In 2013, the United Nations Environment Programme convened the Minamata Convention on Mercury, which prohibits trade in mercury-containing products in order to ensure human health. It will be effectuated in 2020; use of low-pressure mercury lamps will be discontinued and a new UV light source selected to replace the conventional technology. In this regard, UVC-LEDs have been developed and the fundamental inactivating effect has been researched. However, a pulsed UVC-LED system has not been studied, because of the difficulty of generating a UVC-LED pulse wave. An optical chopper system that physically divides the light with an adjustable blade, with personalized frequency and duty ratio settings, was introduced for generation of pulsed UVC-LED irradiation. This study elucidated the efficacy of a pulsed UVC-LED system and investigated its enhanced bactericidal effect in mechanism analyses.
KEYWORDS: UV irradiation, pulsed UVC-LED system, light-emitting diodes, optimization, inactivating mechanism
ABSTRACT
UVC light, a strong surface disinfection technology, is used worldwide to ensure not only environmental safety but also food safety. Several drawbacks associated with the use of mercury-containing UV lamps, especially human and environmental health risks, led to the Minamata Convention on Mercury, which prohibits the manufacture and import/export of products containing mercury. Therefore, light-emitting diode (LED)-based UVC irradiation, a new technology that is ecofriendly and represents an effective UV light source, has been researched recently. To date, however, there has been no report describing pulsed UVC-LED irradiation for improvement of inactivation of foodborne pathogens, although much research regarding conventional pulsed xenon lamps has been published. In this investigation, we evaluated the enhanced bactericidal effect of a pulsed UVC-LED system, compared to continuous irradiation, and optimum conditions for maximizing the effect were determined. Also, the differences in inactivation between pulsed and continuous UVC-LED irradiation were determined by inactivation mechanism analyses. The combination of 20-Hz frequency and 50% duty ratio for pulsed UVC-LED irradiation achieved 4- to 5-log-unit reductions of Escherichia coli O157:H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes; this combination showed the greatest bactericidal effect among various treatment conditions using 2 or 5 mJ/cm2. In mechanism assessments, membrane integrity (propidium iodide uptake) was not affected by UVC-LED treatment but membrane potential [bis-(1,3-dibutylbarbituric acid)trimethine oxonol [DiBAC4(3)] accumulation] showed significantly different values when pulsed and continuous treatments were compared. Changes in membrane lipid peroxidation and respiratory enzyme activity were attributed to generation of more reactive oxygen species by pulsed UVC-LED irradiation.
IMPORTANCE In 2013, the United Nations Environment Programme convened the Minamata Convention on Mercury, which prohibits trade in mercury-containing products in order to ensure human health. It will be effectuated in 2020; use of low-pressure mercury lamps will be discontinued and a new UV light source selected to replace the conventional technology. In this regard, UVC-LEDs have been developed and the fundamental inactivating effect has been researched. However, a pulsed UVC-LED system has not been studied, because of the difficulty of generating a UVC-LED pulse wave. An optical chopper system that physically divides the light with an adjustable blade, with personalized frequency and duty ratio settings, was introduced for generation of pulsed UVC-LED irradiation. This study elucidated the efficacy of a pulsed UVC-LED system and investigated its enhanced bactericidal effect in mechanism analyses.
INTRODUCTION
UV irradiation, which is a powerful surface inactivation technology, has been widely used by the food industry and water disinfection utilities. As one type of UV-light-emitting technology, low-pressure mercury lamps have been utilized for many years and have been thoroughly researched for inactivation of pathogenic microorganisms, including bacteria, viruses, and fungi on food (1–3). Based on its disinfecting efficacy, UVC technology was approved by the U.S. Food and Drug Administration (FDA) in 2000 as an effective inactivating method to control microorganisms in food, water, and beverages (4). UVC irradiation has been applied to control microbial contamination in blood transfusion systems, as well as by the food industry (5–7).
In 2013, the Minamata Convention on Mercury, an international agreement to preserve public health and to protect the environment from mercury contamination, was approved by delegates from approximately 140 countries with the United Nations Environment Programme (UNEP) (8). This treaty regulates the manufacture of products containing mercury and the importation/exportation of mercury, to decrease the amount of mercury released into the environment in order to prevent widespread mercury pollution (9). When the Minamata Convention comes into effect in 2020, use of conventional low-pressure mercury UV lamps will be prohibited; therefore, a new alternative UV-emitting technology should be introduced into the industrial field.
A pulsed-light system that emits a wide range of wavelengths from 200 to 1,100 nm is a powerful surface and water disinfection technology (10). Within a short time, a pulsed xenon lamp can deliver intense UV light, resulting in control of pathogenic microorganisms as effective as that achieved with conventional UV lamps (11–16). Especially high intensity results in physical damage by photons and the photothermal effect (17), which contributes to further inactivation of pathogens. However, the pulsed xenon lamp system still has drawbacks, such as excessively increasing temperatures due to emitting wavelengths in the infrared range, resulting in deterioration of foods (18, 19), and the need for a bulky system to control the pulse and cooling systems, leading to difficulty integrating the system into a conventional processing line (17).
Recently, UVC-light-emitting diode (LED) technology has emerged as a strong candidate to substitute for conventional mercury-containing UV lamps because it can compensate for the limitations of conventional UV lamps, with its ability to be integrated into sterilization systems due to its compact size, its robustness in avoiding breakage from external forces, and, above all, its lack of use of mercury, which eliminates human and environmental health problems (20). Shin et al. showed that UVC-LED did not demonstrate temperature-dependent intensity and no warm-up time to maximize the fluence rate was needed; in contrast, low-pressure UV lamp intensity decreased with lower temperatures and an ∼5-min warm-up time was necessary (20). UVC-LED treatment demonstrated superior bactericidal effects on Escherichia coli O157:H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes, compared to low-pressure lamps at the same dosages with adjusted fluence rates (21). Furthermore, effective viral inactivation using human enteric virus surrogates, such as MS2, Qβ, and ΦX174, were researched in batch-type and continuous-type water disinfection systems (22).
Therefore, the objective of this study was a comprehensive investigation of a pulsed UVC-LED system to enhance its bactericidal effects and to optimize factors such as frequency and duty ratio. Also, elucidation of the mechanisms of increased antimicrobial activity with a pulsed UVC-LED system was performed, and the pulsed irradiation system was applied to food samples such as white mushrooms and commercial ready-to-eat sausages, to inactivate foodborne pathogens.
RESULTS
Bactericidal effects by frequency.
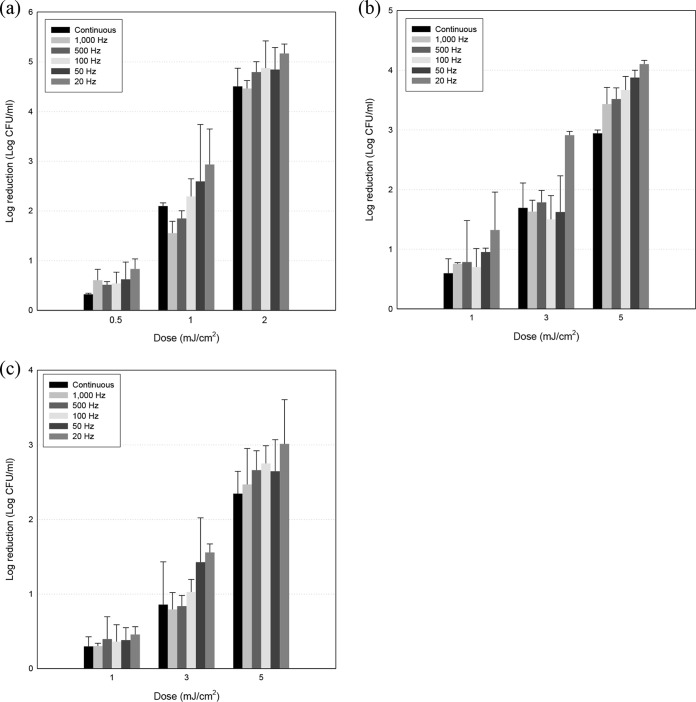
Inactivation of foodborne pathogens at various frequencies is presented in Fig. 1. No reductions were observed for controls (non-UVC-LED-treated groups) and, as treatment dosages increased, greater reductions were induced in all three pathogens, Escherichia coli O157:H7, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes. At the highest UVC-LED dosages, i.e., 2 mJ/cm2 for E. coli O157:H7 and 5 mJ/cm2 for S. Typhimurium and L. monocytogenes, 3- to 5-log-unit reductions were achieved with continuous and pulsed UVC-LED treatments. There was a tendency for greater bactericidal effects with pulsed irradiation, compared to continuous treatment, at 1 and 2 mJ/cm2 for E. coli O157:H7 and 3 and 5 mJ/cm2 for S. Typhimurium and L. monocytogenes, although no significant differences were observed among some treatments (100, 500, and 1,000 Hz). Furthermore, E. coli O157:H7 was much more sensitive to UVC-LED irradiation than were S. Typhimurium and L. monocytogenes, such that a 5-log-unit reduction was achieved with 2 mJ/cm2 for E. coli O157:H7, whereas 3- to 4-log-unit reductions were achieved with 5 mJ/cm2 for S. Typhimurium and L. monocytogenes. Also, no injured cells were generated with continuous or pulsed irradiation, except for some points (Table 1).
FIG 1.
Reductions of E. coli O157:H7 (a), S. Typhimurium (b), and L. monocytogenes (c) cells on selective media (E. coli O157:H7, SMAC agar; S. Typhimurium, XLD agar; L. monocytogenes, OAB agar) after treatment with continuous or pulsed UVC-LED irradiation, with frequencies varying from 20 to 1,000 Hz. Pulsed irradiation was generated using an optical chopper, and the duty ratio was altered with an adjustable chopper blade.
TABLE 1.
Reductions of three pathogens on culture media after treatment with continuous or pulsed UVC-LED irradiation at different frequencies
| Microorganism | Reduction (log CFU/ml)a |
|||||
|---|---|---|---|---|---|---|
| Continuous |
1,000 Hz |
20 Hz |
||||
| Selective | Nonselective | Selective | Nonselective | Selective | Nonselective | |
| E. coli O157:H7 | 4.50 ± 0.37 A | 4.94 ± 0.10 A | 4.46 ± 0.16 A | 5.04 ± 0.04 B | 5.17 ± 0.19 A | 5.10 ± 0.05 A |
| S. Typhimurium | 2.94 ± 0.05 A | 2.84 ± 0.10 A | 3.43 ± 0.28 A | 2.65 ± 0.43 A | 4.10 ± 0.06 A | 3.19 ± 0.37 B |
| L. monocytogenes | 2.35 ± 0.30 A | 1.78 ± 0.10 B | 2.47 ± 0.48 A | 1.77 ± 0.13 A | 3.01 ± 0.59 A | 2.04 ± 0.13 A |
The highest dosages were applied (2 mJ/cm2 for E. coli O157:H7 and 5 mJ/cm2 for S. Typhimurium and L. monocytogenes). Data represent means ± standard deviations from three replications. Values followed by the same uppercase letters within rows for each treatment type are not significantly different.
Bactericidal effects by duty ratio.
Figure 2 demonstrates enhanced bactericidal effects with various duty ratios with steady pulsed UVC-LED irradiation dosages (20 Hz; 1 mJ/cm2 for E. coli O157:H7 and 3 mJ/cm2 for S. Typhimurium and L. monocytogenes) applied to the three pathogens. In contrast to results from frequency-dependent inactivation levels, an enhanced inactivation effect was observed as the duty ratio increased from 10% to 50%. With a 10% duty ratio, inactivation levels for the three pathogens were approximately 2 log units for E. coli O157:H7 and S. Typhimurium and 1 log unit for L. monocytogenes; with a 50% duty ratio, 2.5- to 3-log-unit reductions for E. coli O157:H7 and S. Typhimurium and a 1.5-log-unit reduction for L. monocytogenes occurred. The numerical values for the enhanced bactericidal effects were 41.9, 57.3, and 69.6% for E. coli O157:H7, S. Typhimurium, and L. monocytogenes, respectively. Also, the enhanced bacterial inactivation was log-linear in proportion to increasing duty ratio, so that the treatments achieved approximately 2- to 3-log-unit reductions of E. coli O157:H7 and S. Typhimurium and 1.5-log-unit reductions of L. monocytogenes. Moreover, sublethally injured cells were not produced with continuous or pulsed irradiation (Table 2).
FIG 2.
Reductions of the foodborne pathogens E. coli O157:H7, S. Typhimurium, and L. monocytogenes on selective media after treatment with pulsed UVC-LED irradiation, with duty ratios varying from 10% to 50%. Pulsed irradiation was generated using an optical chopper, and the duty ratio was altered with an adjustable chopper blade.
TABLE 2.
Reductions of three pathogens on culture media after treatment with continuous or pulsed UVC-LED irradiation at different duty ratios
| Microorganism | Reduction (log CFU/ml)a |
|||||
|---|---|---|---|---|---|---|
| Continuous |
10% |
50% |
||||
| Selective | Nonselective | Selective | Nonselective | Selective | Nonselective | |
| E. coli O157:H7 | 2.10 ± 0.06 A | 2.33 ± 0.07 B | 1.91 ± 0.13 A | 2.63 ± 0.13 B | 2.93 ± 0.71 A | 2.80 ± 0.19 A |
| S. Typhimurium | 1.69 ± 0.42 A | 1.35 ± 0.18 A | 1.85 ± 0.28 A | 1.41 ± 0.10 A | 2.91 ± 0.06 A | 2.47 ± 0.48 A |
| L. monocytogenes | 0.86 ± 0.57 A | 0.57 ± 0.24 A | 0.92 ± 0.06 A | 0.96 ± 0.08 A | 1.56 ± 0.11 A | 1.26 ± 0.25 A |
Dosages of 1 mJ/cm2 for E. coli O157:H7 and 3 mJ/cm2 for S. Typhimurium and L. monocytogenes were irradiated onto the media. Data represent means ± standard deviations from three replications. Values followed by the same uppercase letters within rows for each treatment type are not significantly different.
Membrane damage assessments.
Indicators of cell membrane damage, i.e., propidium iodide (PI) uptake and bis-(1,3-dibutylbarbituric acid)trimethine oxonol [DiBAC4(3)] accumulation, are presented in Tables 3 and 4. First, PI uptake values for nontreatment, continuous irradiation, and pulsed UVC-LED irradiation ranged from 90% to 103% for E. coli O157:H7 and from 93% to 101% for L. monocytogenes (Table 3). Among the treatment groups, including nontreated controls, no significant differences in PI uptake proportions were observed for the two foodborne pathogens.
TABLE 3.
PI uptake of E. coli O157:H7 and L. monocytogenes cells suspended in PBS after treatment with continuous or pulsed (1,000 Hz/10% or 20 Hz/50%) UVC-LED irradiation
| Treatment | PI uptake (%)a |
|
|---|---|---|
| E. coli O157:H7 | L. monocytogenes | |
| Control | 100.00 ± 3.63 A | 100.00 ± 1.57 A |
| Continuous | 99.10 ± 5.29 A | 99.29 ± 11.53 A |
| 1,000 Hz/10% | 90.62 ± 5.96 A | 93.11 ± 3.52 A |
| 20 Hz/50% | 103.46 ± 11.20 A | 101.93 ± 5.14 A |
The non-UV-treated groups were designated the controls. Data represent means ± standard deviations from three replications. Values followed by the same uppercase letters within columns for each microorganism are not significantly different.
TABLE 4.
DiBAC4(3) accumulation of E. coli O157:H7 and L. monocytogenes cells suspended in PBS after treatment with continuous or pulsed (1,000 Hz/10% or 20 Hz/50%) UVC-LED irradiation
| Treatment | DiBAC4(3) accumulation (%)a |
|
|---|---|---|
| E. coli O157:H7 | L. monocytogenes | |
| Control | 100.00 ± 0.75 A | 100.00 ± 1.53 A |
| Continuous | 114.30 ± 1.47 B | 105.54 ± 1.06 B |
| 1,000 Hz/10% | 123.02 ± 2.98 C | 115.29 ± 1.76 C |
| 20 Hz/50% | 130.49 ± 2.61 D | 122.24 ± 2.44 D |
The non-UV-treated groups were designated the controls. Data represent means ± standard deviations from three replications. Values followed by the same uppercase letters within columns for each microorganism are not significantly different.
In contrast to PI uptake, DiBAC4(3) accumulation percentages showed statistically significant differences according to UVC-LED treatment types (Table 4). The nontreated controls showed the least DiBAC4(3) accumulation, followed by continuous irradiation and 1,000-Hz/10% pulsed irradiation, and 20-Hz/50% pulsed UVC-LED irradiation showed the highest level of DiBAC4(3) accumulation for both E. coli O157:H7 and L. monocytogenes.
Membrane lipid peroxidation assay.
Levels of thiobarbituric acid-reactive substances (TBARS) (which indicate the level of cell membrane lipid peroxidation) with various UVC-LED treatments are presented in Table 5. Pulsed UVC-LED treatments, including 1,000 Hz/10% and 20 Hz/50%, achieved TBARS accumulation levels of 171% to 323% for E. coli O157:H7 and 1,850% to 2,330% for L. monocytogenes, which were significantly different from values for cells with continuous UVC treatment. The 20-Hz/50% pulsed UVC-LED treatment showed nearly 3- to 23-fold increased TBARS values, compared to continuous UVC-LED treatment for both pathogens.
TABLE 5.
TBARS formation of foodborne pathogen cells suspended in PBS after treatment with continuous or pulsed (1,000 Hz/10% or 20 Hz/50%) UVC-LED irradiation
| Treatment | TBARS formation (%)a |
|
|---|---|---|
| E. coli O157:H7 | L. monocytogenes | |
| Continuous | 100.00 ± 27.01 A | 100.00 ± 4.09 A |
| 1,000 Hz/10% | 171.42 ± 21.86 B | 1,850.76 ± 40.03 B |
| 20 Hz/50% | 323.97 ± 31.89 C | 2,330.27 ± 139.81 C |
The percentage data were obtained by subtracting fluorescence values for untreated cells from those for treated cells, dividing the results by the values for cells treated with continuous UVC-LED irradiation, and expressing those values as percentages (TBARS percentage = [fluorescence value after treatment − fluorescence value for untreated cells]/fluorescence value for continuously treated cells). Data represent means ± standard deviations from three replications. Values followed by the same uppercase letters within columns for each microorganism are not significantly different.
Reactive oxygen species generation.
Table 6 shows different levels of reactive oxygen species (ROS) generation caused by UVC-LED treatment types, i.e., continuous and pulsed UVC irradiation. The values increased with pulsed UVC-LED treatment, compared to continuous treatment, and 20 Hz/50% was significantly different and yielded the highest level of ROS generation among all treatments. For both foodborne pathogens, when the ROS generation value corresponding to continuous treatments was set as a standard, 20-Hz/50% pulsed irradiation yielded approximately 2- to 6-fold increased levels in L. monocytogenes and E. coli O157:H7. The numerical order of increasing ROS generation among the three experimental groups (continuous UVC < 1,000-Hz/10% pulsed UVC < 20-Hz/50% pulsed UVC) was identical to the order of TBARS accumulation increases among treatments.
TABLE 6.
ROS generation by E. coli O157:H7 and L. monocytogenes cells suspended in PBS after treatment with continuous or pulsed (1,000 Hz/10% or 20 Hz/50%) UVC-LED irradiation
| Treatment | ROS generation (%)a |
|
|---|---|---|
| E. coli O157:H7 | L. monocytogenes | |
| Continuous | 100.00 ± 75.56 A | 100.00 ± 34.63 A |
| 1,000 Hz/10% | 265.87 ± 76.05 B | 160.05 ± 22.32 B |
| 20 Hz/50% | 655.07 ± 51.50 C | 269.17 ± 18.38 C |
The percentage data were obtained by subtracting fluorescence values for untreated cells from those for treated cells, dividing the results by the values for cells treated with continuous UVC-LED irradiation, and expressing those values as percentages (ROS percentage = [fluorescence value after treatment − fluorescence value for untreated cells]/fluorescence value for continuously treated cells). Data represent means ± standard deviations from three replications. Values followed by the same uppercase letters within columns for each microorganism are not significantly different.
Succinate-coenzyme Q reductase activity.
Levels of formazan formation induced by the electron-transport-chain-related enzyme succinate-coenzyme Q reductase (SQR) are presented in Table 7. Because nontreated cells maintain SQR activity, the highest level of iodonitrotetrazolium-formazan formation occurs in those cells, and this value was set as a standard. UVC-LED treatment decreased the formazan formation levels by 45% and 85% in E. coli O157:H7 and L. monocytogenes, respectively, and significantly different diminished values were observed with the two pulsed UVC-LED irradiation types, compared to the nontreated control and continuous UVC-LED irradiation.
TABLE 7.
INT-formazan formation by E. coli O157:H7 and L. monocytogenes cells suspended in PBS after treatment with continuous or pulsed (1,000 Hz/10% or 20 Hz/50%) UVC-LED irradiation
| Treatment | Formazan formation (%)a |
|
|---|---|---|
| E. coli O157:H7 | L. monocytogenes | |
| Control | 100.00 ± 2.01 A | 100.00 ± 1.87 A |
| Continuous | 90.12 ± 2.44 B | 98.12 ± 0.90 A |
| 1,000 Hz/10% | 65.18 ± 1.56 C | 93.65 ± 1.59 B |
| 20 Hz/50% | 45.32 ± 2.10 D | 85.37 ± 3.40 C |
The non-UV-treated groups were designated the controls. Data represent means ± standard deviations from three replications. Values followed by the same uppercase letters within columns for each microorganism are not significantly different.
Bactericidal effects of continuous and pulsed UVC-LED treatment of food samples.
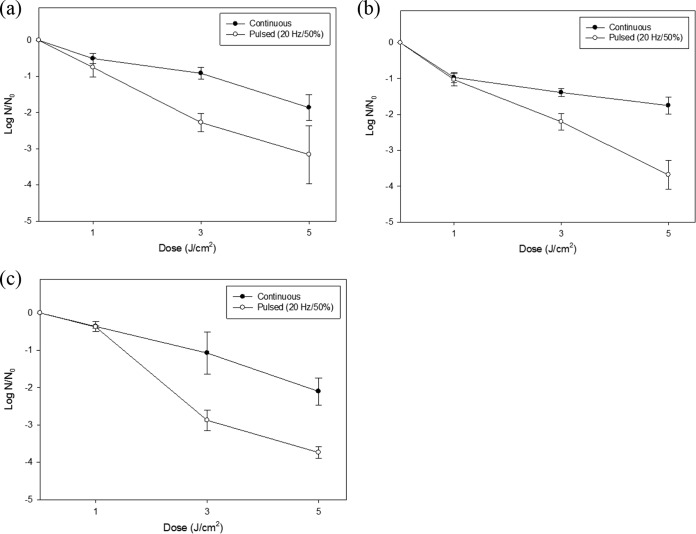
The inactivation of foodborne pathogens, including E. coli O157:H7, S. Typhimurium, and L. monocytogenes, on food surfaces is shown in Fig. 3 and 4. At higher treatment dosages, for both types of food samples, surviving populations of the three foodborne pathogens decreased with a nearly log-linear pattern. Remarkable reductions were observed for ready-to-eat sausages with pulsed irradiation. For E. coli O157:H7, approximately 2-log-unit reductions with continuous UVC-LED irradiation and 3-log-unit reductions with pulsed UVC-LED irradiation were achieved. Similarly, survival of S. Typhimurium decreased by 1.5 log units with continuous treatment and by 4 log units with pulsed UVC-LED treatment. For L. monocytogenes, pulsed UVC-LED treatment produced 4-log-unit reductions but continuous treatment produced 2-log-unit reductions.
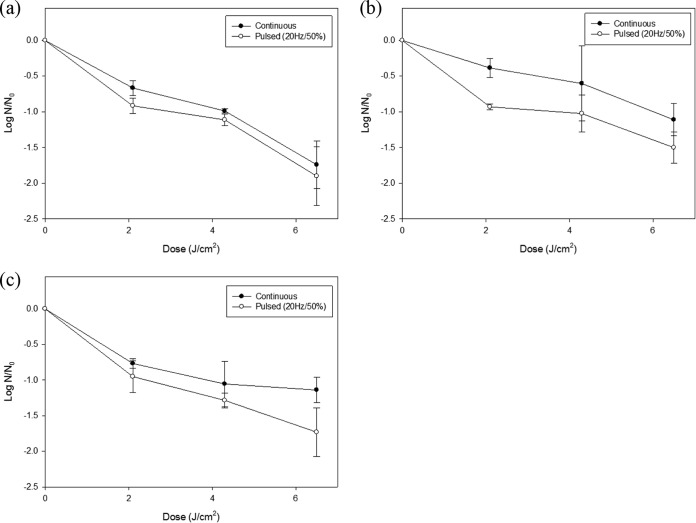
FIG 3.
Reductions of the foodborne pathogens E. coli O157:H7 (a), S. Typhimurium (b), and L. monocytogenes (c) inoculated onto white mushrooms, after treatment with 20-Hz/50% pulsed UVC-LED irradiation with dosages of 2.1, 4.3, and 6.5 J/cm2. The error bars indicate standard deviations.
FIG 4.
Reductions of the foodborne pathogens E. coli O157:H7 (a), S. Typhimurium (b), and L. monocytogenes (c) inoculated onto ready-to-eat sausages, after treatment with 20-Hz/50% pulsed UVC-LED irradiation with dosages of 1, 3, and 5 J/cm2. The error bars indicate standard deviations.
For white mushrooms, approximately 2-log-unit reductions of E. coli O157:H7 were achieved with continuous and pulsed UVC-LED irradiation, and survival of S. Typhimurium decreased by 1.5 log units with pulsed treatment. For L. monocytogenes, pulsed UVC-LED treatment produced 1.8-log-unit reductions. Except for E. coli O157:H7, all pathogens were inactivated by 1.0 log unit with continuous irradiation, and statistically greater reductions were observed with 2.1 J/cm2 for Gram-negative bacteria and with 6.5 J/cm2 for Gram-positive bacteria.
DISCUSSION
Pulsed-light irradiation, using intense light, has been researched and recognized as a powerful surface inactivation and water disinfection technology. However, temperature increases in food samples and the xenon lamp itself are major limitations for it becoming an industrially applicable system, because it needs an additional system to stabilize the pulsed light (17). Also, due to the Minamata Convention, it has become essential to seek a new technology to substitute for traditional low-pressure mercury UV lamps.
LED-based UVC irradiation has been widely recognized as a potential alternative technology, compared to low-pressure mercury lamps, and has been increasingly researched recently. Fundamental characteristics and bactericidal and viricidal effects, relative to irradiance dosages, have been determined, and inactivation mechanisms have been elucidated (20–23). Pulsed LED-based UV irradiation has rarely been investigated; there has only been one research study using an UVA-LED system to inactivate Candida albicans and E. coli K-12 biofilms (24), and scant research has been conducted using UVC-LED systems. Therefore, in this study, inactivation levels achieved with continuous and pulsed UVC-LED irradiation systems were investigated.
Generating pulsed UVC-LED irradiation with a power supply or source meter was not suitable for establishing maximum irradiance output, due to current delay in the turn-off time (data not shown), i.e., the electrical current did not drop sharply with a predictable slope when the electrical power was shut off. Therefore, the optical chopper system, a method to produce full-irradiance pulsed UVC-LED irradiation by interrupting the light with rotating blades, was introduced in this research.
There were differences in pathogen reductions between continuous and 20-Hz pulsed treatments at the lowest dosages (0.5 or 1 mJ/cm2), but no significant differences were observed between the irradiation types (P > 0.05). At higher dosages, such as 2 mJ/cm2 for E. coli O157:H7 and 5 mJ/cm2 for S. Typhimurium and L. monocytogenes, inactivation levels for 20-Hz pulsed UVC-LED irradiation reached an additional 0.5- to 0.7-log-unit reduction, compared to continuous irradiation. The finding of greater inactivation of pathogens produced by pulsed versus continuous UVC-LED irradiation concurs with published research studies, even though the details of conditions yielding maximum bactericidal effects differ. Pulsed xenon lamps (30 Hz) achieved a greater inactivation effect, in that a >4-log-unit reduction of E. coli occurred, while only 2-log-unit reductions were observed with medium-pressure and low-pressure UV lamps at the same UV fluence (25). Within 3 min, 7-log-unit reductions of E. coli O157:H7 and L. monocytogenes were observed with intense pulsed light (5 Hz), whereas 5-log-unit reductions were achieved with a low-pressure UV lamp after treatment for 20 min (26). Also, pulsed UVA-LED irradiation at 100 Hz showed the greatest inactivating effects on E. coli biofilms and C. albicans (24).
Figure 2 shows increased bactericidal effects of pulsed UVC-LED irradiation in terms of duty ratio. Because different inactivation levels for foodborne pathogens were expected, different dosages were employed, namely, 1 mJ/cm2 for E. coli O157:H7 and 3 mJ/cm2 for S. Typhimurium and L. monocytogenes. As the duty ratio increased from 10% to 50%, reductions increased among the three foodborne pathogens. The improved bactericidal effects corresponding to 50% and 10% duty ratios were significantly different (P < 0.05). Li et al. similarly reported that higher duty ratios produced greater inactivation of microorganisms, and statistically different values were observed for continuous and pulsed irradiation (24). The chopper blade system in this study could be adjusted to a 50% duty ratio, and further research, including higher duty ratios, is needed for precise industrial applications.
Analyses of changes in cell physiology, including membrane integrity, enzyme activity, and ROS levels, can elucidate different inactivation levels with treatment technology and synergistic effects caused by combination treatment. Numerous studies used fluorescent dyes to investigate the inactivation process (23, 27–29). In this research, PI uptake assays and DiBAC4(3) analyses were performed to assess alterations in the cell membrane, whereas ROS measurements, TBARS assays, and formazan formation analyses were performed to determine intracellular disruption.
First, percentages of PI uptake were not statistically different; therefore, cell membrane integrity was not affected by the UVC-LED irradiation. It is a well-known phenomenon that UV irradiation disrupts genetic material in cells through the formation of pyrimidine dimers, resulting in interruption of DNA-associated processing (30–32). Neither continuous nor pulsed UVC-LED irradiation influenced cell membrane integrity. However, membrane potential values, which indicate the electrical difference between the inside and outside of the cell membrane, differed in accordance with UV treatment and also the type of UVC irradiation. The greatest accumulation of DiBAC4(3) was observed with the 20-Hz/50% pulsed UVC-LED treatment, and the nontreated group had the lowest level. This result is consistent with other UV research studies using flow cytometric analysis of 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)] accumulation and spectrophotometric measurements of DiBAC4(3) accumulation in foodborne pathogen cells (23, 29, 33). From physiological analysis of the cell membrane, continuous and pulsed UVC-LED irradiation changed the cell membrane charges but did not produce pores. UVC-LED treatment decreased cell activity and ATP formation, so that perturbation of foodborne pathogens treated with UV irradiation occurred, but it was not necessarily coupled to cell death (34).
TBARS analysis was conducted to determine cell membrane lipid peroxidation, because, after UV treatment, vulnerability of membranes was detected from DiBAC4(3) assessments. Three-fold and 23-fold increased TBARS accumulation values were generated for E. coli O157:H7 and L. monocytogenes, respectively, with 20-Hz/50% pulsed irradiation instead of continuous treatments. Membrane lipid peroxidation, as assessed by TBARS analysis, has been reported after treatment with plasma (35–37), chemical agents (38), and visible light (39). From our results, pulsed UVC-LED irradiation obviously affects membrane lipid deterioration.
Because membrane lipid peroxidation was observed with pulsed UVC treatment, ROS generated inside foodborne pathogen cells were analyzed by using 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA). When acetate groups of the agent are cleaved by intracellular esterases, followed by ROS-mediated oxidation within the cell, CM-H2DCFDA is transformed from a nonfluorescent form into a fluorescent form. ROS generation within pathogens is represented in Table 6, and a similar pattern, showing that UVC irradiation causes higher TBARS values, was observed. Li et al. reported that the inactivation level of pulsed UVA-LED irradiation decreased when mannitol, a ROS scavenger, was added to the system (24). The pulsed irradiation system caused significantly greater reductions of microorganisms by greatly increasing the ROS concentrations, because UVA irradiation generates ROS as a major pathogen-inactivating mechanism (30). In the case of UVC-LED irradiation, pulsed irradiation produced intracellular ROS, and a significantly higher level of ROS generation was induced, compared to continuous irradiation. Several studies reported that certain species of ROS induced by UVC irradiation, including superoxide anion, hydrogen peroxide, and hydroxyl ion, readily provide intensive stress to the cell membrane (40–42). In this study, pulsed UVC-LED irradiation increased intracellular ROS generation, and it was linked to enhanced cell membrane lipid peroxidation.
The activity of SQR, an intracellular enzyme that participates in both the tricarboxylic acid (TCA) cycle and the electron transport chain (43), was also investigated, because intracellular ROS can cause multiple failures related to proliferation of cells, and energy formation is the primary factor in cell growth. Iodonitrotetrazolium (INT) is reduced by SQR, as two hydrogen ions are added, and turns into formazan, a color-developing substance. Therefore, low absorbance values imply attenuated SQR activity and a perturbed cell physiological state. Pulsed UVC-LED irradiation caused significantly diminished formazan formation by means of ROS generation (Table 6) and irradiation itself, since protein absorbs light with a peak wavelength of 280 nm, and resulting protein failure in function can be drawn from a UVC-irradiation-induced excitation state (23).
The bactericidal effects of continuous and pulsed UVC-LED irradiation on the white mushroom surface did seem to be similar, although some points had statistically different levels of pathogen inactivation. The averaged difference in reductions in foodborne pathogens between continuous and pulsed irradiation was 0.41 log unit; therefore, the elevated bactericidal effect can be regarded as negligible in this food matrix. Phenolic compounds, such as gallic acid, catechin, caffeic acid, and vitamin groups, in white mushrooms exhibit antioxidant activity and function as ROS scavengers (44, 45). Therefore, inactivation of foodborne pathogens on the surface of white mushrooms declined as a result of diminished activity of ROS. In addition, inefficient bactericidal activity was attributed to a shading effect due to rough food surfaces (10, 46, 47).
However, superior bactericidal effects of pulsed UVC-LED irradiation on ready-to-eat sausage surfaces, compared to continuous irradiation, were observed in the fundamental surface inactivation research. Generally speaking, the shading effect, when light irradiated onto a surface affects the pattern of bacterial inactivation, causes a diminished inactivation rate and tailing (10, 46, 47). For this sample, however, pulsed UVC-LED treatment overcame the shading effect by means of intermittent irradiation, leading to greater penetration depths and ROS generation. Furthermore, Oms-Oliu et al. reported that the thermal effect of conventional pulsed light damaged phenolic contents and vitamin C (48); therefore, UVC-LED irradiation, which does not produce excessive heat, is an alternative to a high-intensity pulsed UV system.
In this study, the bactericidal effects of pulsed UVC-LED irradiation were evaluated with respect to various factors, including frequency and duty ratio, and optimum conditions for the greatest inactivation of foodborne pathogens were determined. Also, the enhanced bactericidal effects were elucidated with analyses of cell physiological changes. There was no effect on cell membrane integrity, but membrane potential, lipid peroxidation, ROS generation, and enzyme activity were affected by pulsed UVC-LED irradiation. Moreover, effective inactivation of foodborne pathogens on the food surface by pulsed irradiation was observed, compared to continuous LED irradiation within treatment dosages.
MATERIALS AND METHODS
Pulsed UVC-LED system.
Three UVC-LEDs (LG Innotek Co., Republic of Korea), emitting the same peak wavelength of approximately 280 nm, were connected and combined on an electrical printed circuit board (PCB). The three UVC-LED packages were arranged linearly to avoid hindrance by an optical chopper system, and a distance of 8 cm was set between the PCB and a 90-mm-diameter petri dish. Immediately below the LED PCB, an optical chopper (MC2000B-EC; Thorlabs, Newton, NJ, USA) was incorporated in order to generate pulse irradiation. A chopper blade with an adjustable duty ratio (1% to 50%) (MC1F10A; Thorlabs), which could produce 20-Hz to 1,000-Hz frequency, was installed and synchronized with the chopper control system.
Bacterial strains.
Escherichia coli O157:H7 (ATCC 35150, ATCC 43889, and ATCC 43890), Salmonella enterica serovar Typhimurium (ATCC 19585, ATCC 43971, and DT 104), and Listeria monocytogenes (ATCC 19111, ATCC 19115, and ATCC 15313) were obtained from the Food Science and Human Nutrition culture collection at Seoul National University (Seoul, Republic of Korea). The stock cultures were kept frozen at −80°C in 0.7 ml of tryptic soy broth (TSB) (MB Cell) and 0.3 ml of 50% glycerol. Working cultures were streaked onto tryptic soy agar (TSA) (MB Cell), incubated for 24 h at 37°C, and stored at 4°C.
Culture preparation.
All strains of E. coli O157:H7, S. Typhimurium, and L. monocytogenes were cultured in 5 ml of TSB for 24 h at 37°C and were harvested by centrifugation at 4,000 × g for 20 min at 4°C. Pelleted cells were washed three times with sterile 0.2% peptone water (PW) (Bacto, Sparks, MD), and the final pellets were resuspended in 9 ml PW, corresponding to approximately 108 to 109 CFU/ml. The mixed-pathogen cocktail was produced by combining the resuspended pelleted cells.
Irradiance measurements.
Irradiance of the UVC-LED packages was measured with a spectrometer (AvaSpec-ULS2048-USB2-UA-50; Avantes, Netherlands) calibrated for a range of 200 to 400 nm to include the entire UV spectrum. For sample treatment, an optical spectrometer probe was placed 3 or 8 cm from the collimated UVC-LEDs and, in order to include the whole intensity that LEDs emit, the integral irradiance of the light source from 240 nm to 280 nm, which covers the UVC range and not just a certain peak wavelength, was measured. The Petri factor, which indicates even distribution of UVC irradiance on the surface of a sample petri dish, was calculated by scanning an area representing one-eighth of the surface (20–23). An 8-cm distance between the probe and LEDs was maintained, the UVC-LED irradiation of each point was divided by the maximum fluence rate, and values were averaged. A Petri factor of >0.9, which indicates >90% uniformity in irradiance distribution, was established using the linear arrangement. Adjusted irradiance values were calculated by multiplying the maximum irradiance by the Petri factor value. Dosages of 0.5 to 3.0 mJ/cm2 were imposed for selective medium experiments, and dosages of 1 to 5 J/cm2 were imposed for model food experiments in this study.
Food sample preparation.
White mushrooms (Agaricus bisporus [J. E. Lange] Imbach) and commercial ready-to-eat (fully cooked) sausages were purchased from a local market (Seoul, Republic of Korea), stored under refrigeration (4°C), and used in experiments for a maximum of 3 days. Stipes of the white mushrooms were removed with a sterile stainless steel knife, and only pilei with diameters ranging from 4 to 5 cm, weighing approximately 10 g, were used in this research. The pilei were arrayed on sterile aluminum foil with the caps facing up and were placed in a biosafety hood without the fan running. The sausage samples were sliced longitudinally into fourths (arcs of 90o) with a sterile stainless steel knife. The sausages were placed on sterile aluminum foil with the circular arcs facing up.
Bacterial inoculation.
Bacterial inoculation was performed according to methods published previously (20, 21, 23). Briefly, the resuspended mixed-pathogen cocktail was serially diluted 10-fold with 0.2% sterile PW so that the initial inoculum concentration ranged approximately from 105 to 106 CFU/ml. Additional 10-fold serial dilutions were performed, and 100-μl aliquots of appropriate diluents were spread plated on the following selective media: sorbitol-MacConkey (SMAC) agar (Oxoid) for E. coli O157:H7, xylose lysine desoxycholate (XLD) agar (Oxoid) for S. Typhimurium, and Oxford agar base with antimicrobial supplement (OAB) (MB Cell) for L. monocytogenes. The same amounts of pathogen suspension diluent were also spread plated on phenol red agar base (Difco) with 1% sorbitol (SPRAB) (MB Cell), to enumerate injured E. coli O157:H7, and on TSA (MB Cell) followed by the overlay technique described below to enumerate injured S. Typhimurium and L. monocytogenes.
For food applications, 100 μl of resuspended cocktail was distributed by spot inoculation with a micropipettor on food surfaces in the biosafety hood, with 16 spots distributed over the entire surface. The inoculated samples were dried for 1 h inside the biosafety hood, with the laminar flow fan running.
Pulsed and continuous UVC treatments.
Inoculated media and food samples were exposed to a linear array of UVC-LED PCBs in the chamber (TH-TG-300; Jeio Tech, Republic of Korea) at selected dosages, at room temperature. For pulsed UVC-LED treatment at select frequencies, the optical chopper system was operated with various frequencies from 20 Hz to 1,000 Hz, with a fixed duty ratio (50%). Also, UVC treatment in terms of various duty ratios was carried out by modifying the gap of the optical chopper blade, resulting in 10% to 50% duty ratios with 20-Hz frequency, which showed the greatest bactericidal effect. For continuous UVC-LED treatment, only the chopper blade was removed from the treatment system. Actual treatment times were calculated by dividing the target dosages by the adjusted irradiance for continuous treatment, and the calculated times were divided by the duty ratios for pulsed UVC treatment in order to maintain conserved UV dosages with various treatments.
A selected form of pulsed UVC irradiation was applied to inactivate foodborne pathogens on food surfaces. Based on preliminary experiments using selective media, pulsed UVC-LED treatment with 20-Hz frequency and a 50% duty ratio, which yielded the greatest inactivating effect, was selected and applied to foods at various dosages. Also, because a need for higher UVC irradiation dosages to control microorganisms in the food matrix was anticipated, the UVC-LED array was placed 3 cm from the samples.
Enumeration.
After the fundamental inactivation experiments on selective medium surfaces were performed, treated agar media were incubated for 24 h at 37°C. For food samples, treated sausages were placed in sterile stomacher bags containing 90 ml PW (Labplas, Inc., Canada) and were thoroughly homogenized with a stomacher (EasyMix; AES Chemunex, France). One-milliliter stomacher-homogenized sample aliquots were serially diluted in 9 ml of sterile 0.2% PW, 100-μl aliquots of selected diluents were spread plated on each selective medium (as described above), the plates were incubated for 24 to 48 h at 37°C, and characteristic colonies were enumerated.
Injured cell analysis.
Enumeration of injured E. coli O157:H7 cells was performed with SPRAB agar, on which sublethally damaged bacteria can be resuscitated. After incubation for 24 h at 37°C, white colonies were enumerated; serological confirmation was performed with an aggregation test (RIM E. coli O157:H7 latex agglutination test; Remel, Lenexa, KS, USA). The overlay method was used to enumerate injured S. Typhimurium and L. monocytogenes cells (20, 21, 49). A nonselective medium, TSA, was used to resuscitate sublethally injured pathogens during short incubations (2 h at 37°C), followed by an overlay of 7 to 10 ml of the selective medium XLD agar for S. Typhimurium or OAB agar for L. monocytogenes. After solidification of the poured selective media, plates were incubated for an additional 22 h at 37°C. Black colonies of both S. Typhimurium and L. monocytogenes were enumerated.
Cell membrane damage assessment.
Cell membrane damage after continuous and pulsed UVC-LED treatments was assessed by using PI (Sigma-Aldrich, St. Louis, MO, USA) and DiBAC4(3) (Molecular Probes, Thermo Fisher Scientific, Waltham, MA, USA), as described in a research study conducted by Kim et al. (23). Quantitative analysis of membrane destruction of microorganisms can be measured by uptake of PI, which is a fluorescent DNA-intercalating agent. E. coli O157:H7 and L. monocytogenes were inoculated into 5 ml of phosphate-buffered saline (PBS) (Corning Inc., NY, USA), and the optical density at 600 nm (OD600) of the suspension was adjusted to approximately 0.4. The maximum dosage for each bacterium (3 mJ/cm2 for E. coli O157:H7 and 5 mJ/cm2 for L. monocytogenes) was delivered to the PBS-bacterium suspension with continuous or 20-Hz/50% pulsed treatments. After treatment, a 1-ml aliquot was transferred to a sterile centrifuge tube and PI solution was added to a final concentration of 2.9 μM, followed by a 10-min incubation in the dark at room temperature.
Membrane potential, an indicator of cellular damage, was evaluated with DiBAC4(3), an anionic fluorescent dye. Treated suspensions of E. coli O157:H7 were incubated for 15 min at 37°C with 2.5 μg/ml DiBAC4(3) in PBS with 4 mM EDTA in the dark. In the case of L. monocytogenes suspensions, cells were incubated for 2 min at 37°C with 0.5 μg/ml DiBAC4(3) in PBS in the dark.
After incubation, cells were collected by centrifugation at 10,000 × g for 10 min and were washed twice with PBS in order to remove excess fluorescent dye. Pelleted cells were resuspended in PBS, and fluorescence was measured using a spectrofluorophotometer (SpectraMax M2e; Molecular Devices, San Jose, CA, USA), with an excitation wavelength of 495 nm and emission wavelength of 615 nm for PI uptake and an excitation wavelength of 488 nm and emission wavelength of 525 nm for DiBAC4(3) accumulation. All measured fluorescence and absorbance values in this study, including PI uptake, DiBAC4(3) accumulation, TBARS accumulation, ROS generation, and SQR activity values, were normalized against the cell density (OD600) of the bacterial suspension and expressed as a percentage of the highest value observed (with 20-Hz/50% treatment).
Membrane lipid peroxidation assessment.
Cell membrane lipid peroxidation was assessed by measuring malondialdehyde using the Oxiselect TBARS assay kit. Bacterium-PBS suspensions were treated with a dosage of 3 mJ/cm2 or 5 mJ/cm2 (for E. coli O157:H7 or L. monocytogenes, respectively), and samples were processed by following the manufacturer's instructions. Fluorescence was measured by using the spectrometer at an excitation wavelength of 540 nm and an emission wavelength of 590 nm.
ROS measurements.
ROS levels were assayed in order to elucidate the reason for different levels of membrane lipid peroxidation with continuous versus pulsed treatments. The ROS detection reagent CM-H2DCFDA (Invitrogen, Eugene, OR, USA) was purchased and processed following the manufacturer's instructions. The final concentration of the agent was set at 5 μM in 1 ml of treated bacterial solution. As for the other mechanism assays, the same levels of UVC irradiation were delivered to the PBS solution. Fluorescence was measured using the spectrometer at an excitation wavelength of 492 nm and an emission wavelength of 520 nm.
SQR activity assay.
The activity of SQR, which is involved in the electron transfer chain for energy production, was assessed in order to evaluate other cellular damage arising from ROS. After UV treatment, 100 μl of 0.5% iodonitrotetrazolium chloride solution (Sigma-Aldrich) was added to 0.9 ml of treated bacterial solution, and the mixture was incubated for 2 h at 37°C in the dark. Bacterial cells were collected by centrifugation (and the supernatant was discarded), followed by resuspension in 1 ml of 1:1 (vol/vol) acetone-ethanol. Based on the amount of formazan formation, SQR activity was determined by measuring the absorbance of INT-formazan at 490 nm with a SpectraMax M2e.
Statistical analysis.
All experiments were performed in duplicate and replicated three times. All data were analyzed with analysis of variance using SAS software (SAS Institute, Cary, NC, USA) and Duncan's multiple range test to determine whether there were significant differences (P < 0.05) in mean values of log-unit reductions in microorganism populations or fluorescence units.
ACKNOWLEDGMENTS
This work was financially supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry through the High Value-Added Food Technology Development Program, funded by the Ministry of Agriculture, Food and Rural Affairs (grant 117064-03-1-HD050). This research was a part of the Development and Commercialization of Marine Products Applicable Rapid Detection Method for Hazardous Microorganisms (Bacteria and Viruses) and Construction Safety Management System by Application New Technology project funded by the Ministry of Oceans and Fisheries, Republic of Korea.
We are grateful for technical support and cooperation from LG Innotek.
REFERENCES
- 1.Caminiti IM, Palgan I, Muñoz A, Noci F, Whyte P, Morgan DJ, Cronin DA, Lyng JG. 2012. The effect of ultraviolet light on microbial inactivation and quality attributes of apple juice. Food Bioprocess Technol 5:680–686. doi: 10.1007/s11947-010-0365-x. [DOI] [Google Scholar]
- 2.Kim Y-H, Jeong S-G, Back K-H, Park K-H, Chung M-S, Kang D-H. 2013. Effect of various conditions on inactivation of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes in fresh-cut lettuce using ultraviolet radiation. Int J Food Microbiol 166:349–355. doi: 10.1016/j.ijfoodmicro.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Mukhopadhyay S, Ukuku D, Juneja V, Fan X. 2014. Effects of UV-C treatment on inactivation of Salmonella enterica and Escherichia coli O157:H7 on grape tomato surface and stem scars, microbial loads, and quality. Food Control 44:110–117. doi: 10.1016/j.foodcont.2014.03.027. [DOI] [Google Scholar]
- 4.Food and Drug Administration. 2013. Code of Federal Regulations, Title 21. Ultraviolet radiation for the processing and treatment of food, chapter I, part 179, subpart B. U.S. Food and Drug Administration, Silver Spring, MD: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=179.39. [Google Scholar]
- 5.van der Meer P, Gravemann U, Korte D, Sumian C, Tolksdorf F, Müller T, Seltsam A. 2016. Effect of increased agitation speed on pathogen inactivation efficacy and in vitro quality in UVC-treated platelet concentrates. Vox Sang 111:127–134. doi: 10.1111/vox.12404. [DOI] [PubMed] [Google Scholar]
- 6.Santo D, Graça A, Nunes C, Quintas C. 2016. Survival and growth of Cronobacter sakazakii on fresh-cut fruit and the effect of UV-C illumination and electrolyzed water in the reduction of its population. Int J Food Microbiol 231:10–15. doi: 10.1016/j.ijfoodmicro.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 7.Yin F, Zhu Y, Koutchma T, Gong J. 2015. Inactivation and potential reactivation of pathogenic Escherichia coli O157:H7 in apple juice following ultraviolet light exposure at three monochromatic wavelengths. Food Microbiol 46:329–335. doi: 10.1016/j.fm.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Kessler R. 2013. The Minamata Convention on Mercury: a first step toward protecting future generations. Environ Health Perspect 121:A304–A309. doi: 10.1289/ehp.121-A304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UN Environment Programme. 2013. New global treaty cuts mercury emissions and releases, sets up controls on products, mines and industrial plants. UN Environment Programme, Châtelaine, Switzerland: http://www.mercuryconvention.org/News/Newglobaltreatycutsmercuryemissions/tabid/3470/language/en-US/Default.aspx. [Google Scholar]
- 10.Elmnasser N, Guillou S, Leroi F, Orange N, Bakhrouf A, Federighi M. 2007. Pulsed-light system as a novel food decontamination technology: a review. Can J Microbiol 53:813–821. doi: 10.1139/W07-042. [DOI] [PubMed] [Google Scholar]
- 11.Anderson JG, Rowan NJ, MacGregor SJ, Fouracre RA, Farish O. 2000. Inactivation of food-borne enteropathogenic bacteria and spoilage fungi using pulsed-light. IEEE Trans Plasma Sci 28:83–88. doi: 10.1109/27.842870. [DOI] [Google Scholar]
- 12.Farrell H, Garvey M, Cormican M, Laffey J, Rowan N. 2010. Investigation of critical inter-related factors affecting the efficacy of pulsed light for inactivating clinically relevant bacterial pathogens. J Appl Microbiol 108:1494–1508. doi: 10.1111/j.1365-2672.2009.04545.x. [DOI] [PubMed] [Google Scholar]
- 13.Gomez-Lopez VM, Devlieghere F, Bonduelle V, Debevere J. 2005. Intense light pulses decontamination of minimally processed vegetables and their shelf-life. Int J Food Microbiol 103:79–89. doi: 10.1016/j.ijfoodmicro.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 14.Levy C, Aubert X, Lacour B, Carlin F. 2012. Relevant factors affecting microbial surface decontamination by pulsed light. Int J Food Microbiol 152:168–174. doi: 10.1016/j.ijfoodmicro.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Rowan N, MacGregor S, Anderson J, Fouracre R, McIlvaney L, Farish O. 1999. Pulsed-light inactivation of food-related microorganisms. Appl Environ Microbiol 65:1312–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodling SE, Moraru CI. 2007. Effect of spectral range in surface inactivation of Listeria innocua using broad-spectrum pulsed light. J Food Prot 70:909–916. doi: 10.4315/0362-028X-70.4.909. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Lopez VM, Ragaert P, Debevere J, Devlieghere F. 2007. Pulsed light for food decontamination: a review. Trends Food Sci Technol 18:464–473. doi: 10.1016/j.tifs.2007.03.010. [DOI] [Google Scholar]
- 18.Jun S, Irudayaraj J, Demirci A, Geiser D. 2003. Pulsed UV-light treatment of corn meal for inactivation of Aspergillus niger spores. Int J Food Sci Technol 38:883–888. doi: 10.1046/j.0950-5423.2003.00752.x. [DOI] [Google Scholar]
- 19.Sharma R, Demirci A. 2003. Inactivation of Escherichia coli O157:H7 on inoculated alfalfa seeds with pulsed ultraviolet light and response surface modeling. J Food Sci 68:1448–1453. doi: 10.1111/j.1365-2621.2003.tb09665.x. [DOI] [Google Scholar]
- 20.Shin J-Y, Kim S-J, Kim D-K, Kang D-H. 2016. Fundamental characteristics of deep-UV light-emitting diodes and their application to control foodborne pathogens. Appl Environ Microbiol 82:2–10. doi: 10.1128/AEM.01186-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S-J, Kim D-K, Kang D-H. 2016. Using UVC light-emitting diodes at wavelengths of 266 to 279 nanometers to inactivate foodborne pathogens and pasteurize sliced cheese. Appl Environ Microbiol 82:11–17. doi: 10.1128/AEM.02092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D-K, Kim S-J, Kang D-H. 2017. Inactivation modeling of human enteric virus surrogates, MS2, Qβ, and ΦX174, in water using UVC-LEDs, a novel disinfecting system. Food Res Int 91:115–123. doi: 10.1016/j.foodres.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 23.Kim D-K, Kim S-J, Kang D-H. 2017. Bactericidal effect of 266 to 279 nm wavelength UVC-LEDs for inactivation of Gram positive and Gram negative foodborne pathogenic bacteria and yeasts. Food Res Int 97:280–287. doi: 10.1016/j.foodres.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Hirota K, Yumoto H, Matsuo T, Miyake Y, Ichikawa T. 2010. Enhanced germicidal effects of pulsed UV-LED irradiation on biofilms. J Appl Microbiol 109:2183–2190. doi: 10.1111/j.1365-2672.2010.04850.x. [DOI] [PubMed] [Google Scholar]
- 25.Bohrerova Z, Shemer H, Lantis R, Impellitteri CA, Linden KG. 2008. Comparative disinfection efficiency of pulsed and continuous-wave UV irradiation technologies. Water Res 42:2975–2982. doi: 10.1016/j.watres.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Cheigh C-I, Park M-H, Chung M-S, Shin J-K, Park Y-S. 2012. Comparison of intense pulsed light-and ultraviolet (UVC)-induced cell damage in Listeria monocytogenes and Escherichia coli O157:H7. Food Control 25:654–659. doi: 10.1016/j.foodcont.2011.11.032. [DOI] [Google Scholar]
- 27.Anvarian AH, Smith MP, Overton TW. 2016. The effects of orange juice clarification on the physiology of Escherichia coli: growth-based and flow cytometric analysis. Int J Food Microbiol 219:38–43. doi: 10.1016/j.ijfoodmicro.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Ha J-W, Lee J-I, Kang D-H. 2017. Application of a 222-nm krypton-chlorine excilamp to control foodborne pathogens on sliced cheese surfaces and characterization of the bactericidal mechanisms. Int J Food Microbiol 243:96–102. doi: 10.1016/j.ijfoodmicro.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Kim S-S, Choi W, Kang D-H. 2017. Application of low frequency pulsed ohmic heating for inactivation of foodborne pathogens and MS-2 phage in buffered peptone water and tomato juice. Food Microbiol 63:22–27. doi: 10.1016/j.fm.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Bintsis T, Litopoulou-Tzanetaki E, Robinson RK. 2000. Existing and potential applications of ultraviolet light in the food industry: a critical review. J Sci Food Agric 80:637–645. doi:. [DOI] [PubMed] [Google Scholar]
- 31.Friedberg EC, Walker GC, Siede W, Wood RD, Schultz RA, Ellenberger T. 2006. DNA repair and mutagenesis, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 32.Lopez-Malo A, Palou E. 2005. Ultraviolet light and food preservation, p 405–422. In Barbosa-Canovas GV, Tapia MS, Cano MP (ed), Novel food processing technologies. CRC Press, Boca Raton, FL. [Google Scholar]
- 33.Chan WH, Wu CC, Yu JS. 2003. Curcumin inhibits UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermoid carcinoma A431 cells. J Cell Biochem 90:327–338. doi: 10.1002/jcb.10638. [DOI] [PubMed] [Google Scholar]
- 34.Díaz M, Herrero M, García LA, Quirós C. 2010. Application of flow cytometry to industrial microbial bioprocesses. Biochem Eng J 48:385–407. doi: 10.1016/j.bej.2009.07.013. [DOI] [Google Scholar]
- 35.Dolezalova E, Lukes P. 2015. Membrane damage and active but nonculturable state in liquid cultures of Escherichia coli treated with an atmospheric pressure plasma jet. Bioelectrochemistry 103:7–14. doi: 10.1016/j.bioelechem.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Joshi SG, Cooper M, Yost A, Paff M, Ercan UK, Fridman G, Friedman G, Fridman A, Brooks AD. 2011. Nonthermal dielectric-barrier discharge plasma-induced inactivation involves oxidative DNA damage and membrane lipid peroxidation in Escherichia coli. Antimicrob Agents Chemother 55:1053–1062. doi: 10.1128/AAC.01002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machala Z, Jedlovský I, Chládeková L, Pongrác B, Giertl D, Janda M, Šikurová L, Polčic P. 2009. DC discharges in atmospheric air for bio-decontamination: spectroscopic methods for mechanism identification. Eur Phys J D 54:195–204. doi: 10.1140/epjd/e2009-00035-7. [DOI] [Google Scholar]
- 38.Rajapakse N, Kim M-M, Mendis E, Kim S-K. 2007. Inhibition of free radical-mediated oxidation of cellular biomolecules by carboxylated chitooligosaccharides. Bioorg Med Chem 15:997–1003. doi: 10.1016/j.bmc.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 39.Kim M-J, Bang WS, Yuk H-G. 2017. 405±5 nm light emitting diode illumination causes photodynamic inactivation of Salmonella spp. on fresh-cut papaya without deterioration. Food Microbiol 62:124–132. doi: 10.1016/j.fm.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Cadet J, Wagner JR. 2013. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol 5:a012559. doi: 10.1101/cshperspect.a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krisko A, Radman M. 2010. Protein damage and death by radiation in Escherichia coli and Deinococcus radiodurans. Proc Natl Acad Sci U S A 107:14373–14377. doi: 10.1073/pnas.1009312107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharffetter-Kochanek K, Wlaschek M, Brenneisen P, Schauen M, Blaudschun R, Wenk J. 1997. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol Chem 378:1247–1258. [PubMed] [Google Scholar]
- 43.Oyedotun KS, Lemire BD. 2004. The quaternary structure of the Saccharomyces cerevisiae succinate dehydrogenase homology modeling, cofactor docking, and molecular dynamics simulation studies. J Biol Chem 279:9424–9431. doi: 10.1074/jbc.M311876200. [DOI] [PubMed] [Google Scholar]
- 44.Jagadish LK, Krishnan VV, Shenbhagaraman R, Kaviyarasan V. 2009. Comparitive study on the antioxidant, anticancer and antimicrobial property of Agaricus bisporus (J. E. Lange) Imbach before and after boiling. Afr J Biotechnol 8:654–661. [Google Scholar]
- 45.Liu J, Jia L, Kan J, Jin C-H. 2013. In vitro and in vivo antioxidant activity of ethanolic extract of white button mushroom (Agaricus bisporus). Food Chem Toxicol 51:310–316. doi: 10.1016/j.fct.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Gómez-López V, Devlieghere F, Bonduelle V, Debevere J. 2005. Factors affecting the inactivation of micro-organisms by intense light pulses. J Appl Microbiol 99:460–470. doi: 10.1111/j.1365-2672.2005.02641.x. [DOI] [PubMed] [Google Scholar]
- 47.Yi JY, Lee N-H, Chung M-S. 2016. Inactivation of bacteria and murine norovirus in untreated groundwater using a pilot-scale continuous-flow intense pulsed light (IPL) system. LWT Food Sci Technol 66:108–113. doi: 10.1016/j.lwt.2015.10.027. [DOI] [Google Scholar]
- 48.Oms-Oliu G, Aguiló-Aguayo I, Martín-Belloso O, Soliva-Fortuny R. 2010. Effects of pulsed light treatments on quality and antioxidant properties of fresh-cut mushrooms (Agaricus bisporus). Postharvest Biol Technol 56:216–222. doi: 10.1016/j.postharvbio.2009.12.011. [DOI] [Google Scholar]
- 49.Lee S-Y, Kang D-H. 2001. Suitability of overlay method for recovery of heat-injured Listeria monocytogenes and Salmonella Typhimurium. Food Sci Biotechnol 10:323–326. [Google Scholar]