingivalis LPS-stimulated macrophages. MiR-21 mimic inhibits the pro-inflammatory cytokine production by macrophages, while miR-21 deficiency elevates the production of pro-inflammatory cytokines. Moreover, absence of miR-21 promotes activation of nuclear factor-κB (NF-κB) in P. gingivalis LPS- stimulated cells. In a murine periodontitis model, ligation induced exacerbated gingival inflammation and alveolar bone loss in miR-21 deficient mice than their wild-type littermates. These results demonstrated the anti-inflammatory function of miR-21 in vitro and in vivo, indicating miR-21 could be an interventional target for the control of periodontitis.
Keywords: NF-κB, microRNA, Inflammation, Bone loss, Macrophage
1. Introduction
MicroRNAs (miRNAs) are a class of small non-coding ribonucleic acid (RNA) molecules, which are implicated in various developmental, physiological and pathogenic events. They are about 22 nucleotides long and broadly evolutionarily conserved. MiRNAs function as post-transcriptional regulators by binding to the complementary sequences in target messenger RNA transcripts (mRNAs) and resulting in the translational repression and/or target mRNA transcript degradation. Periodontitis is one of the most common infection-driven inflammatory disease characterized by gingival inflammation and bone loss (Papapanou, 2012). Infection of sub-gingival etiological bacteria induces the periodontal host response, which leads to periodontal tissue damage and inflammatory bone loss (Baker, 2000; Taubman et al., 2005). MiRNAs are important regulators of inflammation and play important roles in inflammatory diseases. One of the most widely investigated miRNAs, MicroRNA-21, has been reported to be a key for various cancers and infectious diseases, through a variety of regulatory functions on inflammation, osteogenesis, and osteoclastogenesis (Asangani et al., 2008; Bhatti et al., 2011; Chan et al., 2005; Damania et al., 2014; Wei et al., 2015; Zhu et al., 2014). Recent reports have shown that various miRNAs are involved in periodontitis though the miRNA expression pattern was distinct in different reports (Lee et al., 2011; Perri et al., 2012; Stoecklin-Wasmer et al., 2012). Research on miRNA express profiling revealed that miR-21 is upregulated in response to periodontitis pathogen stimulation (Du et al., 2016). Although publications showed that miR-21 is involved in the development of periodontal ligament cell and gingival mesenchymal stem cell (Li et al., 2012; Li et al., 2018; Wei et al., 2015; Wei et al., 2017), the functional roles of miR-21 throughout the periodontitis pathogenesis, especially its effect on the inflammatory responses, remain undetermined.
The regulatory functions of microRNAs are complicated, since one microRNA might target different mRNAs and modulate different physiological responses. The regulation of certain signaling pathways by microRNAs can be cell and tissue specific. MiR-21 showed its complicated influences on the activation of nuclear factor-κB (NF-κB) signaling in different cell types (Asangani et al., 2008; Bhatti et al., 2011; Damania et al., 2014; Sheedy et al., 2010; Zhu et al., 2014). NF-κB is pivotal in the signaling network in infection-induced inflammatory responses. The NF-κB pathway is important in pro-inflammatory signaling and the production of cytokines, chemokines, and adhesion molecules. Higher NF-κB activation in the tissues of the lesion site from periodontitis patients indicates an important pathogenic role of NF-κB in periodontitis (Arabaci et al., 2010). Virulence factors from period-ontal pathogen Porphyromonas gingivalis (P. gingivalis) signal through Toll- like receptors, activate the NF-κB pathway, and induce in-flammation (Darveau et al., 2004). NF-κB activation leads to the up-regulation of miR-21 (Niu et al., 2012). P. gingivalis lipopolysaccharide (LPS) has been shown to induce the miR-21 up-regulation (Du et al., 2016; Venugopal et al., 2017; Yao et al., 2009), implying a close association of miR-21 and periodontal pathogen-induced inflammation. Indeed, NF-κB was predicted to be regulated by miR-21 and other miRNAs during periodontitis pathogenesis (Du et al., 2016; Venugopal et al., 2017). MiR-21′s regulation on NF-κB is complicated and may inhibit NF-κB signaling (Jia et al., 2017; Marquez et al., 2010; Sheedy et al., 2010) or promote NF-κB activation (Iliopoulos et al., 2010; Wu et al., 2015), depending on the different cell types and diseases.
In this study, we manifested that miR-21 is important in down-regulating P. gingivalis LPS-induced NF-κB activation and pro-in-flammatory cytokine production, by targeting programmed cell death protein 4 (PDCD4). Furthermore, in a well-established animal period-ontitis model, we showed that miR-21 deficiency leads to more severe gingival inflammation and alveolar bone loss. Our results in this manuscript revealed miR-21 as an important mechanism in the negative feedback loop during periodontal pathogen-induced inflammation and periodontitis progression.
2. Material and methods
2.1. Mice
MiR-21 deficient mice (B6;129S6-Mir21atm1Yoli/J) were purchased from Jackson Lab and bred with C57BL/6 mice to generate heterozygous mice. Wild-type (WT) and homozygous knockout littermates (KO) were obtained from heterozygous breeding. Animals were kept in animal facilities at University of Louisville. All handling and processing were approved by Institutional Animal Care and Use Committee, and in appliance with the established Federal and State policies.
2.2. Human tissue miRNA assay
Periodontal ligament tissues were obtained from 7 periodontitis patients diagnosed by clinical signs and symptoms, including panoramic pantomography or cone-beam computed tomographic imaging before surgery. Healthy periodontal ligament tissue controls were isolated from 7 patients who received extraction premolars or third molars for orthodontic treatment. Subjects were free from severe autoimmune diseases, cardiovascular diseases, neurologic diseases, or cancers. Total RNAs (including small RNAs such as miRNAs) were extracted from tissues using Trizol (Life Technologies, Carlsbad, CA) lysis methods; MiRNAs were reverse transcribed using miScript II RT Kit (Qiagen, Germantown, MD). Real-time polymerase chain reaction (qPCR) amplifications were performed using miScript SYBR Green PCR Kit (Qiagen) in a CFX96 qPCR Detection System (Bio-Rad, Hercules, CA) according to the manufacturer’s protocol. The primer sequences for miR-21 is 5′-GCCCGCTAGCTTATCAGACTGATG-3′ (forward) and 5′-GTGCAGGGTCCGAGGT-3′ (reverse). Results were normalized to the expression of RNU6B (U6) (Qiagen). The procedure was approved by the ethics committee of West China College of Stomatology, Sichuan University, China, and informed consent was obtained from each patient.
2.3. Transfection
Mmu-miR-21a-5p mirVana® miRNA mimic (4464066), mmu-miR-21a-5p mirVana® miRNA inhibitor (4464084), and their negative control oligonucleotides (4464076) were purchased from Thermofisher. RAW 264.7 cells were transfected by electroporation using SF Cell Line 4D-NucleofectorTM X Kit (Lonza, Allendale, NJ, USA) and 4DNucleofector™ X Unit (Lonza, Allendale, NJ, USA), following the manufacturer’s protocol. Transfected cells were cultured for 2 days before further experiments.
2.4. Cell culture and assay
Bone marrow-derived macrophages (BMDMs) were prepared following previously published protocol (Trouplin et al., 2013). Eight weeks old mice were sacrificed to harvest femurs. Bone marrow was flushed out of the femur with PBS using a 3-mL syringe and a 23-gauge needle. Bone marrow was then pipetted several times to make a single-cell suspension. Cells were washed with PBS twice before being plated onto a culture dish. The cells were cultured in DMEM supplemented with 10% fetal bovine serum and 10 ng/ml murine granulocyte macrophage colony stimulating factor (M-CSF, R&D systems, Minneapolis, MN) and incubated at 37 °C with 5% CO2. Wash cells every other day and add fresh medium. The differentiated macrophages were harvested on day 7 for later experiments. The purity of macrophage preparations (> 90%) was confirmed by flow cytometry using phycoerythrin-labeled anti-F4/80 (clone BM8; eBioscience). Cell viability was monitored using the CellTiter-Blue™ assay kit (Promega).
2.5. MiR-21 targets array analysis
The expression of potential miR-21 targets was measured using a RT2 PCR Array (Qiagen) by qPCR on an ABI 7500 Real Time PCR System (Applied Biosystems, Foster City, CA) following the manufacturer’s protocol. Samples were grouped and analyzed using the PCR Array Data Analysis Excel Template with the ΔΔCt method (Qiagen).
2.6. Western blots
Cell lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). Protein expression was detected by chemiluminescence. Antibodies to p-NF-κB p65 (Ser 536), IκB, PDCD4, and GAPDH were purchased from Cell Signaling Technology (Beverly, MA, USA). Densities of bands were quantified by Eagle Eye II software.
2.7. Ligature-induced periodontitis model
A murine ligature-induced periodontitis model is used as described in our and other’s previous publication (Duan et al., 2016; Eskan et al., 2012; Matsuda et al., 2016). Sex-matched miR-21 KO mice and WT littermates (8–10 weeks) were ligated around the maxillary 2nd molar with 6–0 silk suture, with the ligature placed in the gingival sulcus. Ten days after the ligature placement, at the termination of the experiments, the distance from cemento-enamel junction to alveolar bone crest (CEJ/ABC) on the ligated second molar (three sites corresponding to mesial cusp, buccal groove, and distal cusp) and the affected adjacent regions (sites corresponding to distal cusp and distal groove of the first molar, and buccal cusp of the third molar) were measured. To calculate proinflammatory periodontal bone loss, the mean CEJ-ABC distance from the group of sham-ligated mice was subtracted from the CEJ-ABC distance for each mouse.
2.8. Mouse gingival mRNA and miRNA expression assay
Gingival tissues of the mice were excised from around the maxillary molars for mRNA harvest. The expression of periodontal disease markers and other molecules of interest were determined by qPCR. Briefly, mRNA was extracted from gingival tissue, using the RNeasy Mini Kit (Qiagen). The RNA was reverse-transcribed using the High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA, USA) and qPCR was performed using the ABI 7500 System, following the manufacturer’s protocol (Applied Biosystems). TaqMan probes, sense primers, and antisense primers for genes of interest or a housekeeping gene (glyceraldehyde 3-phosphate dehydrogenase, GAPDH) were purchased from Applied Biosystems. The following inventoried gene expression primers (Applied Biosystems) were used: IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), TNFα (Mm00443258_m1), Rankl (Mm00441906_m1). Gene expression has been normalized to that of GAPDH.
For miRNA expression, miRNA was extracted using mirVana™ miRNA Isolation Kit (ambion, life technologies) and reverse transcription was performed on the isolated total RNA using miRNA cDNA Synthesis Kit (Applied Biological Materials, Vancouver, Canada). And BrightGreen miRNA qPCR MasterMix (Applied Biological Materials, Vancouver, Canada) was used for quantitative analysis of miRNA. Reverse transcription was performed at 37 °C for 30 min., 65 °C for 5 min., 42 °C for 15 min and at 70 °C for 10 min. The PCR conditions were as follows: enzyme activaition at 95 °C for 10 min., denaturation at 95 °C 10 s., annealing at 63 °C for 15 s., followed by extension at 72 °C for 32 s. The procedure was performed on ABI 7500(Thermo Scientific, Wilmington, DE, USA). U6 was amplified as an internal control. The mmu-miR-21 and U6 were supplied by Applied Biological Materials (Vancouver, Canada).
2.9. Statistics
Data were evaluated by ANOVA and the Dunnett multiple-comparison test (InStat v3.06 program, GraphPad). Two-tailed t tests were also performed where appropriate (comparison of two groups only). Differences were considered statistically significant at the p < 0.05 level.
3. Results
3.1. Higher miR-21 expression in periodontitis patients and animals
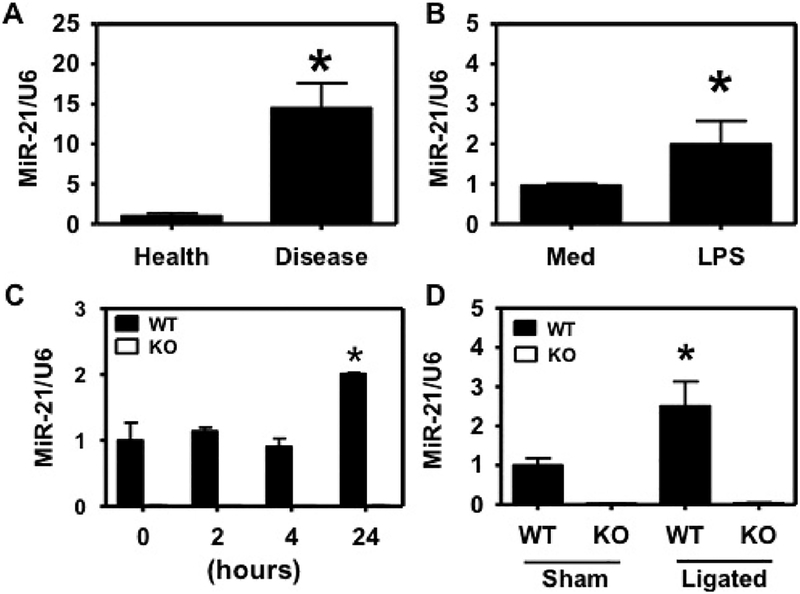
We harvested periodontal tissues from periodontitis patients as well as healthy tissues from the patients who had received extraction premolars or third molars for orthodontic treatment. We tested the miR-21 expression using qPCR and found higher miR-21 expression in period-ontitis patients (Fig. 1A). Furthermore, we found that P.gingivalis LPS stimulation induces upregulated miR-21 in murine macrophage cell line RAW264.7 (Fig. 1B). We next generated bone marrow derived macrophages (BMDM) from miR-21 KO mice and their WT littermates, and stimulated the BMDMs with P. gingivalis LPS (2 μg/ml). At different time points (2 h, 4 h, and 24 h), the BMDMs were harvested and tested for miR-21 expression. The miR-21 expression was tested at the time points indicated and we found P. gingivalis LPS significantly increased miR-21 in WT BMDMs at 24 h (Fig. 1C), while the miR-21 expression in KO cells was minimal, as we expected. We next tested the miR-21 expression in a widely used ligation induced periodontitis animal model, in which ligation leads to gingival inflammation and alveolar bone loss in the mice (Duan et al., 2016; Eskan et al., 2012; Matsuda et al., 2016). Similar to our result from human patients, in WT mice, miR-21 expression in the gingival tissues from ligated mice was higher than the control sham-ligated mice (Fig. 1D). The miR-21 expression in the gingival tissues from miR-21 KO mice was neglectable.
Fig. 1.
Periodontal pathogen and periodontitis prompts miR-21 upregulation. MiR-21 is upregulated in (A) periodontitis patients, (B, C) P. gingivalis LPS-stimulated macrophages, and (D) ligated mice. MiR-21 expression was tested by qPCR in (A) periodontal ligament tissues from 7 periodontitis patients (Disease) and 7 healthy subjects (Health), *, p < 0.05. (B) RAW 264.7 cells stimulated with P. gingivalis LPS (LPS), *, p < 0.05. (C) BMDM cells from WT or miR-21 KO mice were stimulated with P. gingivalis LPS (LPS) for 2 hs, 4 hs, or 24 hs or before stimulation (0 h), n = 3. Asterisk indicated statistically significant (p < 0.05) differences versus 0 h samples. *, p < 0.05. Or (D) gingival tissues from ligated WT or KO mice (Ligated; n = 5) and sham-ligated WT or KO mice (Sham; n = 6). Asterisks indicate statistically significant (p < 0.05) differences between ligated and sham WT mice. *, p < 0.05. The data were normalized to U6 and are presented as fold change in the transcript levels relative to their controls, set as 1. The data shown represent mean ± standard deviation.
3.2. MiR-21 down-regulates P. gingivalis LPS-induced pro-inflammatory cytokine production
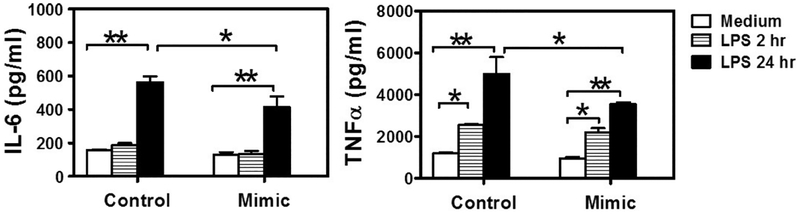
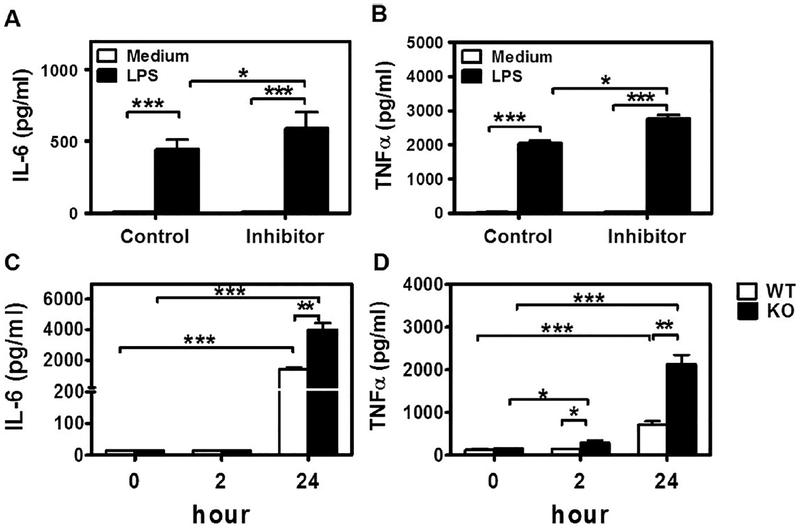
Pro-inflammatory cytokines are regulated directly or indirectly by pathogen-stimulated NF-κB activation. Levels of pro-inflammatory cytokines also indicate the severity of periodontitis. To test the roles of miR-21 in P. gingivalis LPS- induced pro-inflammatory cytokine production, we transfected RAW 264.7 cells with miR-21 mimic, miR-21 inhibitor, or their negative controls, followed by 2 μg/ml P. gingivalis LPS challenge. Cell culture supernatants were collected 2 h or 24 h later and tested for IL-6 and TNFα. We found that miR-21 transfection inhibited IL-6 and TNFα production (Fig. 2A & B). On the contrast, cells transfected with miR-21 inhibitor produced higher levels of IL-6 and TNFα (Fig. 3A & B). To better illustrate the roles of MiR-21 deficiency in pathogen-induced inflammation, we harvested BMDMs from miR-21 KO mice and their WT littermates, and stimulated the BMDMs with P. gingivalis LPS (2 μg/ml). Higher TNFα and IL-6 production were detected in LPS-stimulated miR-21 KO BMDM cells (Fig. 3C & D).
Fig. 2.
MiR-21 inhibits P. gingivalis LPS-induced IL-6 and TNFα production. RAW 264.7 cells were transfected with miR-21 mimic. Cells transfected with miR-21 negative control were used as controls. The cells were then stimulated with 2 μg/ml P. gingivalis LPS for 2 hs or 24 hs before tested for the production of (A) IL-6 and (B) TNFα in the supernatants by ELISA. The data shown represent mean ± standard deviation, *, p < 0.05; **, p < 0.01; ***, p < 0.001; n = 3.
Fig. 3.
P. gingivalis LPS induced higher level of IL-6 and TNFα with miR-21 ablation. (A, B) MiR-21 inhibitor transfected RAW264.7 cells were stimulated with 2 μg/ml P. gingivalis LPS for 24 hs; Or (C, D) BMDM cells from miR-21 KO mice and their WT littermates were stimulated with 2 μg/ml P. gingivalis LPS for 2 h or 24 hs. (A, C) IL-6 and (B, D) TNFα in the supernatants were tested by ELISA. The data shown represent mean ± standard deviation, *, p < 0.05; **, p < 0.01; n = 3.
3.3. MiR-21 ablation promotes P. gingivalis LPS-induced NF-κB activation
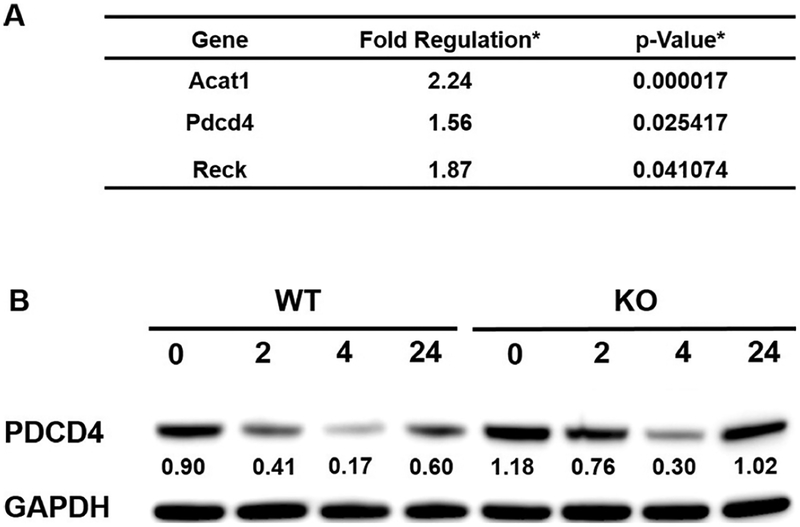
In order to determine the targets of miR-21 in P. gingivalis LPS-stimulated macrophages, we used mouse miR-21 targets RT2 profiler PCR Array (Qiagen), which included experimentally verified or bioinformatically predicted miR-21 target genes. BMDMs from miR-21 KO mice and their WT littermates were stimulated with P. gingivalis LPS (2 μg/ml) for 4 h before harvested for mRNA isolation. Using qPCR, our results showed the expression of Acat1 (Acetyl-CoA acetyltransferase), PDCD4, and Reck (Reversion-inducing-cysteine-rich protein with kazal motifs) was significantly higher in miR-21 KO cells, indicating they are regulated by miR-21 (Fig. 4A). Among these targets, PDCD4 has been shown to be involved into NF-κB signaling (Sheedy et al., 2010).
Fig. 4.
MiR-21 inhibited PDCD4 expression. BMDM cells from miR-21 KO mice and their WT littermates were stimulated with P. gingivalis LPS (2 μg/ml). (A) List of upregulated genes in P. gingivalis LPS-stimulated miR-21 KO BMDM cells versus stimulated WT cells. Upregulation is defined as Fold regulation > 1.5 and p < 0.05. (B) Cell lysates were collected at the time points (hours) indicated for Western blot analysis. Blots were probed with the antibodies to PDCD4, and GAPDH as a loading control. The number showed the densitometric quantification of the ratio of PDCD4 to GAPDH.
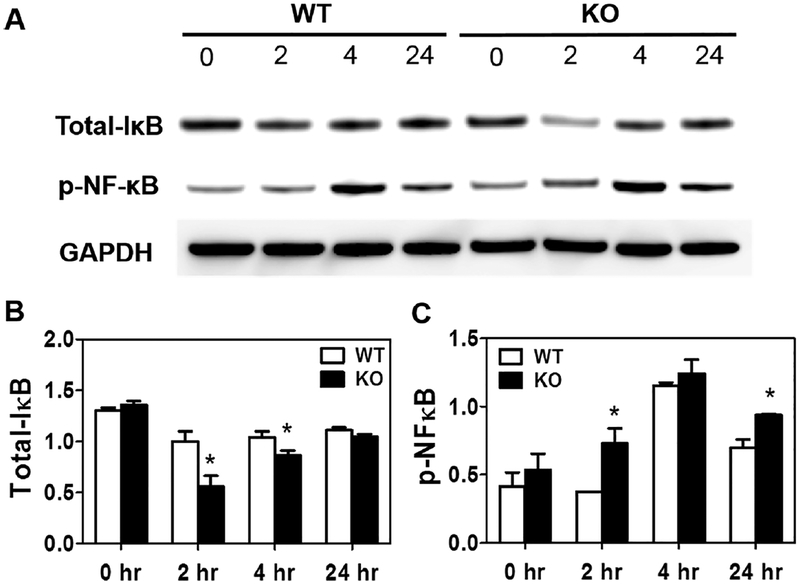
MiR-21 has shown different modulatory mechanisms in NF-κB signaling in different diseases and cells (Asangani et al., 2008; Bhatti et al., 2011; Damania et al., 2014; Zhu et al., 2014). NFκB signaling plays a pivotal role in inflammatory responses and periodontitis progression (Arabaci et al., 2010; Pacios et al., 2015). Higher NF-κB activation at the lesion site in periodontitis patients indicates an important pathogenic role for NF-κB in periodontitis (Arabaci et al., 2010). P. gingivalis and its virulence factors, such as fimbria and LPS, activate the NF-κB pathway (Darveau et al., 2004; Diomede et al., 2017). To confirm PDCD4 as the target of miR-21 regulation, we stimulated BMDMs from miR-21 KO mice and their WT littermates with P. gingivalis LPS (2 μg/ml) and tested for the protein expression of PDCD4. PDCD4 level was higher in miR-21 KO cells, indicating that miR-21 down-regulated PDCD4 (Fig. 4B). To investigate the role of MiR-21 in periodontal pathogen-induced NF-κB activation, BMDMs from miR-21 KO mice and their WT littermates were stimulated with P. gingivalis LPS (2 μg/ml) and tested for the protein expression of phosphorylated (p)-NF-κB and total IκB. Higher level of (p)-NF-κB and lower level of total IκB were detected in miR-21 KO BMDM cells, indicating higher NF-κB activation in these cells (Fig. 5). Considering the critical role of NF-κB for the transcription of TNFα and IL-6 (Neurath et al., 1996), miR-21-deficiency mediated increase of TNFα and IL-6 could be attributed to the increased activation of NF-κB in LPS-stimulated BMDMs.
Fig. 5.
MiR-21 ablation promotes P. gingivalis LPS-induced NF-κB activation. BMDM cells from miR-21 KO mice and their WT littermates were stimulated with P. gingivalis LPS (2 μg/ml). Cell lysates were collected at the time points (hours) indicated for Western blot analysis. (A) Blots were probed with the antibodies to IκB, phosphrylated NF-κBp65, and GAPDH as a loading control. Densitometric quantification of the ratio of (B) total- IκB, and (C) p-NF-κBp65 to GAPDH. Data represent mean ± S.E.M. from three experiments. Asterisks indicate statistically significant (p < 0.05) differences between miR-21 KO mice and WT mice.
3.4. Elevated gingival inflammation in miR-21 KO mice
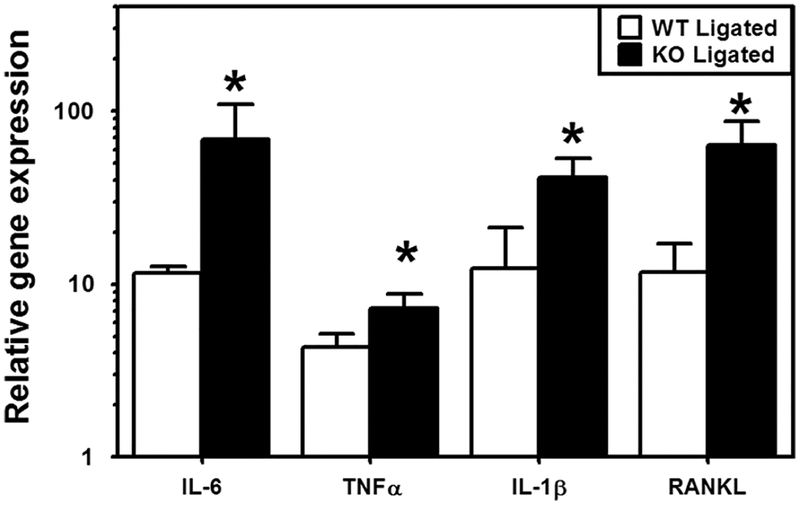
Gingival inflammation was induced in a murine ligature-induced periodontitis model, as described in our previous publication (Eskan et al., 2012). The mice with sham ligature placement were used as controls. At termination of the experiments, gingival tissues were harvested for mRNA extraction. Pro-inflammatory cytokines, including IL-6, TNFα, IL-1β, and RANKL, are critical for osteoclastogenesis and periodontitis pathogenesis. Expressions of these cytokines in gingiva were determined by qPCR. Ligation induced gingival inflammation in both miR-21 KO and WT mice. We observed significantly higher transcript expression of IL-1β, IL-6, TNFα, and RANKL, indicating more severe inflammation, in miR-21 KO mice than WT mice (Fig. 6).
Fig. 6.
Higher gingival inflammation in miR-21 KO mice. Gingival mRNA expression levels for the indicated molecules were determined by qPCR (normalized against GAPDH mRNA levels). The gingivae used were excised from miR-21 KO mice and their WT littermates. Results are shown as fold change relative to WT sham-ligated mice. The data represent the mean ± SD of expression values corresponding to qPCR analysis of at least 5 mice in each group. Asterisks indicate statistically significant (p < 0.05) differences between miR-21 KO mice and WT mice.
3.5. Exacerbated alveolar bone loss in miR-21 KO mice
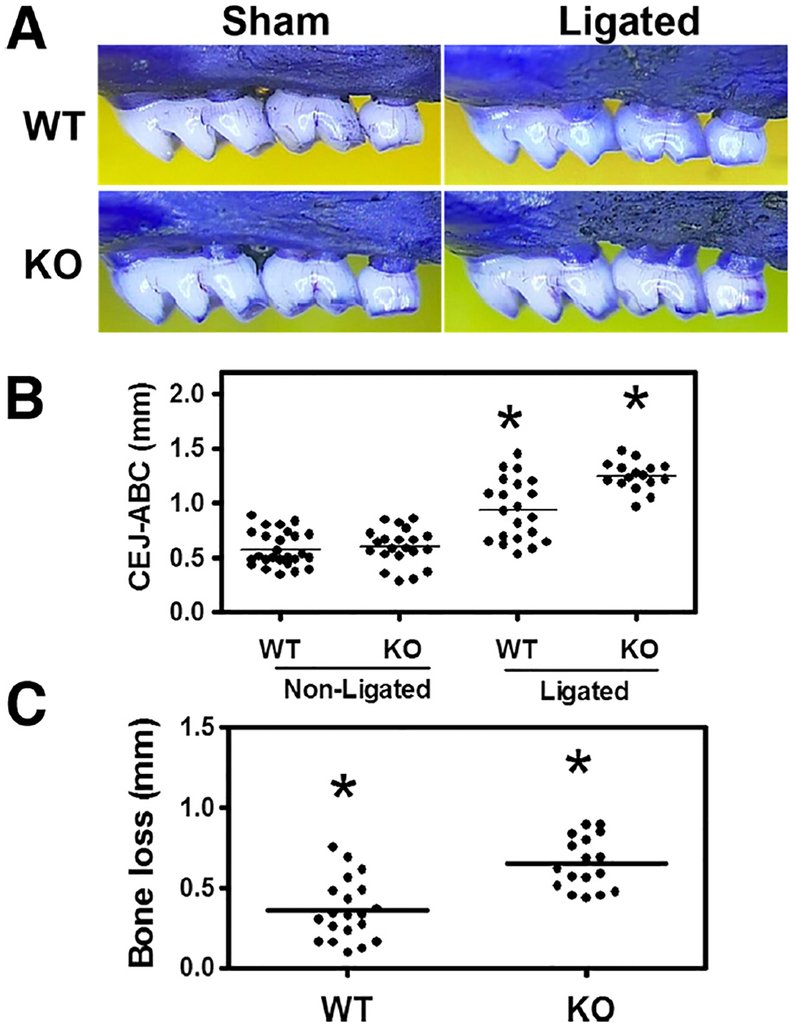
We next investigated whether miR-21 deficiency led to more severe bone loss. In the ligature-induced periodontitis model, silk ligature placement results in massive local accumulation of bacteria and the induction of rapid bone loss in conventional rodents (Duan et al., 2016; Eskan et al., 2012; Graves et al., 2008). We observed higher CEJ-ABC in ligated miR-21 KO mice than their WT littermates. At the same time, there was no difference in CEJ-ABC readings between sham-ligated miR-21 KO and WT mice (Fig. 7A, B). We calculated bone loss by subtracting the CEJ-ABC distance of the sham-ligated mice from that of the ligated mice. Indeed, miR-21 KO mice showed higher bone loss (Fig. 7C), indicating that dysregulated host immune responses to oral bacteria in miR-21 KO mice result in exacerbated periodontal bone loss.
Fig. 7.
Periodontal bone loss in ligated miR-21 KO and WT mice. (A) Representative images from the maxillae of ligated (right panels) or sham-li-gated (Sham; left panels) WT (upper panels) and miR-21 KO (lower panels) mice. (B) The mm distance from the cementoenamel junction (CEJ) to the alveolar bone crest (ABC) was measured at 6 most affected maxillary buccal sites and the readings were totaled for each mouse. (C) The data from CEJ-ABC readings were transformed to indicate bone loss, as outlined in Materials and Methods. The reading of each mouse was represented by each dot. Asterisks indicate statistically significant (p < 0.05) differences between ligated miR-21 KO mice and WT mice.
4. Discussion
MiR-21 has been widely investigated on its roles in various cancers and inflammatory diseases. While some reports showed that miR-21 is involved in bone formation and resorption (Wei et al., 2015; Yang et al., 2013), its roles in periodontitis remain unclear. Similar to many other miRNAs, miR-21 can bind and regulate various transcripts, and function differently in different cell types and diseases (Barnett et al., 2016; Chan et al., 2005; Chen et al., 2013). Our results showed upregulated miR-21 in periodontitis patients (Fig. 1A) and the ligated mice in ligature-induced periodontitis model (Fig. 1D). Furthermore, stimulating the macrophage with periodontal pathogen P. gingivalis LPS also caused increased miR-21 expression (Fig. 1B and C). All these imply that miR-21 plays an important role in periodontitis.
Periodontitis is initiated by periodontal pathogens and caused by dysregulated host immune responses. P. gingivalis is one of the most important and widely investigated periodontitis pathogens. P. gingivalis LPS is able to induce pro-inflammatory cytokines in host immune cells (Darveau et al., 2004), which are important for periodontitis pathogenesis and bone resorption (Di Benedetto et al., 2013; Graves and Cochran, 2003; Kato et al., 2014). We tested the roles of miR-21 in inflammation by testing the production of pro-inflammatory cytokines in P. gingivalis LPS- stimulated macrophages, which have been transfected with miR-21 mimic or miR-21 inhibitor. Forced expression of miR-21 in macrophages leads to decreased IL-6 and TNFα production in responding to LPS stimulation, while inhibition of miR-21 increased IL-6 and TNFα production (Figs. 2 & 3). While miR-21 transfection does not totally block miR-21 function, we took advantage of miR-21 KO mice and derived BMDM cells. We found that miR-21 KO BMDM cells produced higher level of IL-6 and TNFα than WT cells, further confirming that miR-21 is important to the down-regulation of LPS-induced inflammation (Fig. 3).
NF-κB plays an important pathogenic role in periodontitis (Arabaci et al., 2010; Pacios et al., 2015). Under normal conditions, NF-κB is sequestered in the cytoplasm as an inactive complex with the inhibitory protein IκB. Upon stimulation, IκB is phosphorylated, ubiquitinated and degraded. Consequently, NF-κB becomes free to translocate to the nucleus where it initiates the expression of NF-κB -dependent pro-in-flammatory cytokines. Previous publications have shown the complicated interplay between miR-21 and NF-κB (Ma et al., 2011). NF-κB activation leads to the transcription of miR-21 (Niu et al., 2012; Shin et al., 2011). In turn, miR-21 can either up-regulate or down-regulate the activation of NF-κB signaling pathway through targeting different genes. It has been shown that miR-21 can activate NF-κB via the inhibition of Phosphatase and tensin homolog (PTEN) (Iliopoulos et al., 2010; Wu et al., 2015). On the other hand, NF-κB is down-regulated by miR-21 through PDCD4 or PELI1 (Pellino E3 ubiquitin protein ligase 1) (Asangani et al., 2008; Marquez et al., 2010; Sheedy et al., 2010). The difference might be caused by cell-specificity. Because the important roles of NF-κB in inflammation and periodontitis, it is critical to understand the regulation of NF-κB by miR-21 in this disease. Indeed, we found that miR-21 ablation cause elevated NF-κB activation after LPS challenge, indicated by decreased total-IκB and elevated phosphorylated NF-κB. Our result implies that NF-κB activation results in upregulated pro-inflammatory cytokine production. A mouse miR-21 targets RT2 profiler PCR Array (Qiagen) included experimentally verified or bioinformatically predicted miR-21 target genes. We tested P. gingivalis LPS- stimulated WT and miR-21 KO BMDMs with the array and found that Acat1, PDCD4 and Reck might be the targets of miR-21 (Fig. 4A). Because PDCD4 has been shown to be involved into TLR and NF-κB signaling (Sheedy et al., 2010), we further tested for the protein expression of PDCD4 and confirmed that PDCD4 level is upregulated in miR-21 KO cells (Fig. 4B). Previous reports (Sheedy et al., 2010) and our results showed that miR-21 inhibition could block LPS-induced PDCD4 down-regulation, therefore increase NF-κB activation and pro-inflammatory cytokine production. Our results implied that miR-21 acted as an anti-inflammatory agent within a negative regulatory loop during periodontitis.
Animal models have been widely used to the research on the pathogenesis of various diseases, including periodontitis. Ligation around the 2nd molar of mouse induces gingival inflammation and periodontal bone loss, which has been used in a model for periodontitis. Pro-in-flammatory cytokines such as IL-1β, IL-6, TNFα are pro-osteoclastogenic factors. Along with RANKL, excessive amount of these cytokines destroy the periodontal tissue and result in the attachment loss of the gingiva and alveolar bone loss (Di Benedetto et al., 2013; Graves and Cochran, 2003; Kato et al., 2014). We tested the expression of IL-1β, IL-6, TNFα, and RANKL in the gingival tissues of ligated miR-21 KO and WT mice. Similar to our in vitro results, ligation induced higher level of pro-inflammatory cytokines in miR-21 KO mouse tissues. This indicates that miR-21 is fulfilling an anti-inflammatory role in periodontitis, while its deprivation causes inflammation. In ligature-induced period-ontitis model, ligation and dysregulated inflammation lead to period-ontal bone loss. We measured the distance from CEJ to ABC (CEJ-ABC) in each group. Ligation induced higher CEJ-ABC in miR-21 KO mice than in WT mice. In sham-ligated groups, the CEJ-ABC readings were comparable in KO mice and WT mice. Our findings indicate that miR-21 is a feedback mechanism to mitigate pathogen-induced excessive in-flammation during periodontitis.
5. Conclusion
In summary, we found the higher expression of miR-21 in period-ontitis patients, ligated mice, and P. gingivalis LPS challenged cells, all suggesting the involvement of miR-21 in periodontitis. Our results showed that miR-21 down-regulated P. gingivalis LPS-induced in-flammation, while miR-21 absence elevated PDCD4 expression, NF-κB activation and pro-inflammatory cytokine production. These findings manifested the negative regulatory functions of miR-21 on pathogen-induced pro-inflammatory responses. Furthermore, in an in vivo periodontitis animal model, we demonstrated the protective roles of miR-21 on periodontitis progression. Our findings provide a potential immunotherapeutic method for periodontitis treatment.
Acknowledgements
This research was supported by grants KSEF-3283-RDE-018, KSEF-3835-RDE-020, DE025388 (S.L.), and DE 026727 (H.W.). The funding agencies have no role in the study design, collection, analysis and interpretation of the data.
Footnotes
Conflict of interest
The authors report no conflicts of interest related to this work.
References
- Arabaci T, Cicek Y, Canakci V, Canakci CF, Ozgoz M, Albayrak M, Keles ON, 2010. Immunohistochemical and stereologic analysis of NF-kappaB activation in chronic periodontitis. Eur. J. Dentistry 4, 454–461. [PMC free article] [PubMed] [Google Scholar]
- Asangani IA, Rasheed SA, Nikolova DA, Leupold JH, Colburn NH, Post S, Allgayer H, 2008. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 27, 2128–2136. [DOI] [PubMed] [Google Scholar]
- Baker PJ, 2000. The role of immune responses in bone loss during periodontal disease. Microbes Infect 2, 1181–1192. [DOI] [PubMed] [Google Scholar]
- Barnett RE, Conklin DJ, Ryan L, Keskey RC, Ramjee V, Sepulveda EA, Srivastava S, Bhatnagar A, Cheadle WG, 2016. Anti-inflammatory effects of miR-21 in the macrophage response to peritonitis. J. Leukocyte Biol 99, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti I, Lee A, James V, Hall RI, Lund JN, Tufarelli C, Lobo DN, Larvin M, 2011. Knockdown of microRNA-21 inhibits proliferation and increases cell death by targeting programmed cell death 4 (PDCD4) in pancreatic ductal adenocarcinoma. J. Gastrointest. Surg 15, 199–208. [DOI] [PubMed] [Google Scholar]
- Chan JA, Krichevsky AM, Kosik KS, 2005. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res 65, 6029–6033. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen J, Wang H, Shi J, Wu K, Liu S, Liu Y, Wu J, 2013. HCV-induced miR-21 contributes to evasion of host immune system by targeting MyD88 and IRAK1. PLoS Pathog 9, e1003248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damania P, Sen B, Dar SB, Kumar S, Kumari A, Gupta E, Sarin SK, Venugopal SK, 2014. Hepatitis B virus induces cell proliferation via HBx-induced microRNA-21 in hepatocellular carcinoma by targeting programmed cell death protein4 (PDCD4) and phosphatase and tensin homologue (PTEN). PloS One 9, e91745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau RP, Pham TT, Lemley K, Reife RA, Bainbridge BW, Coats SR, Howald WN, Way SS, Hajjar AM, 2004. Porphyromonas gingivalis lipopolysaccharide contains multiple lipid A species that functionally interact with both toll-like receptors 2 and 4. Infect. Immun 72, 5041–5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto A, Gigante I, Colucci S, Grano M, 2013. Periodontal disease: linking the primary inflammation to bone loss. Clin. Develop. Immunol 2013, 503754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomede F, Zingariello M, Cavalcanti M, Merciaro I, Pizzicannella J, De Isla N, Caputi S, Ballerini P, Trubiani O, 2017. MyD88/ERK/NFkB pathways and proinflammatory cytokines release in periodontal ligament stem cells stimulated by Porphyromonas gingivalis. Eur. J. Histochem.: EJH 61, 2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du A, Zhao S, Wan L, Liu T, Peng Z, Zhou Z, Liao Z, Fang H, 2016. MicroRNA expression profile of human periodontal ligament cells under the influence of Porphyromonas gingivalis LPS. J. Cell. Mol. Med 20, 1329–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Gleason RC, Li F, Hosur KB, Duan X, Huang D, Wang H, Hajishengallis G, Liang S, 2016. Sex dimorphism in periodontitis in animal models. J. Periodontal Res 51, 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskan MA, Jotwani R, Abe T, Chmelar J, Lim JH, Liang S, Ciero PA, Krauss JL, Li F, Rauner M, et al. , 2012. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nat. Immunol 13, 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves DT, Cochran D, 2003. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J. Periodontol 74, 391–401. [DOI] [PubMed] [Google Scholar]
- Graves DT, Fine D, Teng YT, Van Dyke TE, Hajishengallis G, 2008. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol 35, 89–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K, 2010. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol. Cell 39, 493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia P, Wu X, Dai Y, Teng J, Fang Y, Hu J, Zou J, Liang M, Ding X, 2017. MicroRNA-21 Is required for local and remote ischemic preconditioning in multiple organ protection against sepsis. Crit. Care Med 45, e703–e710. [DOI] [PubMed] [Google Scholar]
- Kato H, Taguchi Y, Tominaga K, Umeda M, Tanaka A, 2014. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch. Oral Biol 59, 167–175. [DOI] [PubMed] [Google Scholar]
- Lee YH, Na HS, Jeong SY, Jeong SH, Park HR, Chung J, et al. , 2011. Comparison of inflammatory microRNA expression in healthy and periodontitis tissues. Biocell 35, 43–49. [PubMed] [Google Scholar]
- Li C, Li C, Yue J, Huang X, Chen M, Gao J, Wu B, 2012. miR-21 and miR-101 regulate PLAP-1 expression in periodontal ligament cells. Mol. Med. Rep 5, 1340–1346. [DOI] [PubMed] [Google Scholar]
- Li X, Guo L, Liu Y, Su Y, Xie Y, Du J, Wang S, Wang H, Liu Y, 2018. MicroRNA-21 promotes wound healing via the Smad7-Smad2/3-Elastin pathway. Exp. Cell Res 362, 245–251. [DOI] [PubMed] [Google Scholar]
- Ma X, Becker Buscaglia LE, Barker JR, Li Y, 2011. MicroRNAs in NF-kappaB signaling. J. Mol. Cell. Biol 3, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez RT, Wendlandt E, Galle CS, Keck K, McCaffrey AP, 2010. MicroRNA-21 is upregulated during the proliferative phase of liver regeneration, targets Pellino-1, and inhibits NF-kappaB signaling. Am. J. Physiol. Gastrointest. Liver Physiol 298, G535–G541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Kato T, Takahashi N, Nakajima M, Arimatsu K, Minagawa T, Sato K, Ohno H, Yamazaki K, 2016. Ligature-induced periodontitis in mice induces elevated levels of circulating interleukin-6 but shows only weak effects on adipose and liver tissues. J. Periodontal Res 51, 639–646. [DOI] [PubMed] [Google Scholar]
- Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W, 1996. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa b abrogates established experimental colitis in mice. Nat. Med 2, 998–1004. [DOI] [PubMed] [Google Scholar]
- Niu J, Shi Y, Tan G, Yang CH, Fan M, Pfeffer LM, Wu ZH, 2012. DNA damage induces NF-kappaB-dependent microRNA-21 up-regulation and promotes breast cancer cell invasion. J. Biol. Chem 287, 21783–21795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pacios S, Xiao W, Mattos M, Lim J, Tarapore RS, Alsadun S, Yu B, Wang CY, Graves DT, 2015. Osteoblast lineage cells play an essential role in periodontal bone loss through activation of nuclear factor-Kappa B. Sci. Rep 5, 16694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapanou PN, 2012. The prevalence of periodontitis in the US: forget what you were told. J. Dent. Res 91, 907–908. [DOI] [PubMed] [Google Scholar]
- Perri R, Nares S, Zhang S, Barros SP, Offenbacher S, 2012. MicroRNA modulation in obesity and periodontitis. J. Dental Res 91, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, Johnson DS, Chen Y, O’Neill LA, 2010. Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nat. Immunol 11, 141–147. [DOI] [PubMed] [Google Scholar]
- Shin VY, Jin H, Ng EK, Cheng AS, Chong WW, Wong CY, Leung WK, Sung JJ, Chu KM, 2011. NF-kappaB targets miR-16 and miR-21 in gastric cancer: involvement of prostaglandin E receptors. Carcinogenesis 32, 240–245. [DOI] [PubMed] [Google Scholar]
- Stoecklin-Wasmer C, Guarnieri P, Celenti R, Demmer RT, Kebschull M, Papapanou PN, 2012. MicroRNAs and their target genes in gingival tissues. J. Dent. Res 91, 934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman MA, Valverde P, Han X, Kawai T, 2005. Immune response: the key to bone resorption in periodontal disease. J. Periodontol 76, 2033–2041. [DOI] [PubMed] [Google Scholar]
- Trouplin V, Boucherit N, Gorvel L, Conti F, Mottola G, Ghigo E, 2013. Bone marrow-derived macrophage production. J. Visual. Exp.: JoVE, e50966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal P, Koshy T, Lavu V, Ranga Rao S, Ramasamy S, Hariharan S, Venkatesan V, 2017. Differential expression of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction with NF-kB pathway genes in periodontitis pathogenesis. J. Cell. Physiol [DOI] [PubMed] [Google Scholar]
- Wei F, Liu D, Feng C, Zhang F, Yang S, Hu Y, Ding G, Wang S, 2015. microRNA-21 mediates stretch-induced osteogenic differentiation in human periodontal ligament stem cells. Stem Cells Dev 24, 312–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Yang S, Guo Q, Zhang X, Ren D, Lv T, Xu X, 2017. MicroRNA-21 regulates osteogenic differentiation of periodontal ligament stem cells by targeting Smad5. Sci. Rep 7, 16608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Liu Y, Fan Z, Xu J, Jin L, Gao Z, Wu Z, Hu L, Wang J, Zhang C, et al. , 2015. miR-21 modulates the immunoregulatory function of bone marrow mesenchymal stem cells through the PTEN/Akt/TGF-beta1 pathway. Stem Cells 33, 3281–3290. [DOI] [PubMed] [Google Scholar]
- Yang N, Wang G, Hu C, Shi Y, Liao L, Shi S, Cai Y, Cheng S, Wang X, Liu Y, et al. , 2013. Tumor necrosis factor alpha suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J. Bone Miner. Res 28, 559–573. [DOI] [PubMed] [Google Scholar]
- Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH, 2009. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem. Biophys. Res. Commun 388, 539–542. [DOI] [PubMed] [Google Scholar]
- Zhu HY, Li C, Bai WD, Su LL, Liu JQ, Li Y, Shi JH, Cai WX, Bai XZ, Jia YH, et al. , 2014. MicroRNA-21 regulates hTERT via PTEN in hypertrophic scar fibroblasts. PloS One 9, e97114. [DOI] [PMC free article] [PubMed] [Google Scholar]