Cell surface structure stabilization is important for extracellular secretion of proteins in Escherichia coli. As the main constituent of the cell wall, peptidoglycan contributes to cell structure robustness and stability. Here, we perturbed the peptidoglycan network by deleting dacA and dacB genes encoding d,d-carboxypeptidase enzymes to improve extracellular protein secretion. This new strategy could enhance the capacity of E. coli as a microbial cell factory for extracellular secretion of proteins and chemicals.
KEYWORDS: d,d-carboxypeptidase; gene deletion; extracellular secretion; cell wall; Escherichia coli
ABSTRACT
Escherichia coli is one of the most widely used host microorganisms for recombinant protein expression and metabolic engineering, but it cannot efficiently secrete recombinant proteins to extracellular space. Here, extracellular protein secretion was enhanced in E. coli by deleting two d,d-carboxypeptidase genes (dacA and dacB, single and double deletions) to perturb the cell wall peptidoglycan network. Deletion of dacA and dacB enhanced the accumulation of intracellular soluble peptidoglycan in E. coli and affected cell morphology, resulting in a more irregular cell shape and the appearance of transparent bulges. Deletion of dacA and dacB appears to disrupt the normal rigid structure, presumably due to perturbation and destruction of the cell wall peptidoglycan network. The extracellular green fluorescent protein (GFP) fluorescence intensity of deletion mutants was increased by >2.0-fold compared with that of control cells, and that of the double deletion mutant was increased by 2.7-fold. Extracellular recombinant fibroblast growth factor receptor 2 (FGFR2) and collagen E4 secretion in deletion mutants was also enhanced compared with that in the control cells. Additionally, the extracellular recombinant amylase activity of single-deletion mutants BL21 ΔdacA pETDuet-amyk and BL21 ΔdacB pETDuet-amyk was increased 2.5- and 3.1-fold, respectively. The extracellular distribution of α-galactosidase by deletion mutants was also increased by >2.0-fold. Deletion of dacA and dacB increased outer membrane permeability, which could explain the enhanced extracellular protein secretion.
IMPORTANCE Cell surface structure stabilization is important for extracellular secretion of proteins in Escherichia coli. As the main constituent of the cell wall, peptidoglycan contributes to cell structure robustness and stability. Here, we perturbed the peptidoglycan network by deleting dacA and dacB genes encoding d,d-carboxypeptidase enzymes to improve extracellular protein secretion. This new strategy could enhance the capacity of E. coli as a microbial cell factory for extracellular secretion of proteins and chemicals.
INTRODUCTION
Escherichia coli is one of the most important host microorganisms used for recombinant protein expression and metabolic engineering due to several advantages, including the ability to achieve high expression levels and rapid growth. Extracellular secretion is desirable for many proteins to avoid intracellular proteolytic degradation and to facilitate simpler purification (1–4). Moreover, when substrates, such as toxic pollutants, are not adequately taken up by E. coli cells, extracellular secretion of recombinant enzymes is also useful for metabolic engineering (4). However, most recombinant proteins are transported into the periplasmic space, except for some toxins and erythrocytolysin, which are immediately secreted into the extracellular environment (5).
E. coli uses two strategies to introduce proteins into the extracellular medium (6). One strategy involves transport through E. coli membranes by active transport, as occurs in pathogenic E. coli and other Gram-negative bacteria (6–8). The other strategy is a two-stage translocation process involving active transporters in the cytoplasmic membrane that transport proteins into the periplasmic space, followed by secretion by passive transport into the extracellular medium through outer membrane proteins (6). External or internal destabilization of E. coli structural components can result in passive transport. There are several methods that can partially break the outer membrane or cell wall to release periplasmic proteins via selective permeabilization or disruption, including chemical methods (e.g., Triton X-100), enzymatic treatments (e.g., lysozyme), and mechanical methods (e.g., ultrasound) (2, 5, 6, 9).
Peptidoglycan is the main constituent of the cell wall and contributes to cell structure robustness and stability (10). Bacterial L-forms, representing the most drastic example of disturbing the cell surface structure, have been used to improve the secretion of murein staphylokinase and penicillin G acylase (11, 12). Bacterial L-forms are formed by completely deleting the cell wall through natural or artificial induction (e.g., by penicillin) (13). However, since bacterial L-forms have several limitations, such as low protein expression levels, slow growth, and poor robustness, they are not used widely in industrial production (14, 15). Twelve penicillin binding proteins (PBPs) have been characterized in E. coli, and they are divided into high-molecular-weight (HMW) and low-molecular-weight (LMW) classes (16, 17). The HMW PBPs PBP1a, PBP1b, PBP2, and PBP3 possess both d-alanyl–d-alanine transpeptidase (d,d-transpeptidase) and transglycosidase activity, and are essential for cell growth, whereas none of the LMW PBPs are essential for E. coli growth. The LMW PBPs PBP4, PBP5, PBP6, and PBP6b, known as d-alanyl–d-alanine carboxypeptidases (d,d-carboxypeptidases; Dac) DacB, DacA, DacC, and DacD, respectively (18), play important roles in the synthesis and maintenance of the E. coli cell wall by mediating peptidoglycan crosslinking, structure stabilization, and cell wall modification (19).
In the present work, the d,d-carboxypeptidase genes dacA and dacB in E. coli were deleted to perturb the cell wall peptidoglycan network (Fig. 1). We investigated the effects of deleting the d,d-carboxypeptidase genes dacA and dacB on extracellular secretion of recombinant proteins in E. coli by using recombinant green fluorescent protein (GFP; 26.8 kDa), recombinant fibroblast growth factor receptor 2 (FGFR2; 28.2 kDa), recombinant collagen E4 (12.8 kDa), and recombinant amylase (AmyK; 62.8 kDa) as model proteins. Cell growth, morphology, intracellular soluble peptidoglycan accumulation, extracellular distribution of α-galactosidase, and outer membrane permeability were also evaluated.
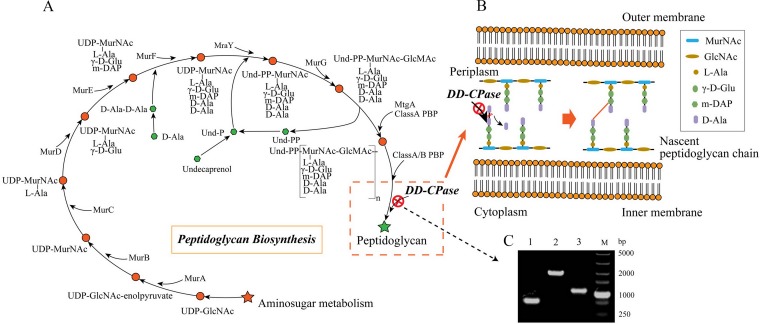
FIG 1.
E. coli peptidoglycan synthesis and d,d-carboxypeptidase gene deletion. (A) The peptidoglycan biosynthesis pathway of E. coli. UDP-GlcNAc, UDP-N-acetylglucosamine; UDP-MurNAc, UDP-N-acetylmuramate; MurA, UDP-N-acetylglucosamine 1-carboxyvinyltransferase; MurB, UDP-N-acetylmuramate dehydrogenase; MurC, UDP-N-acetylmuramate-alanine ligase; MurD, UDP-N-acetylmuramoylalanine–d-glutamate ligase; MurE, UDP-N-acetylmuramoyl-l-alanyl–d-glutamate-2,6-diaminopimelate ligase; MurF, UDP-N-acetylmuramoyl-tripeptide-d-alanyl–d-alanine ligase; MraY, phospho-N-acetylmuramoyl-pentapeptide-transferase; MurG, UDP-N-acetylglucosamine–N-acetylmuramyl-(pentapeptide) pyrophosphoryl-undecaprenol N-acetylglucosamine transferase; MtgA, monofunctional glycosyltransferase; ClassA PBP, penicillin-binding protein 1A; ClassA/B PBP, penicillin-binding protein 1A and penicillin-binding protein 2; DD-CPase, d,d-carboxypeptidases. (B) Nascent peptidoglycan chain synthesis with DD-CPase in the periplasm. During peptidoglycan synthesis, d,d-carboxypeptidases delete the C-terminal d-Ala from the majority of peptidoglycan precursor pentapeptide stem molecules. (C) Deletion of DD-CPase genes dacA and dacB (detailed gene deletion methods and data are included in the supplemental material). 1, BL21 ΔdacA ΔdacB; 2, BL21 ΔdacA ΔdacB::kan; 3, BL21 ΔdacB; and M, standard molecular weight markers.
RESULTS
Effects of dacA and dacB deletion on cell growth.
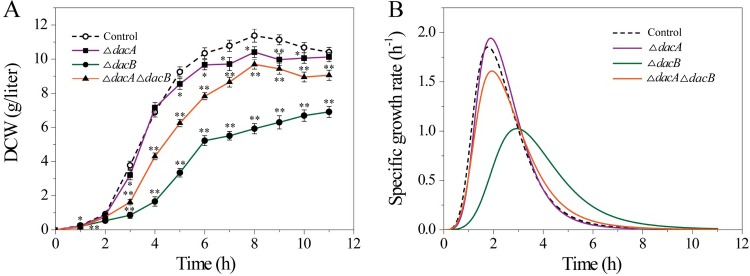
d,d-Carboxypeptidase genes dacA and dacB in E. coli BL21 were deleted using the Red homologous recombination system (Fig. 1), and two single-deletion mutants, BL21 ΔdacA and BL21 ΔdacB, and one double-deletion mutant, BL21 ΔdacA ΔdacB, were constructed (see supplemental material). We investigated the effects of deleting dacA and dacB on E. coli cell growth (Fig. 2). Deletion of dacA did not significantly inhibit cell growth, whereas the highest dry cell weight (DCW) of BL21 ΔdacB was 6.9 g/liter, compared with 11.4 g/liter for control cells (Fig. 2A), and the highest specific growth rate was decreased from 1.8 h−1 for control cells to 1.0 h−1 for BL21 ΔdacB (Fig. 2B). Meanwhile, after deleting dacA and dacB, the cell growth of BL21 ΔdacA ΔdacB was also inhibited compared with that of control cells, indicating that dacB is more critical than dacA for the growth of E. coli BL21. The BL21 ΔdacA ΔdacB double-deletion mutant grew faster than the dacB single-deletion mutant.
FIG 2.
Effects of dacA and dacB deletion on cell growth. (A) Dry cell weight (DCW). Asterisks indicate a significant difference compared with control cells (none, P > 0.05; *, P < 0.05; and **, P < 0.01). (B) Specific growth rate, also known as relative growth rate (RGR), exponential growth rate, and continuous growth rate. RGR was calculated using the following equation from Hoffmann and Poorter (43): RGR = (lnW2 − lnW1)/(t2 − t1) where ln = natural logarithm, t1 = time 1 (e.g., in days), t2 = time 2 (e.g., in days), W1 = size at time 1, and W2 = size at time 2. Control, wild-type E. coli; ΔdacA, BL21 ΔdacA; ΔdacB, BL21 ΔdacB; and ΔdacA ΔdacB, BL21 ΔdacA ΔdacB.
Effects of dacA and dacB deletion on intracellular soluble peptidoglycan accumulation.
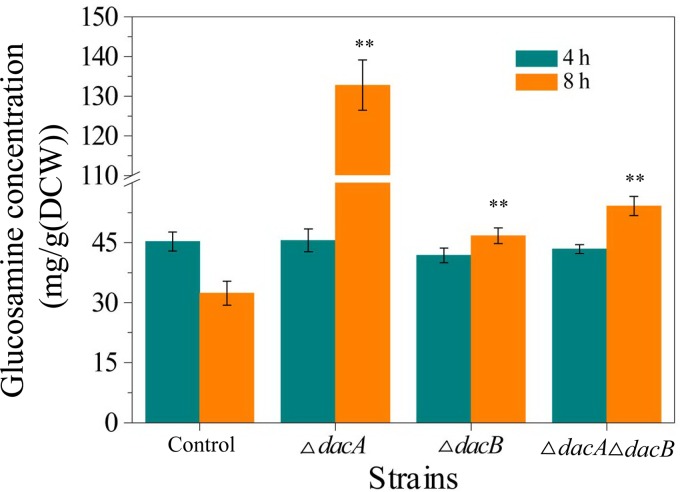
Peptidoglycan is an essential cell wall component of nearly all bacteria, which protects the cell from bursting due to turgor pressure (20, 21). In the first stage of peptidoglycan synthesis, soluble precursors are synthesized in the cytoplasm (22). The growth of the peptidoglycan sacculus is a dynamic process, and soluble fragments removed from the sacculus are reused by an efficient peptidoglycan recycling pathway (23). Peptidoglycan of the E. coli cell wall is a polymer consisting of alternating N-acetylglucosamine and N-acetylmuramic acid residues, to which peptide side chains are linked to form the sacculus network. d,d-Carboxypeptidases DacA and DacB play an important role in peptidoglycan synthesis (23). In this work, we determined the glucosamine concentration of soluble peptidoglycan to investigate changes in intracellular soluble peptidoglycan concentration in the different strains. Glucosamine concentrations of dacA or dacB single-deletion mutants and the double-deletion mutant were 132.8, 46.7, and 54.2 mg/g (DCW), respectively, at 8 h, compared with only 32.4 mg/g (DCW) for control cells (Fig. 3). These results indicated that deletion of dacA and dacB promoted the accumulation of intracellular soluble peptidoglycan, which accumulated to a greater extent in the double-deletion mutant than in the dacB single-deletion mutant.
FIG 3.
Analysis of glucosamine concentration. The y axis represents the glucosamine concentration of soluble peptidoglycan to indicate changes in intracellular soluble peptidoglycan concentration in different stains. Control, BL21-pETDuet; ΔdacA, BL21 ΔdacA pETDuet; ΔdacB, BL21 ΔdacB pETDuet; ΔdacA ΔdacB, BL21 ΔdacA ΔdacB pETDuet. Asterisks indicate a significant difference compared with control cells (none, P > 0.05; *, P < 0.05; and **, P < 0.01).
Effects of dacA and dacB deletion on cell morphology.
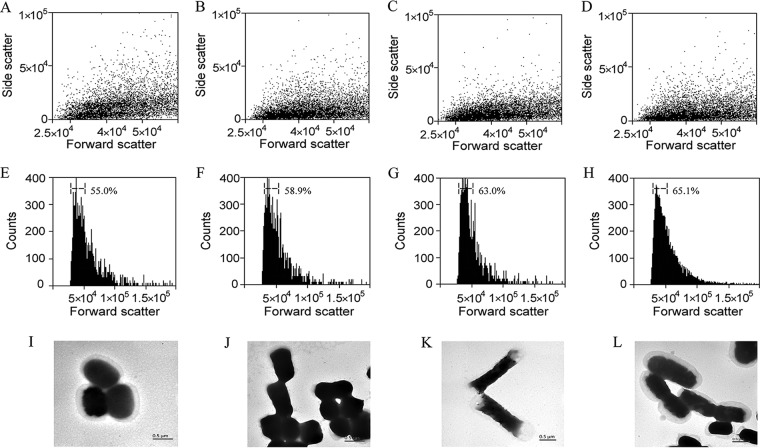
Fluorescence-activated cell sorting (FACS) can be used to quantify differences in E. coli cell shape (24). Here, the cell population distribution of mutant strains was investigated using forward-scattered and side-scattered light at a cell density of 1.0 × 104. The population of mutant E. coli strains clustered in a two-dimensional scatter plot of forward- versus side-scattered light (Fig. 4A to D). However, the actual densities of data points for mutant strains were not truly reflected in the scatter plots. Meberg et al. found that differences in E. coli cell shape are visualized most easily by graphing only the distribution of forward-scattered light (24). The cell population distribution of mutant E. coli strains lacking dacA and dacB displayed a forward-scattered light range between 0 and 5.0 × 104, which was changed significantly compared with that of control cells (Fig. 4E to H). FACS gates can be drawn to give a relative comparison of cell distribution among gates, and differences generally become more evident after this quantification (24). Fractions give the distribution (proportion [%]) of cells within a FACS gate among total cells. The fractions of dacA and dacB single-deletion mutants and the double-deletion mutant falling within the gates were 58.9, 63.0, and 65.1%, respectively, compared with only 55.0% for control cells (Fig. 4E to H). As shown in Fig. 4I to L, the cell shape of dacA and dacB single-deletion mutants was more irregular than that of control cells, with a wider cell width and the appearance of localized transparent bulges at cell poles in the case of the dacB single deletion. Double deletion of dacA and dacB resulted in transparent globular swelling in E. coli cells (Fig. 4L).
FIG 4.
Effects of dacA and dacB deletion on cell morphology. (A through D) Dot plot of cells using forward-scattered light (x axis) and side-scattered light (y axis). (E through H) Numbers of cells (y axis) according to the amount of forward-scattered light (x axis). (I through L) Effect of dacA and dacB deletion on cell morphology assessed by transmission electron microscopy (TEM). (A, E, and I) BL21-pETDuet (control cells). (B, F, and J) BL21 ΔdacA pETDuet. (C, G, and K) BL21 ΔdacB pETDuet. (D, H, and L) BL21 ΔdacA ΔdacB pETDuet.
Effects of dacA and dacB deletion on extracellular recombinant GFP, FGFR2, and E4 secretion.
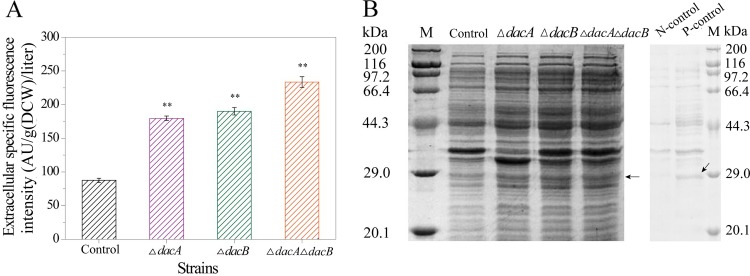
Recombinant GFP (26.8 kDa) was used to investigate the effects of deleting dacA and dacB on extracellular protein secretion in E. coli. The recombinant plasmid pETDuet-gfp was transformed into mutant strains BL21 ΔdacA, BL21 ΔdacB, and BL21 ΔdacA ΔdacB to generate recombinant mutant strains BL21 ΔdacA pETDuet-gfp, BL21 ΔdacB pETDuet-gfp, and BL21 ΔdacA ΔdacB pETDuet-gfp. As shown in Fig. 5, deletion of dacA and dacB increased the extracellular GFP concentration of BL21 ΔdacA pETDuet-gfp, BL21 ΔdacB pETDuet-gfp, and BL21 ΔdacA ΔdacB pETDuet-gfp compared with control cells. The extracellular GFP fluorescence intensity of BL21 ΔdacA pETDuet-gfp, BL21 ΔdacB pETDuet-gfp, and BL21 ΔdacA ΔdacB pETDuet-gfp was 179.7, 190.0, and 233.5 AU/g (DCW)/liter, respectively, compared with 87.5 AU/g (DCW)/liter for control cells (Fig. 5A). The extracellular protein concentration of BL21 ΔdacA pETDuet-gfp, BL21 ΔdacB pETDuet-gfp, and BL21 ΔdacA ΔdacB pETDuet-gfp were also enhanced compared with that of control cells (see Table S1 in the supplemental material). The concentrations of extracellular recombinant GFP of mutants and control cells were further analyzed by SDS-PAGE (Fig. 5B) and was higher than that of control cells, further verifying that extracellular secretion of recombinant GFP was enhanced after deletion of dacA and dacB. The effects of deleting dacA and dacB on extracellular recombinant FGFR2 and E4 secretion were also determined by SDS-PAGE (see Fig. S2 in the supplemental material), and extracellular secretion of both proteins was enhanced in the deletion mutants compared with that in the control cells.
FIG 5.
Effects of dacA and dacB deletion on extracellular recombinant green fluorescent protein (GFP) secretion. (A) Extracellular specific fluorescence intensity. AU, arbitrary units. Asterisks indicate a significant difference compared with control cells (none, P > 0.05; *, P < 0.05; and **, P < 0.01). (B) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis. M, standard molecular weight markers; arrow, GFP; control, E. coli BL21-pETDUET-gfp (extracellular); ΔdacA, BL21 ΔdacA pETDuet-gfp; ΔdacB, BL21 ΔdacB pETDuet-gfp; ΔdacA ΔdacB, BL21 ΔdacA ΔdacB pETDuet-gfp; P-control, E. coli BL21-pETDuet-gfp (positive control, intracellular); N-control, E. coli BL21-pETDuet (negative control, intracellular).
Effects of dacA and dacB deletion on extracellular recombinant amylase secretion.
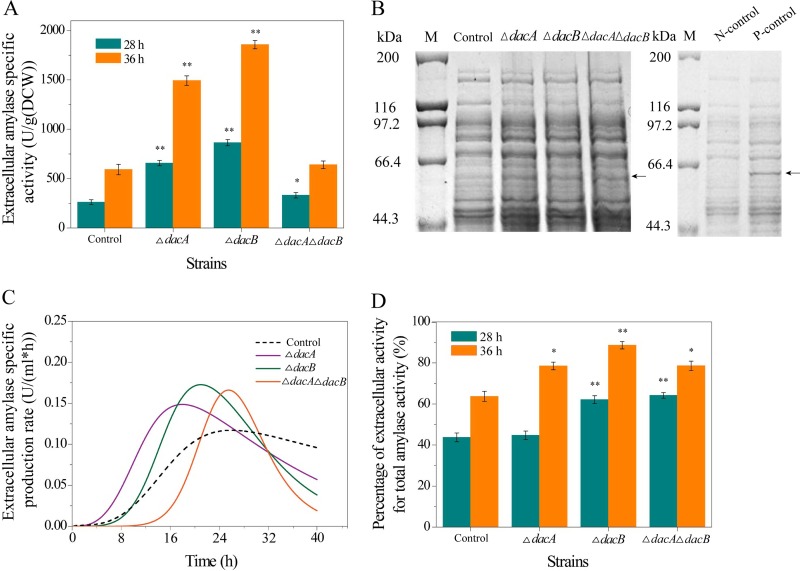
Recombinant amylase AmyK (62.8 kDa) was also used to study the effects of deleting dacA and dacB on extracellular protein secretion in E. coli. As shown in Fig. 6A, the extracellular amylase activity of E. coli was improved by deleting dacA and dacB, especially by single deletion of dacA or dacB. The specific activity of extracellular amylase of BL21 ΔdacA pETDuet-amyk and BL21 ΔdacB pETDuet-amyk reached 1,492.9 and 1,857.4 U/g (DCW), respectively, following isopropyl-β-d-thiogalactopyranoside (IPTG) induction for 36 h, compared with only 592.7 U/g (DCW) for control cells (see Table S2 in the supplemental material). SDS-PAGE was also used to analyze effects of dacA and dacB deletion on extracellular recombinant amylase secretion (Fig. 6B). Apparent protein bands corresponding to the molecular mass (62.8 kDa) of AmyK were observed in deletion mutants, further indicating that extracellular secretion of recombinant amylase was enhanced by deletion of dacA and dacB. Meanwhile, as shown in Table S1 in the supplemental material, the extracellular protein concentrations of mutants lacking dacA and dacB was also significantly enhanced compared with that of control cells. The specific production rates of extracellular amylase of mutants was investigated, and deletion of dacA and dacB increased extracellular secretion of amylase in E. coli BL21, especially in the case of dacB deletion (Fig. 6C). The specific production rates of extracellular amylase of BL21 ΔdacA pETDuet-amyk, BL21 ΔdacB pETDuet-amyk, and BL21 ΔdacA ΔdacB pETDuet-amyk were significantly boosted compared with that of control cells, and the rate was highest for BL21 ΔdacB pETDuet-amyk (Fig. 6C), reaching 0.17 U/(ml · h).
FIG 6.
Effects of dacA and dacB deletion on extracellular recombinant amylase secretion. (A) Specific activity of extracellular amylase. Asterisks indicate a significant difference compared with control cells (none, P > 0.05; *, P < 0.05; and **, P < 0.01). (B) SDS-PAGE analysis of extracellular amylase. M, standard molecular weight markers; arrow, amylase; control, E. coli BL21-pETDuet-amy (extracellular); P-control, E. coli BL21-pETDuet-amy (positive control, intracellular); N-control, E. coli BL21-pETDuet (negative control, intracellular). (C) Extracellular amylase production rate. (D) Percentage of extracellular activity of total amylase activity. ΔdacA, BL21 ΔdacA pETDuet-amy; ΔdacB, BL21 ΔdacB pETDuet-amy; ΔdacA ΔdacB, BL21 ΔdacA ΔdacB pETDuet-amy.
The distribution of extracellular amylase was also determined to analyze the effect of deleting dacA and dacB on extracellular protein secretion in E. coli (Fig. 6D). Extracellular secretion of recombinant amylase in E. coli was enhanced by deletion of dacA and dacB. Compared with that of control cells, the extracellular amylase distribution for BL21 ΔdacA pETDuet-amyk, BL21 ΔdacB pETDuet-amyk, and BL21 ΔdacA ΔdacB pETDuet-amyk was increased from 63.7% for control cells to 78.5, 88.6, and 78.6%, respectively, at 36 h. However, the total amylase activity of BL21 ΔdacA ΔdacB pETDuet-amyk was not high compared with that of control cells (Table S2), indicating that double deletion (both dacA and dacB) might not be advantageous for heterologous expression of amylase in E. coli.
Effects of dacA and dacB deletion on extracellular α-galactosidase activity.
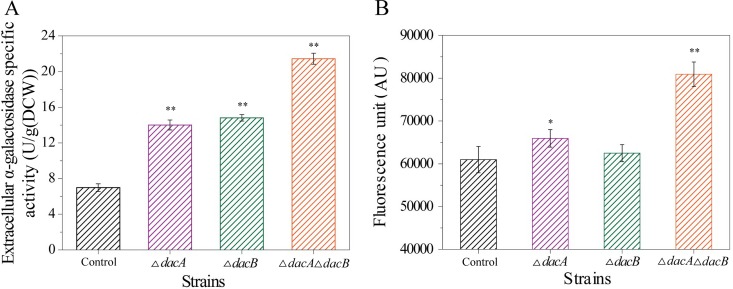
The extracellular distribution of α-galactosidase, an intracellular enzyme in E. coli, was also determined in order to investigate the effect of deleting dacA and dacB on extracellular protein secretion in E. coli. As shown in Fig. 7A, the extracellular α-galactosidase activity of mutants was boosted compared with that of control cells. Extracellular α-galactosidase activity of mutant strains BL21 ΔdacA pETDuet, BL21 ΔdacB pETDuet and BL21 ΔdacA ΔdacB pETDuet was 14.0, 14.8, and 21.4 U/g(DCW), respectively, compared with only 6.9 U/g (DCW) for control cells.
FIG 7.
Effects of dacA and dacB deletion on extracellular α-galactosidase activity and outer membrane permeability. (A) Effects of dacA and dacB deletion on extracellular α-galactosidase activity. (B) Effects of dacA and dacB deletion on outer membrane permeability. Control, BL21-pETDuet; ΔdacA, BL21 ΔdacA pETDuet; ΔdacB, BL21 ΔdacB pETDuet; ΔdacA ΔdacB, BL21 ΔdacA ΔdacB pETDuet. Asterisks indicate a significant difference compared with control cells (none, P > 0.05; *, P < 0.05; and **, P < 0.01).
Effects of dacA and dacB deletion on cell outer membrane permeability.
The effect of dacA and dacB deletion on cell outer membrane permeability was also investigated. Loh et al. previously used N-phenyl-α-naphthylamine (NPN) as a probe to assess outer membrane permeability (25). NPN fluoresces weakly in an aqueous environment, and its intensity increases in a nonpolar or hydrophobic environment. Deletion of dacA and dacB caused an increase in the fluorescence intensity of NPN bound to E. coli cells, especially to cells of the double-deletion mutant (Fig. 7B). The NPN fluorescence intensity of the double-deletion mutant was 8.1 × 104 AU, representing an increase of 1.3-fold compared with that of control cells. These results suggest that deletion of dacA and dacB increased the permeability of the cell outer membrane, which could explain the enhanced extracellular secretion of recombinant proteins in deletion mutants.
DISCUSSION
In this work, two d,d-carboxypeptidase genes, dacA and dacB, were successfully deleted from the E. coli BL21 parent strain. Cell growth was not significantly inhibited by deleting dacA. Similarly, Broome-Smith constructed a double-deletion E. coli mutant lacking dacA and dacC, and the growth rate was comparable to that of the wild-type strain (26). However, although d,d-carboxypeptidases DacA and DacB are not essential for bacterial survival (27), deletion of dacB in the present study did result in a lower growth rate compared to that of control cells. The BL21 ΔdacA ΔdacB double-deletion mutant grew faster than the dacB single-deletion strain. There are at least four LMW PBPs with d,d-carboxypeptidase activity in E. coli, namely, DacA, DacB, DacC, and DacD, but there are at least two LMW PBPs (DacB and PBP7) with d,d-endopeptidase activity. The roles of LMW PBPs are less clear than those of HMW PBPs (19). It has been reported that DacA, DacC, and DacD are monofunctional d,d-carboxypeptidases, and DacB is a bifunctional enzyme (endopeptidase and d,d-carboxypeptidase) (19, 24, 27, 28). PBP7 appears to be a d,d-endopeptidase with no known d,d-carboxypeptidase activity (19) for one of the DacB isozymes. E. coli is a Gram-negative bacterium encoding multiple d,d-carboxypeptidase isozymes (24), including DacA, DacB, DacC, and DacD. It was presumed that the d,d-carboxypeptidase activity of DacA d,d-carboxypeptidase isozymes and the endopeptidase activity of DacB d,d-endopeptidase isozymes in the double-deletion mutant might be in better equilibrium for cell growth in LB medium compared with that in the dacB single-deletion mutant, since the double-deletion mutant grew faster.
Synthesis of peptidoglycan includes three overall stages (23); first, soluble, activated nucleotide precursors are synthesized in the cytoplasm (22); second, the lipid-anchored disaccharide-pentapeptide monomer subunit is formed by assembling nucleotide precursors (after flipping across the membrane) with undecaprenyl phosphate (29, 30); and third, glycan chains are inserted into the peptidoglycan sacculus (23). The d,d-carboxypeptidases DacA and DacB play important roles in peptidoglycan synthesis by removing excess pentapeptide donors in newly synthesized peptidoglycan (23). Deletion of dacA and dacB increased the intracellular soluble peptidoglycan concentration in E. coli at 8 h, especially in the dacA single-deletion mutant. Soluble peptidoglycan in the double-deletion mutant accumulated to a greater extent than in the dacB single-deletion strain. It was therefore presumed that deletion of dacA and dacB affected the synthesis and metabolic stability of the peptidoglycan network in E. coli, especially single deletion of dacA. DacB possesses endopeptidase activity and can degrade subunits crosslinked to monomeric muropeptides, the lifetimes of which may be prolonged after deleting DacB (24). d,d-Carboxypeptidases can remove the terminal d-alanine from pentapeptide side chains and excess pentapeptide donors in newly synthesized peptidoglycan (23, 24). DacA is one of the main d,d-carboxypeptidases in E. coli, and in the absence of DacA, pentapeptide peptidoglycan subunits (muropeptides) accumulate to higher levels than in wild-type strains (31).
In order to investigate the effects of dacA and dacB deletion on cell morphology, FACS analysis was employed in this work. The results of FACS analysis indicated that deleting dacA and dacB affected E. coli cell morphology. Maintenance of cell shape is tightly regulated by the growth of the mesh-like peptidoglycan sacculus between inner and outer membranes, which provides mechanical strength to resist osmotic pressure (23, 32). d,d-Carboxypeptidases are crucial peptidoglycan synthases that help to maintain bacterial cell shape. Huang et al. (2008) found that deleting d,d-carboxypeptidase genes damaged the cell wall in Gram-negative bacteria (33), and Nelson and Young (2000) reported that loss of d,d-carboxypeptidase DacA altered the cell diameter and contour of E. coli cells and caused morphological defects (34).
In order to further analyze the effects of deleting dacA and dacB on E. coli cell morphology and perturbation of the cell wall peptidoglycan network structure, transmission electron microscopy (TEM) was also employed. Compared with control cells, the deletion mutants adopted a more irregular shape, and local transparent bulges at the poles of the dacB deletion strain became evident. Meanwhile, transparent globular swelling occurred with the double-deletion mutant. The d,d-carboxypeptidases DacA and DacB are involved in maintaining cell morphology (17). In Gram-negative bacteria, the peptidoglycan network is responsible for cell wall tensile strength, and it is necessary to maintain cell robustness in the face of intracellular stress (19, 35). The rigid structure of native E. coli cells was not maintained in the dacA or dacB deletion mutants, indicating perturbation and destruction of the cell wall peptidoglycan network.
The extracellular GFP fluorescence intensity of deletion mutants BL21 ΔdacA pETDuet-gfp, BL21 ΔdacB pETDuet-gfp, and BL21 ΔdacA ΔdacB pETDuet-gfp was increased by 2.1-, 2.2-, and 2.7-fold, respectively, compared with that of the control cells. Extracellular secretion of recombinant FGFR2 and E4 in deletion mutants was also improved compared with that of control cells. Similarly, the extracellular amylase activity of single-deletion mutants BL21 ΔdacA pETDuet-amyk and BL21 ΔdacB pETDuet-amyk was significantly increased by 2.5- and 3.1-fold, respectively, compared with that of control cells. However, the extracellular amylase activity of the BL21 ΔdacA ΔdacB pETDuet-amyk double-deletion mutant was only increased by 1.1-fold from that of control cells, indicating that simultaneous deletion of both dacA and dacB inhibited extracellular amylase secretion compared with that of single-deletion mutants. Furthermore, the extracellular amylase specific production rate of deletion mutants was increased compared with that of control cells.
The proportion of extracellular enzyme activity relative to total enzyme activity is often used to characterize cell integrity (4). The proportion of extracellular amylase in deletion mutants was also improved compared with that of control cells, further indicating that deletion of dacA and dacB improved extracellular secretion of amylase in E. coli. However, it was presumed that double deletion (both dacA and dacB) might inhibit production of amylase in E. coli. This amylase does not have an N-terminal signal peptide targeting translocation across both the inner and outer cell membranes into the extracellular space under osmotic stress and translation stress conditions (36). The temporal expression of DacA and DacB in native strains occurs during log phase and early log phase (19). In the absence of DacA and DacB, the amount of specific crosslinked products might increase, and their lifetimes might be prolonged (24). Less efficient activation of PBPs in double-deletion mutant might lead to high peptidoglycan density during the early phase (e.g., early log phase) of cell growth (23), resulting in smaller peptidoglycan pores than in control cells, which could inhibit extracellular secretion of the HMW recombinant amylase during the early phase. This hypothesis is consistent with the results (shown in Fig. 6C) that the specific production rate of extracellular amylase in the double-deletion mutant was low during the early phase (<16 h). A decrease in extracellular secretion of recombinant amylase during the early phase presumably inhibited the production of amylase in the double-deletion mutant.
Meanwhile, the extracellular distribution of α-galactosidase, an intracellular enzyme in E. coli, was significantly improved by deleting dacA and dacB. The extracellular α-galactosidase activity of mutant strains BL21 ΔdacA pETDuet, BL21 ΔdacB pETDuet, and BL21 ΔdacA ΔdacB pETDuet was increased by 2.0-, 2.2-, and 3.1-fold, respectively, compared with that of control cells, suggesting that double deletion (dacA and dacB) more significantly increased extracellular α-galactosidase activity than single deletion (dacA or dacB). It was therefore presumed that deleting dacA and dacB destroyed the integrity and stability of the peptidoglycan network to favor the extracellular secretion of proteins in E. coli.
d,d-Carboxypeptidases DacA and DacB cleave the terminal d-alanine of the pentapeptide side chains (l-Ala–d-iso-Glu–m-Dap–d-Ala–d-Ala for E. coli; m-Dap, meso-diaminopimelic acid) of murein components of the cell wall peptidoglycan sacculus (18, 20, 27). The meshlike peptidoglycan sacculus consists of glycan chains crosslinked by short peptides with synthases (23). DacA is membrane-anchored and presumably acts to trim peptidoglycan (17). DacB has an active site that differs slightly from those of other d,d-carboxypeptidases (37, 38). E. coli DacB is not directly related to DacBs from Gram-positive bacteria, which are functionally equivalent to DacA from E. coli (17). DacB possesses both d,d-endopeptidase and d,d-carboxypeptidase activities and is responsible for breaking crosslinks to facilitate insertion of new glycan chains (39). Here, deleting dacA and dacB disrupted the cell wall integrity of E. coli, resulting in enhanced extracellular protein secretion. Similarly, Horne et al. (1977) found that inhibition of peptidoglycan synthesis in Staphylococcus epidermidis and Bacillus subtilis resulted in the secretion of lipids into the extracellular medium (40), and Shin and Chen (2008) improved extracellular recombinant protein secretion in E. coli by deleting the lpp gene (4).
Deletion of dacA and dacB caused an increase in fluorescence intensity of NPN bound to E. coli cells, especially to cells of the double-deletion mutant. The fluorescence intensity of NPN is increased in a nonpolar or hydrophobic environment (25). The stress-bearing peptidoglycan sacculus of bacterial cells provides mechanical strength to resist osmotic pressure (23, 32). Thus, deletion of dacA and dacB could disrupt the peptidoglycan network, resulting in an incomplete cell wall and increasing the permeability of the cell outer membrane, especially in the case of the double-deletion mutant. This could explain the enhanced extracellular secretion of recombinant proteins in dacA and dacB deletion strains compared with that of the parent strain.
MATERIALS AND METHODS
Strains and vectors.
Strains, plasmids, and primers used in this work are listed in Tables 1 and 2. E. coli JM109 was used as the host for plasmid construction, and E. coli BL21 was used as the parent strain from which mutants were derived. The pMD19-T vector was used for TA cloning. The kanamycin resistance gene was cloned from plasmid pKD13. The temperature-sensitive pKD46 helper plasmid was used to express Red recombinases, and the helper plasmid pCP20 was used to express FLP recombinase to delete the resistance gene. The pETDuet plasmid was used to express GFP (GenBank accession no. U70496) and amylase AmyK (GenBank accession no. KF751392).
TABLE 1.
Plasmids and strains used in this work
| Plasmid or strain | Relevant genotype and/or characteristic(s) | Source or reference |
|---|---|---|
| Plasmids | ||
| pMD19-T vector | TA cloning | TaKaRa |
| pKD13 | R6KY ori, Kanr, Ampr, Cmr | CGSC |
| pKD46 | Ampr, helper plasmid | CGSC |
| pCP20 | Ampr, Cmr, helper plasmid | CGSC |
| pETDuet | T7 promoters, pBR322 ori, Ampr | Novagen |
| pET28a | T7 promoters, pBR322 ori, Kanr | Novagen |
| pETDuet-gfp | pETDuet derivate with gfp cloned | This work |
| pET28a-amy | pET28a derivate with amy cloned | This work |
| Gene knockout cassettes | ||
| ΔdacA::kan | Kanr, knockout of gene dacA | This work |
| ΔdacA::kan′ | Kanr, knockout of gene dacA of BL21 ΔdacB to obtain double deletion mutant | This work |
| ΔdacB::kan | Kanr, knockout of gene dacB | This work |
| Strains | ||
| E. coli JM109 | Cloning host | Novagen |
| E. coli BL21 | Wild-type E. coli BL21(DE3) | Novagen |
| BL21-pETDuet | E. coli BL21 with plasmid pETDuet | This work |
| BL21-pETDuet-gfp | E. coli BL21 with plasmid pETDuet-gfp | This work |
| BL21-pETDuet-amy | E. coli BL21 with plasmid pETDuet-amy | This work |
| BL21-pKD46 | E. coli BL21(DE3) derivate, including plasmid pKD46, Ampr | This work |
| BL21 ΔdacA::kan pKD46 | BL21-pKD46 derivate, deleting dacA | This work |
| BL21 ΔdacB::kan pKD46 | BL21-pKD46 derivate, deleting dacB | This work |
| BL21 ΔdacA ΔdacB::kan pKD46 | BL21 ΔdacB pKD46 derivate, deleting ΔdacA ΔdacB | This work |
| BL21 ΔdacA::kan | BL21 ΔdacA::kan pKD46 derivate, deleting plasmid pKD46 | This work |
| BL21 ΔdacB::kan | BL21 ΔdacB::kan pKD46 derivate, deleting plasmid pKD46 | This work |
| BL21 ΔdacA ΔdacB::kan | BL21 ΔdacA ΔdacB::kan pKD46 derivate, deleting plasmid pKD46 | This work |
| BL21 ΔdacA | BL21 ΔdacA::kan derivate, deleting plasmid pKD46 and Kanr | This work |
| BL21 ΔdacB | BL21 ΔdacB::kan derivate, deleting plasmid pKD46 and Kanr | This work |
| BL21 ΔdacA ΔdacB | BL21 ΔdacA ΔdacB::kan derivate, deleting plasmid pKD46 and Kanr | This work |
| BL21 ΔdacA pETDuet | BL21 ΔdacA derivate with plasmid pETDuet | This work |
| BL21 ΔdacB pETDuet | BL21 ΔdacB derivate with plasmid pETDuet | This work |
| BL21 ΔdacA ΔdacB pETDuet | BL21 ΔdacA ΔdacB derivate with plasmid pETDuet | This work |
| BL21 ΔdacA pETDuet-gfp | BL21 ΔdacA derivate with plasmid pETDuet-gfp | This work |
| BL21 ΔdacB pETDuet-gfp | BL21 ΔdacB derivate with plasmid pETDuet-gfp | This work |
| BL21 ΔdacA pETDuet-amy | BL21 ΔdacA derivate with plasmid pETDuet-amy | This work |
| BL21 ΔdacB pETDuet-amy | BL21 ΔdacB derivate with plasmid pETDuet-amy | This work |
| BL21 ΔdacA ΔdacB pETDuet-gfp | BL21 ΔdacA ΔdacB derivate with plasmid pETDuet-gfp | This work |
| BL21 ΔdacA ΔdacB pETDuet-amy | BL21 ΔdacA ΔdacB derivate with plasmid pETDuet-amy | This work |
TABLE 2.
Nucleotide sequences of primers
| Oligonucleotide primer or purposea | Sequence (5′ to 3′)b |
|---|---|
| Plasmid construction | |
| GFP-FW | CGGAATTCATGAGTAAAGGAGAAGAACTTTTC |
| GFP-RV | GAAGATCTTTATTTGTATAGTTCATCCATGC |
| Amy-FW | CCGGAATTCATGAGCGAGCTGCCGCAAATC |
| Amy-RV | CCGCTCGAGTTAAAAACCGCCATTGAAGGACG |
| Gene knockout | |
| ΔdacA-FW | GGCTCTTTGCACAGCCTTTATCTCTGCTGCACATGCCGATGACCTGAATAGTGTAGGCTGGAGCTGCTTC |
| ΔdacA-RV | CGAAGAAGTTACCTTCCGGGATTTCTTGCAGTACAACCAACGGGCGTTGTATTCCGGGGATCCGTCGACC |
| ΔdacB-FW | GATTACCACAGTCAGCAGATGGCGCAGCCCGCCAGTACGCAGAAAGTGATGTGTAGGCTGGAGCTGCTTC |
| ΔdacB-RV | CATCCACGCCCGCCTGATGCAGACCTGCACGGTACTGCAAAGAGCCGTCAATTCCGGGGATCCGTCGACC |
| ΔdacA′-1-FWc | GCTCTTTGCACAGCCTTTATCT |
| ΔdacA′-1-RV | GAAGCAGCTCCAGCCTACACGAGGAACATCAGCGAAGAACC |
| ΔdacA′-2-FW | GGTTCTTCGCTGATGTTCCTCGTGTAGGCTGGAGCTGCTTC |
| ΔdacA′-2-RV | CAGTCGCAGAAGCAACAAGGATTCCGGGGATCCGTCGACC |
| ΔdacA′-3-FW | GGTCGACGGATCCCCGGAATCCTTGTTGCTTCTGCGACTG |
| ΔdacA′-3-RV | GTTACCTTCCGGGATTTCTTG |
FW, forward primer; RV, reverse primer.
Italicized letters represent the restriction enzyme sites. Underlined letters represent homologous sequences used for gene knockout.
Primers ΔdacA′-1 through ΔdacA′-3 were used to delete dacA of BL21 ΔdacB, and were redesigned in order to improve the efficiency of deletion. ΔdacA′-1, ΔdacA′-3, and ΔdacA′-2 were used to amplify forward and reverse homologous sequences and the Kanr gene.
Gene deletion.
The Red homologous recombination system was used to delete d,d-carboxypeptidase genes dacA and dacB in E. coli BL21 (41). Gene knockout cassettes (ΔdacA::kan and ΔdacB::kan), flanked by Flp recombination target (FRT) sites and homologous arms of target genes, were constructed by PCR amplification, using plasmid pKD13 as the template and the primers listed in Table 2. PrimeSTAR HS DNA polymerase (TaKaRa, Dalian, China) was used for PCR, following the standard procedure of the PrimeSTAR HS DNA polymerase kit. Plasmid pKD46 containing exo, bet, and gam genes from λ bacteriophage was used for homologous recombination of gene knockout cassettes in the E. coli BL21 genome. Gene knockout cassettes were electroporated into E. coli BL21 cells harboring plasmid pKD46 expressing Red recombinases (see supplemental material). Verified plasmids were introduced into competent E. coli cells by the CaCl2 method (see supplemental material). Positive clones were selected using ampicillin and kanamycin antibiotics and confirmed by PCR analysis. The temperature-sensitive plasmid pKD46 was removed by overnight growth at 37°C, and the kanamycin resistance gene was subsequently removed from the chromosome with FLP recombinase from plasmid pCP20.
Construction of recombinant plasmids.
The gfp gene was amplified by PCR with primers GFP-FW and GFP-RV (Table 2). Recombinant plasmid pUC57-gfp containing the gfp gene (synthesized by Sangon Biotech Co., Ltd.) was used as a PCR template. Conditions for PCR were as follows: 94°C for 4 min, followed by 30 circles at 98°C for 10 s, 55°C for 15 s, 72°C for 48 s, and a final extension at 72°C for 10 min. The gfp sequence was ligated into plasmid pETDuet between the EcoRI and BglII restriction enzyme sites to construct pETDuet-gfp. The amy gene was amplified by PCR with primers Amy-FW and Amy-RV (Table 2). Recombinant plasmid pUC57-amy, harboring the amy gene synthesized by Sangon Biotech Co., Ltd., was used as a PCR template. Conditions for PCR were as follows: 94°C for 4 min, followed by 30 cycles at 98°C for 10 s, 61°C for 15 s, 72°C for 96 s, and a final elongation at 72°C for 10 min. The amy sequence was ligated into plasmid pETDuet between the EcoRI and XhoI sites to construct pETDuet-amy. Verified plasmids were introduced into competent E. coli cells by the CaCl2 method (see supplemental material). PrimeSTAR HS DNA polymerase (TaKaRa) was used for PCR with standard conditions. Recombinant plasmids pETDuet-gfp and pETDuet-amy did not include a signal peptide. DNA ligase solution I was used to ligate amplified protein-encoding genes into plasmids. All constructed plasmids were verified by restriction enzyme analysis and DNA sequencing.
Media and culture conditions.
Luria-Bertani (LB) medium comprising 5 g/liter yeast extract, 10 g/liter tryptone, and 10 g/liter NaCl was used to cultivate E. coli JM109 and E. coli BL21 cells. Terrific broth (TB) medium was comprised of 24 g/liter yeast extract, 10 g/liter tryptone, 4 g/liter glycerol, 2.31 g/liter KH2PO4, and 12.54 g/liter K2HPO4·H2O. Ampicillin and kanamycin were used at final concentrations of 50 and 30 μg/ml, respectively. After culturing recombinant E. coli BL21 cells for 8 h in LB medium at 37°C, an aliquot was inoculated into TB medium to 1% (vol/vol) in a shake flask (25 ml/250 ml) and cultured at 37°C to an optical density at 600 nm (A600) of 0.8. Protein expression was induced with 1 mM isopropyl β-d-thiogalactoside (IPTG) at 25°C.
Cell density determination.
E. coli culture broth (5 ml) was centrifuged at 1.0 × 104 × g for 10 min at 4°C to determine the dry cell weight (DCW). Harvested cells were washed with 10 mM phosphate-buffered saline (PBS; Na2HPO4, NaH2PO4, and NaCl [pH 7.4]). After washing, cells were centrifuged at 1.0 × 104 × g for 10 min at 4°C and dried to a constant weight at 105°C for 2 h.
Intracellular and extracellular sample preparation.
E. coli cultures were centrifuged at 1.0 × 104 × g for 10 min at 4°C. The supernatant was used for extracellular GFP concentration determination and enzyme activity assays. Cells were harvested to assay the outer membrane permeability, intracellular enzyme activity, GFP concentration, and soluble peptidoglycan concentration. Harvested cells were washed, resuspended in 10 mM PBS, and lysed using a JXFSTPRP automatic sample rapid grinding machine (Shanghai Jingxin Industrial Development Co., Ltd., Shanghai, China) at 70 Hz for 11 min. Lysed cells were centrifuged at 1.5 × 104 × g for 15 min at 4°C, and the supernatant was used for intracellular enzyme activity, GFP concentration, and soluble peptidoglycan concentration assays.
Glucosamine concentration assay.
Glucosamine was used to generate a standard curve from known concentrations of 0, 5.0, 10.0, 15.0, and 20.0 mg/liter (wt/vol). Glucosamine and acetylacetone solutions (1 ml) were mixed and boiled for 25 min, and para-dimethylaminobenzaldehyde (1 ml) and absolute ethyl alcohol (1 ml) were added and incubated at 20°C for 1 h. The A440 was determined. E. coli cell walls were disrupted using five freeze-thaw cycles and ultrasonication for 10 min, and lysed cells were centrifuged at 1.0 × 104 × g for 20 min. The supernatant was freeze dried, and 2 mg was dissolved in 6 M hydrochloric acid (1.5 ml) and boiled for 1 h. The solution was cooled and neutralized with NaOH, and distilled water was added to 10 ml. The glucosamine concentration of the solution was measured based on the glucosamine standard curve.
Fluorescence-activated cell sorting (FACS) assay.
Recombinant E. coli cells were cultured in TB medium at 37°C in a shake flask (25 ml/250 ml). When the optical density at 600 nm (OD600) reached 0.8, protein expression was induced by 1 mM IPTG and culturing continued at 25°C for 8 h. E. coli cells (30 ml) were collected by centrifugation at 1.0 × 104 × g for 10 min at 4°C. Harvested cells were washed and resuspended in 30 ml 10 mM PBS. The solution was diluted to an OD600 of 1.6 × 10−2, and a FACSCalibur Flow Cytometer (BD Accuri C6; Becton Dickinson, NJ, USA) was used for cell counting.
Transmission electron microscopy (TEM) assay.
Recombinant E. coli cells were cultured in LB medium at 200 rpm and 37°C for 8 h in a shake flask (25 ml/250 ml). A 20-μl aliquot of the culture was diluted and spread on the surface of an LB medium plate, and expression was induced by 1 mM IPTG for 24 h. A Hitachi H-7650 (Hitachi, Tokyo, Japan) was used for TEM analysis.
GFP assay.
Recombinant strains were cultured in LB medium at 37°C for 8 h, and 1.0% (vol/vol) was inoculated into TB medium in a shake flask (25 ml/250 ml) for expression of recombinant GFP. When the OD600 reached 0.8, recombinant GFP was induced by 1 mM IPTG, and culturing was continued at 25°C for 4 h. A Cytation 3 multimode microplate reader (BioTek Instruments, Inc., Winooski, VT) and a 96-well plate were used to measure the GFP fluorescence intensity. Excitation and absorption wavelengths were 488 and 533 nm, respectively.
Enzyme activity assay.
Amylase activity was determined by a modified method based on measuring reducing sugars during the hydrolysis of soluble starch (1). One unit (U) of amylase was defined as the amount of enzyme required to release 1 μmol of reducing sugar (glucose) from starch per min at 50°C and pH 9.5. The reaction mixture contained 28 mM glycine-NaOH buffer (pH 9.5) and 7.4 g/liter (wt/vol) soluble starch preheated at 50°C for 5 min. After adding the enzyme solution to the preheated mixture, the sample (1.35 ml) was incubated at 50°C for an additional 5 min. Reducing sugars released were measured using a modified dinitrosalicylic acid (DNS) method (42). The DNS solution consisted of 6.5 g/liter (wt/vol) 3,5-dinitrosalicylic acid and 45.0 g/liter (wt/vol) glycerin. Glucose standard concentrations were 0, 1.1, 2.2, 3.3, 4.4, and 5.5 mM. The mixture containing 1.0 ml DNS solution and 1.0 ml reaction solution was boiled for 15 min and cooled in ice water. Deionized water was added to the mixture until the total volume reached 10 ml, and the A540 was determined using a BioTek Cytation 3 microplate reader.
One unit of α-galactosidase activity was defined as the amount of enzyme required to liberate 1 μmol of paranitrophenol per min under the conditions described. A 1.0% (vol/vol) sample of each of the recombinant strains cultured in LB medium at 37°C for 8 h was inoculated into TB medium in a shake flask (25 ml/250 ml). When the OD600 reached 0.8, 1 mM IPTG was added and strains were cultured at 25°C for a further 4 h. The α-galactosidase reaction mixture contained 100 μl enzyme solution, 50 μl o-nitrophenyl β-d-galactopyranoside (10 mM, wt/vol), and 50 μl citrate buffer (pH 5.8; 100 mM [wt/vol]). Reaction mixtures were incubated at 45°C for 15 min, and 3 ml of 0.25 mM (wt/vol) NaCO3 solution was added to stop the reaction. The absorbance at 400 nm was measured and compared with a standard curve made using 0.2, 0.4, 0.6, 0.8, and 1.0 mM paranitrophenol standards (Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China) prepared in 100 mM citrate buffer (pH 5.8).
SDS-PAGE assay.
The effect of deleting dacA and dacB genes on extracellular proteins secretion were also investigated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Molecular weight protein markers were purchased from TaKaRa. The cell density (OD600) of the fermentation broth was diluted to 5.0 with 10 mM PBS. After centrifugation at 1.0 × 104 × g and 4°C for 10 min, equal volumes of supernatants containing extracellular proteins were separated by SDS-PAGE. Samples of 8 μl were mixed with 2 μl of 5× sample buffer (Beyotime Biotech Co., Ltd., Shanghai, China) and mixtures were incubated at 100°C for 10 min. SDS-PAGE was performed with a 12% separating gel, a 5% stacking gel, and a Mini-PROTEAN Tetra electrophoresis tank (Bio-Rad, California, USA). Gels were stained using Coomassie brilliant blue R250 (1.2 g/liter) in 10% (vol/vol) acetic acid and 45% (vol/vol) methanol at 25°C for 2 h. Stained gels were destained with destaining solution consisting of 10% (vol/vol) acetic acid and 45% (vol/vol) methanol at 25°C for 10 h.
Cell outer membrane permeability assay.
N-Phenyl-α-naphthylamine (NPN) can be used to analyze outer membrane permeability (25). Here, 100 mM NPN (20 μl, Shanghai Aladdin Bio-Chem Technology Co., Ltd.) was mixed with 200 μl of cell suspension (OD600 = 5.0 × 10−1). The fluorescence intensity of the mixture was immediately determined using a BioTek Cytation 3 microplate reader. The intensity of the fluorescence emitted was measured at excitation and absorption wavelengths of 350 nm and 420 nm, respectively.
Statistical analysis.
All experiments were independently performed at least three times. Data are expressed as means ± standard deviation (SD). Statistical analyses were performed using the Student t test, and a P value < 0.05 was considered statistically significant. The F test was conducted first to identify overall significant differences, and, if those were detected, the t test was used to determine differences between mutants and the parent strain. Once the t value and degrees of freedom are determined, a P value can be ascribed using the table of values from the Student t test distribution (see https://en.wikipedia.org/wiki/Student%27s_t-distribution#Table_of_selected_values).
Supplementary Material
ACKNOWLEDGMENTS
This work, including the efforts of H.Y., X.L., J.H., Y.C., W.S., and L.L., was funded by the National Natural Science Foundation of China (grant 21406089), the Natural Science Foundation of Jiangsu Province (grant BK20140152), the Open Project Program of the Key Laboratory of Industrial Biotechnology, Ministry of Education, China (grant KLIB-KF201509), the Open Project Program of the Key Laboratory of Carbohydrate Chemistry and Biotechnology, Ministry of Education, China (grants KLCCB-KF201607 and KLCCB-KF201802), and the 111 Project (grant 111-2-06).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01382-18.
REFERENCES
- 1.Ma YF, Shen W, Chen XZ, Liu L, Zhou ZM, Xu F, Yang HQ. 2016. Significantly enhancing recombinant alkaline amylase production in Bacillus subtilis by integration of a novel mutagenesis-screening strategy with systems-level fermentation optimization. J Biol Eng 10:13. doi: 10.1186/s13036-016-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mergulhao F, Summers D, Monteiro G. 2005. Recombinant protein secretion in Escherichia coli. Biotechnol Adv 23:177–202. doi: 10.1016/j.biotechadv.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman S. 1996. Proteases and their targets in Escherichia coli. Annu Rev Genet 30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- 4.Shin HD, Chen RR. 2008. Extracellular recombinant protein production from an Escherichia coli lpp deletion mutant. Biotechnol Bioeng 101:1288–1296. doi: 10.1002/bit.22013. [DOI] [PubMed] [Google Scholar]
- 5.Choi J, Lee S. 2004. Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol 64:625–635. doi: 10.1007/s00253-004-1559-9. [DOI] [PubMed] [Google Scholar]
- 6.Shokri A, Sandén A, Larsson G. 2003. Cell and process design for targeting of recombinant protein into the culture medium of Escherichia coli. Appl Microbiol Biotechnol 60:654–664. doi: 10.1007/s00253-002-1156-8. [DOI] [PubMed] [Google Scholar]
- 7.Sauvonnet N, Vignon G, Pugsley AP, Gounon P. 2000. Pilus formation and protein secretion by the same machinery in Escherichia coli. EMBO J 19:2221–2228. doi: 10.1093/emboj/19.10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stathopoulos C, Hendrixson DR, Thanassi DG, Hultgren SJ, St Geme JW, Curtiss R. 2000. Secretion of virulence determinants by the general secretory pathway in Gram-negative pathogens: an evolving story. Microbes Infect 2:1061–1072. doi: 10.1016/S1286-4579(00)01260-0. [DOI] [PubMed] [Google Scholar]
- 9.Bao RM, Yang HM, Yu CM, Zhang WF, Tang JB. 2016. An efficient protocol to enhance the extracellular production of recombinant protein from Escherichia coli by the synergistic effects of sucrose, glycine, and Triton X-100. Protein Expr Purif 126:9–15. doi: 10.1016/j.pep.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Lee M, Hesek D, Llarrull LI, Lastochkin E, Pi H, Boggess B, Mobashery S. 2013. Reactions of all Escherichia coli lytic transglycosylases with bacterial cell wall. J Am Chem Soc 135:3311–3314. doi: 10.1021/ja309036q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoischen C, Fritsche C, Gumpert J, Westermann M, Gura K, Fahnert B. 2002. Novel bacterial membrane surface display system using cell wall-less L-forms of Proteus mirabilis and Escherichia coli. Appl Environ Microbiol 68:525–531. doi: 10.1128/AEM.68.2.525-531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gumpert J, Hoischen C. 1998. Use of cell wall-less bacteria (L-forms) for efficient expression and secretion of heterologous gene products. Curr Opin Biotechnol 9:506–509. doi: 10.1016/S0958-1669(98)80037-2. [DOI] [PubMed] [Google Scholar]
- 13.Taubeneck U. 1962. Susceptibility of Proteus mirabilis and its stable L-forms to erythromycin and other macrolides. Nature 196:195–196. [DOI] [PubMed] [Google Scholar]
- 14.Kujau M, Hoischen C, Riesenberg D, Gumpert J. 1998. Expression and secretion of functional miniantibodies McPC603scFvDhlx in cell-wall-less L-form strains of Proteus mirabilis and Escherichia coli: a comparison of the synthesis capacities of L-form strains with an E. coli producer strain. Appl Microbiol Biotechnol 49:51–58. doi: 10.1007/s002530051136. [DOI] [PubMed] [Google Scholar]
- 15.Ray MV, Meenan CP, Consalvo AP, Smith CA, Parton DP, Sturmer AM, Shields PP, Mehta NM. 2002. Production of salmon calcitonin by direct expression of a glycine-extended precursor in Escherichia coli. Protein Expr Purif 26:249–259. doi: 10.1016/S1046-5928(02)00523-5. [DOI] [PubMed] [Google Scholar]
- 16.Denome SA, Elf PK, Henderson TA, Nelson DE, Young KD. 1999. Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J Bacteriol 181:3981–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishida H, Unzai S, Roper DI, Lloyd A, Park SY, Tame JR. 2006. Crystal structure of penicillin binding protein 4 (dacB) from Escherichia coli, both in the native form and covalently linked to various antibiotics. Biochem 45:783–792. doi: 10.1021/bi051533t. [DOI] [PubMed] [Google Scholar]
- 18.Baquero MR, Ayala JA, Moreno F. 1996. dacD, an Escherichia coli gene encoding a novel penicillin-binding protein (PBP6b) with DD-carboxypeptidase activity. J Bacteriol 178:7106–7111. doi: 10.1128/jb.178.24.7106-7111.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh AS, Chowdhury C, Nelson DE. 2008. Physiological functions of d-alanine carboxypeptidases in Escherichia coli. Trends Microbiol 16:309–317. doi: 10.1016/j.tim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Egan AJ, biboy J, van 't Veer I, Breukink E, Vollmer W. 2015. Activities and regulation of peptidoglycan synthases. Philos Trans R Soc Lond B Biol Sci 370:20150031. doi: 10.1098/rstb.2015.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vollmer W, Blanot D, de Pedro MA. 2008. Peptidoglycan structure and architecture. FEMS Microbiol Rev 32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 22.Barreteau H, Kovač A, Boniface A, Sova M, Gobec S, Blanot D. 2008. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol Rev 32:168–207. doi: 10.1111/j.1574-6976.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 23.Typas A, Banzhaf M, Gross CA, Vollmer W. 2011. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol 10:123–136. doi: 10.1038/nrmicro2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meberg BM, Paulson AL, Priyadarshini R, Young KD. 2004. Endopeptidase penicillin-binding proteins 4 and 7 play auxiliary roles in determining uniform morphology of Escherichia coli. J Bacteriol 186:8326–8336. doi: 10.1128/JB.186.24.8326-8336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loh B, Grant C, Hancock R. 1984. Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother 26:546–551. doi: 10.1128/AAC.26.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broome-Smith JK. 1985. Construction of a mutant of Escherichia coli that has deletions of both the penicillin-binding protein 5 and 6 genes. J Gen Microbiol 131:2115–2118. [DOI] [PubMed] [Google Scholar]
- 27.Nelson DE, Young KD. 2001. Contributions of PBP 5 and dd-carboxypeptidase penicillin binding proteins to maintenance of cell shape in Escherichia coli. J Bacteriol 183:3055–3064. doi: 10.1128/JB.183.10.3055-3064.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. 2008. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol Rev 32:234–258. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 29.Bouhss A, Trunkfield AE, Bugg TD, Mengin-Lecreulx D. 2008. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol Rev 32:208–233. doi: 10.1111/j.1574-6976.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- 30.Mohammadi T, Van Dam V, Sijbrandi R, Vernet T, Zapun A, Bouhss A, Diepeveen-de Bruin M, Nguyen-Distèche M, de Kruijff B, Breukinka E. 2011. Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane. EMBO J 30:1425–1432. doi: 10.1038/emboj.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kraus W, Holtje J-V. 1987. Two distinct transpeptidation reactions during murein synthesis in Escherichia coli. J Bacteriol 169:3099–3103. doi: 10.1128/jb.169.7.3099-3103.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Typas A, Banzhaf M, van Saparoea BV, Verheul J, Biboy J, Nichols RJ, Zietek M, Beilharz K, Kannenberg K, von Rechenberg M, Breukink E, den Blaauwen T, Gross CA, Vollmer W. 2010. Regulation of peptidoglycan synthesis by outer-membrane proteins. Cell 143:1097–1109. doi: 10.1016/j.cell.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang KC, Mukhopadhyay R, Wen B, Gitai Z, Wingreen NS. 2008. Cell shape and cell-wall organization in Gram-negative bacteria. Proc Natl Acad Sci U S A 105:19282–19287. doi: 10.1073/pnas.0805309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson DE, Young KD. 2000. Penicillin binding protein 5 affects cell diameter, contour, and morphology of Escherichia coli. J Bacteriol 182:1714–1721. doi: 10.1128/JB.182.6.1714-1721.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dmitriev B, Toukach F, Ehlers S. 2005. Towards a comprehensive view of the bacterial cell wall. Trends Microbiol 13:569–574. doi: 10.1016/j.tim.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Morra R, Del Carratore F, Muhamadali H, Horga LG, Halliwell S, Goodacre R, Breitling R, Dixon N. 2018. Translation stress positively regulates MscL-dependent excretion of cytoplasmic proteins. mBio 9:e02118-17. doi: 10.1128/mBio.02118-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakagawa J, Tamaki S, Tomioka S, Matsuhashi M. 1984. Functional biosynthesis of cell wall peptidoglycan by polymorphic bifunctional polypeptides. Penicillin-binding protein 1Bs of Escherichia coli with activities of transglycosylase and transpeptidase. J Biol Chem 259:13937–13946. [PubMed] [Google Scholar]
- 38.Spratt BG, Pardee AB. 1975. Penicillin-binding proteins and cell shape in E. coli. Nature 254:516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- 39.Burman LG, Park JT. 1984. Molecular model for elongation of the murein sacculus of Escherichia coli. Proc Natl Acad Sci U S A 81:1844–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horne D, Hakenbeck R, Tomasz A. 1977. Secretion of lipids induced by inhibition of peptidoglycan synthesis in Streptococci. J Bacteriol 132:704–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang C, Ye L, Gu J, Yang X, Li A, Yu H. 2017. Directed evolution of mandelate racemase by a novel high-throughput screening method. Appl Microbiol Biotechnol 101:1063–1072. doi: 10.1007/s00253-016-7790-3. [DOI] [PubMed] [Google Scholar]
- 42.Voigt CA, Martinez C, Wang ZG, Mayo SL, Arnold FH. 2002. Protein building blocks preserved by recombination. Nat Struct Biol 9:553–558. doi: 10.1038/nsb805. [DOI] [PubMed] [Google Scholar]
- 43.Hoffmann WA, Poorter H. 2002. Avoiding bias in calculations of relative growth rate. Ann Bot 90:37–42. doi: 10.1093/aob/mcf140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.