This study analyzed the influence of finR deletion on the transcriptomic profile of Pseudomonas putida KT2440. The FinR regulator is widely distributed but poorly studied in diverse proteobacteria. Here, we found 11 operons that potentially are regulated by FinR in KT2440. We further demonstrated that FinR played a positive role and cooperated with the NicR repressor in bacterial nicotinic acid (NA) degradation via regulating the expression of nicC and nicX operons. Furthermore, a transcriptomic analysis also indicated a potentially negative role of FinR in the expression of the hut cluster involved in bacterial histidine utilization. The work deepened our knowledge of FinR function and nicotinic acid degradation in P. putida.
KEYWORDS: FinR, Pseudomonas putida KT2440, nicC and nicX operons, nicotinic acid, transcriptomic profile
ABSTRACT
Many proteobacteria harbor FinR homologues in their genomes as putative LysR-type proteins; however, the function of FinR is poorly studied except in the induction of fpr-1 under superoxide stress conditions in Pseudomonas putida and Pseudomonas aeruginosa. Here, by analyzing the influence of finR deletion on the transcriptomic profile of P. putida KT2440 through RNA sequencing and real-time quantitative PCR (RT-qPCR), we found 11 operons that are potentially regulated by FinR. Among them, the expression of nicC and nicX operons, which were reported to be responsible for the aerobic degradation of nicotinic acid (NA), was significantly decreased in the finR mutant, and complementation with intact finR restored the expression of the two operons. The results of bacterial NA utilization demonstrated that the deletion of finR impaired bacterial growth in minimal medium supplemented with NA/6HNA (6-hydroxynicotinic acid) as the sole carbon source and that complementation with intact finR restored the growth of the mutant strain. The expression of nicC and nicX operons was previously revealed to be repressed by the NicR repressor and induced by NA/6HNA. Our transcriptional assay revealed that the deletion of finR weakened the induction of nicC and nicX by NA/6HNA. Meanwhile, the deletion of finR largely decreased the effect of nicR deletion on the expression of nicC and nicX operons. These results suggest that finR plays a positive role and cooperates with NicR in the regulation of nicC and nicX operons. In vitro experiments showed that both FinR and NicR bound to nicX and nicC promoter regions directly. The results of this study deepened our knowledge of FinR function and nicotinic acid degradation in P. putida.
IMPORTANCE This study analyzed the influence of finR deletion on the transcriptomic profile of Pseudomonas putida KT2440. The FinR regulator is widely distributed but poorly studied in diverse proteobacteria. Here, we found 11 operons that potentially are regulated by FinR in KT2440. We further demonstrated that FinR played a positive role and cooperated with the NicR repressor in bacterial nicotinic acid (NA) degradation via regulating the expression of nicC and nicX operons. Furthermore, a transcriptomic analysis also indicated a potentially negative role of FinR in the expression of the hut cluster involved in bacterial histidine utilization. The work deepened our knowledge of FinR function and nicotinic acid degradation in P. putida.
INTRODUCTION
The LysR-type transcriptional regulators (LTTRs) represent the most abundant type of transcriptional regulator with an N-terminal DNA-binding helix-turn-helix motif and a C-terminal coinducer-binding domain as conserved structures (1). LTTRs exhibit negative autoregulation and regulate a diverse set of genes, including those involved in virulence, metabolism, quorum sensing, and cell division (2–6). Many proteobacterial genomes contain a LysR-type oxidative stress-sensing transcriptional regulator, FinR, which is located next to fpr-1, encoding ferredoxin NADP+ reductase, an enzyme catalyzing the reversible electron-transferring reaction between NADPH and one-electron carriers such as ferredoxin or flavodoxin (7–10). Previous studies revealed that FinR binds directly to the promoter region of fpr-1 and is essential for the induction of fpr-1 during exposure to superoxide stress in Pseudomonas putida KT2440 (8). Fpr-1 is important in maintaining the cellular NADP+/NADPH ratio by mediating a reversible redox reaction between NADP+/NADPH and electron carriers such as ferredoxin or flavodoxin (7). An interruption of finR or fpr-1 increased bacterial sensitivity to oxidative stress in Escherichia coli, Salmonella, and Pseudomonas (9–12).
Normally, redox-sensing proteins harbor conserved cysteine residues to control their regulation, as is the case for SoxR and OxyR (13). FinR in P. putida has three well-conserved cysteine residues and two additional cysteine residues, but the five cysteines are not associated with FinR activation, since strains with mutations in each of these cysteine residues show transcription activity similar to that of the wild-type strain (8). These results suggest that FinR has a unique redox-sensing mechanism which is different from the well-characterized mechanisms of OxyR and SoxR.
Although FinR is widely distributed in diverse proteobacteria, its function is poorly studied except in the induction of fpr-1 under superoxide stress conditions in P. putida and P. aeruginosa. In this study, we analyzed the influence of finR deletion on the transcriptomic profile of P. putida KT2440 through RNA sequencing and real-time quantitative PCR (RT-qPCR) and found 11 operons potentially regulated by FinR. Of these, nicC and nicX were positively regulated by FinR. nicC and nicX operons belong to the nic cluster, which is responsible for the aerobic degradation of nicotinic acid (NA). In this study, we mainly focused on characterizing the role of FinR in the expression of nicC and nicX operons and nicotinic acid utilization.
RESULTS
Transcriptomic analysis revealed genes under the influence of FinR.
FinR is a LysR-type transcriptional factor positioned divergently adjacent to fpr-1 in the P. putida KT2440 genome. Many bacteria possess a FinR homologue in their genomes, but except in the induction of fpr-1 in response to superoxide (9, 10), the function of FinR has been poorly studied. To identify the set of genes under the influence of FinR, we constructed a finR deletion mutant and performed transcriptomic analysis. The results of the transcriptomic analysis revealed that expression of 189 genes was statistically significantly different (log2 fold change of ≥1 or ≤−1) in the finR mutant compared to that in the wild-type KT2440 (see Table S1 in the supplemental material). Among the 189 genes, 56 genes exhibited larger differences in expression levels (log2 fold change of ≥2 or ≤−2), with 16 genes showing decreased expression, including the formerly reported fpr-1, and 40 genes showing increased expression (Table 1). The 56 genes belong to 26 operons on the basis of the organization of the KT2440 genome. To verify the results of the transcriptomic analysis, we compared the transcriptions of the 26 operons in a finR mutant and the wild-type KT2440 by RT-qPCR. One gene was chosen and tested for each operon. As revealed in Fig. 1, the expression levels of 11 genes were different between the finR mutant and the wild type in the RT-qPCR assay, with trends similar to those observed in the transcriptomic analysis, while the expression of the other 15 genes showed no obvious difference in the RT-qPCR assay.
TABLE 1.
Genes upregulated or downregulated in finR deletion mutant versus in wild-type P. putida KT2440a
| Gene IDb | Log2 fold change | P value | Gene name | Description |
|---|---|---|---|---|
| PP_0057 | −2.207 | 1.72E−39 | Major facilitator family transporter | |
| PP_0137 | 2.856 | 1.25E−103 | gltP | Glutamate/aspartate-proton DAACS transporter |
| PP_0596 | 3.701 | 0 | Omega-amino acid–pyruvate aminotransferase | |
| PP_0597 | 3.623 | 1.02E−290 | mmsA-I | Methylmalonate-semialdehyde dehydrogenase |
| PP_0614 | 2.447 | 2.58E−08 | Bifunctional N-carbamoyl-beta-alanine amidohydrolase/allantoin amidohydrolase | |
| PP_0615 | 3.250 | 5.79E−05 | ABC transporter ATP-binding protein | |
| PP_0616 | 3.154 | 2.77E−10 | ABC transporter ATP-binding protein | |
| PP_0617 | 4.385 | 3.32E−07 | Branched-chain amino acid ABC transporter permease | |
| PP_0618 | 4.646 | 3.32E−10 | Branched-chain amino acid ABC transporter permease | |
| PP_0619 | 3.991 | 1.39E−46 | Branched-chain amino acid ABC transporter substrate-binding protein | |
| PP_0620 | 5.553 | 3.63E−206 | GntR family transcriptional regulator | |
| PP_0817 | −2.297 | 5.46E−33 | alaC | Aminotransferase |
| PP_1039 | 4.143 | 0.0075892 | tatC-I | Sec-independent protein translocase protein |
| PP_1638 | −3.811 | 2.01E−149 | fpr-I | Ferredoxin-NADP+ reductase |
| PP_2034 | 3.046 | 8.06E−77 | Membrane protein | |
| PP_2035 | 3.295 | 2.17E−52 | benE-I | Benzoate transport protein |
| PP_2036 | 3.045 | 8.77E−101 | 4-Hydroxy-tetrahydrodipicolinate synthase | |
| PP_2037 | 2.091 | 3.92E−10 | Aldolase | |
| PP_2052 | 2.233 | 3.20E−14 | Bifunctional sugar-phosphatase/mannitol-1-phosphate 5-dehydrogenase | |
| PP_2397 | 4.002 | 3.31E−68 | EF hand domain-containing protein | |
| PP_2404 | 3.914 | 1.29E−18 | Hypothetical protein | |
| PP_2453 | 2.024 | 6.07E−148 | ansB | Glutaminase-asparaginase |
| PP_2454 | −2.195 | 2.45E−119 | rbsB | Ribose ABC transporter, periplasmic ribose-binding subunit |
| PP_3073 | −3.332 | 1.27E−84 | hbdH | 3-Hydroxybutyrate dehydrogenase |
| PP_3074 | −4.063 | 3.12E−164 | bhbP | d-Beta-hydroxybutyrate permease |
| PP_3191 | −2.213 | 4.57E−134 | Threonine ammonia-lyase/dehydratase | |
| PP_3589 | 2.618 | 1.16E−36 | sdaC | Serine:H+ symport permease |
| PP_3661 | 3.956 | 1.44E−90 | Membrane protein | |
| PP_3745 | −3.031 | 6.86E−37 | glcD | Glycolate oxidase FAD-linked subunit |
| PP_3940 | −2.455 | 3.63E−21 | nicT | Metabolite transport protein NicT |
| PP_3941 | −2.753 | 1.35E−33 | nicF | Maleamate amidohydrolase |
| PP_3942 | −2.414 | 5.88E−22 | nicE | Maleate isomerase |
| PP_3943 | −2.694 | 1.60E−16 | nicD | N-Formylmaleamate deformylase |
| PP_3944 | −3.601 | 2.45E−44 | nicC | 6-Hydroxynicotinate 3-monooxygenase |
| PP_3945 | −2.474 | 2.26E−58 | nicX | 2,5-Dihydroxypyridine 5,6-dioxygenase |
| PP_4232 | 2.120 | 1.93E−14 | Cytochrome c family protein | |
| PP_4233 | 2.640 | 1.50E−05 | Oxidoreductase small subunit | |
| PP_4434 | 3.476 | 1.57E−41 | dadA-I | d-Amino acid dehydrogenase small subunit |
| PP_4435 | 4.100 | 0 | Hypothetical protein | |
| PP_4548 | 2.585 | 6.57E−14 | Oxidoreductase | |
| PP_4625 | 3.054 | 4.80E−34 | cidB | Holin CidA anti-holin regulator |
| PP_4626 | 3.358 | 3.29E−12 | cidA | Murein hydrolase holin regulator |
| PP_4735 | 3.332 | 0.00E+00 | lldP | l-Lactate permease |
| PP_4736 | 3.441 | 4.45E−105 | lldD | l-Lactate dehydrogenase |
| PP_4737 | 4.059 | 1.27E−94 | dld2 | d-Lactate dehydrogenase |
| PP_5029 | 4.210 | 5.79E−145 | hutG | N-Formylglutamate deformylase |
| PP_5030 | 4.031 | 1.76E−213 | hutI | Imidazolonepropionase |
| PP_5031 | 3.454 | 3.46E−109 | proY | Proline (histidine) APC transporter |
| PP_5032 | 3.937 | 0 | hutH | Histidine ammonia-lyase |
| PP_5033 | 4.132 | 6.99E−245 | hutU | Urocanate hydratase |
| PP_5036 | 4.591 | 0 | hutF | Formiminoglutamate deiminase |
| PP_5073 | −3.290 | 6.77E−50 | Hypothetical protein | |
| PP_5269 | 4.310 | 0.00E+00 | dadX | Alanine racemase |
| PP_5270 | 3.167 | 0 | dadA-II | d-Amino acid:quinone oxidoreductase |
| PP_5338 | −2.377 | 1.80E−156 | aspA | Aspartate ammonia-lyase |
| PP_5549 | 2.424 | 1.95E−36 | Hypothetical protein |
Genes with log2 fold changes of >2 or <−2 are shown.
ID, identifier.
FIG 1.
Analysis of the relative transcriptional levels of 26 operons in wild-type KT2440 and the finR deletion mutant by RT-qPCR. Transcriptional levels in the wild type were used as a reference. The results are the averages from three independent assays. The data values represent mean values with standard deviations. *, P ≤ 0.05; **, P ≤ 0.01.
FinR positively regulates nicC and nicX operons.
Among the 11 verified operons, three operons with decreased expression, PP_3940 (nicT), PP_3942 (nicE), and PP_3945 (nicX), were of interest. The three operons belong to the nic cluster (Fig. 2A), which consists of 11 genes and is responsible for the aerobic degradation of nicotinic acid (NA) (14). To further test the role of FinR in the expression of the nic cluster, we compared the expression levels of all 11 nic genes in the finR mutant and the wild type by RT-qPCR. The results showed that the expression of eight genes was decreased in the finR mutant relative to that in the wild-type strain (Fig. 2B), while the expression of the other three genes, nicA, nicB, and nicS, showed no difference. Complementation with intact finR under the control of its own promoter restored the expression of the eight genes to wild-type levels.
FIG 2.
FinR regulates expression of nicC and nicX operons. (A) Schematic representation of the nic cluster from P. putida. Arrows above the genes represent promoters of indicated operons. (B) Analysis of the relative transcriptional levels of nic cluster genes in wild-type KT2440, the finR deletion mutant, and the finR complemented strain (cΔfinR) by RT-qPCR. Transcriptional levels in the wild type were used as a reference. The results are the averages from three independent assays. (C) Promoter activity of nic cluster in the wild type and the finR mutant. β-Galactosidase activity was measured from stationary-phase (24 h) LB broth cultures. ND, not detected. The results are the averages from three independent assays. The data values represent mean values with standard deviations. *, P ≤ 0.05; **, P ≤ 0.01.
The results of a previous study suggested that the nic cluster from P. putida is organized in three NA-inducible operons, i.e., nicAB under the control of the Pa promoter (promoter of nicA), nicXR under the control of the Px promoter (promoter of nicX), and nicCDEFTP under the control of the Pc promoter (promoter of nicC). Furthermore, the NA-independent expression of the nicS, nicTP, and nicR operons suggests the existence of three additional constitutive promoters upstream of the nicS, nicT, and nicR genes, respectively (15). To test the role of FinR in the expression activity of these promoters, we constructed seven nic promoter-lacZ fusion reporters and introduced them to the finR mutant and the wild-type strain. Then, β-galactosidase activity was measured. As shown in Fig. 2C, the promoter activities for nicC and nicX were significantly lower in the finR mutant than in the wild type. Meanwhile, the promoter activities for nicR, nicA, and nicS showed no difference and the promoter activities for nicT and nicP-II were too low to be detected. Altogether, both results from the RT-qPCR and the promoter-lacZ fusion reporters reveal that FinR positively regulates the expression of nicC and nicX operons.
FinR deletion impaired NA utilization.
The initial hydroxylation of NA is catalyzed by two hydroxylases, NicA and NicB, which can convert NA to 6-hydroxynicotinic acid (6HNA). The second enzymatic step for aerobic NA degradation is the oxidative decarboxylation of 6HNA to render 2,5-dihydroxypyridine (2,5DHP) catalyzed by the 6HNA-3-monooxygenase NicC. Then, the Fe2+-dependent dioxygenase NicX converts 2,5DHP to N-formylmaleamic acid. Further conversion of N-formylmaleamic acid to formic and maleamic acids is catalyzed by the NicD protein (14). Since FinR positively regulates the expression of nicX and nicC operons, we wondered whether finR deletion would influence bacterial NA degradation and lead to impaired growth of KT2440 in minimal medium containing NA or 6HNA as the sole carbon source. The results from previous studies showed that the finR mutant had longer lag phases than the wild type in minimal medium containing benzoate (10 mM) or glucose (111 mM) as the carbon source because of the decreased expression of fpr-1 (10). Thus, to eliminate the influence of the decreased expression of fpr-1 on the growth of the finR mutant in minimal medium, we introduced an fpr-1 overexpression vector (403-fpr-1 [see Table S2 in the supplemental material]) into the finR mutant. As showed in Fig. 3A, complementation with fpr-1 restored the growth trend of the finR mutant to the wild-type level in minimal medium containing glucose as the sole carbon source. Then, the growth trend of the wild-type strain, the finR mutant complemented with fpr-1 (ΔfinR+403-fpr-1 strain), and the finR complementary strain (cΔfinR strain) in minimal medium containing NA or 6HNA as the sole carbon source was tested. A noticeably longer growth delay in the finR mutant was observed in a liquid growth assay as revealed in Fig. 3B and C, and the complementation restored the growth to a level close to that of the wild type. Similar results were obtained from a plate growth assay, in which the growth of the finR mutant was severely impaired in both NA- and 6HNA-containing plates. Complementation with wild-type finR under the control of its own promoter restored the growth (Fig. 3D and E). These results suggest that FinR deletion impaired NA utilization.
FIG 3.
finR deletion impaired NA/6HNA utilization. Growth trends of wild-type KT2440, the finR mutant, the finR mutant complemented with 403-fpr-1, and the finR complemented strain in liquid minimal medium containing 0.4% glucose (A), 5 mM NA (B), or 5 mM 6HNA (C) as the sole carbon source. Growth of strains on solid minimal medium containing 5 mM NA (D) or 5 mM 6HNA (E) as the sole carbon source after 48 h of incubation.
FinR cooperates with NicR in the regulation of nicC and nicX operons.
The results from a previous study revealed that NicR, a MarR-like protein, repressed the activity of nicC and nicX promoters and that 6HNA was the inducer molecule (15). Here, we found that FinR positively regulated the activity of nicC and nicX promoters. To investigate the relationship of the two regulators in the induction of nicC and nicX operons in the absence or presence of inducers, we constructed a nicR signal mutant and a nicR finR double mutant and compared the expression levels of nic genes in the presence or absence of NA/6HNA by RT-qPCR. As previously reported, the expression of nicC, nicX, and nicA operons was significantly higher in the nicR deletion mutant than in the wild-type strain in the absence of inducers (Fig. 4A). Meanwhile, the deletion of finR largely decreased the effect of nicR deletion on the expression of nicC and nicX operons, since the expression of the two operons was lower in the nicR finR double mutant than in the nicR single mutant in the absence of inducers (Fig. 4A). We also noticed that the expression of the two operons in the double deletion mutant was still approximately 100-fold higher than in the wild-type strain, suggesting that the repressive function of NicR played a chief role in regulating the expression of the two operons, while the positive role of FinR was needed for full induction.
FIG 4.
Induction of the nic cluster by NA/6HNA. (A) Analysis of the relative transcriptional levels of nic cluster genes in wild-type KT2440, the finR mutant, the nicR mutant, and the finR nicR double mutant in the absence of inducer by RT-qPCR. Induction of the nic cluster by 5 mM NA/6HNA in wild-type (B), finR mutant (C), nicR mutant (D), and finR nicR double mutant (E) strains. Control check (CK) represents the addition of water instead of NA/6HNA to the culture. The results are the averages from three independent assays. The data values represent mean values with standard deviations. *, P ≤ 0.05; **, P ≤ 0.01.
In the presence of NA/6HNA, the expression of nicC and nicX operons was induced in the wild type and the finR mutant but not in the nicR mutant and the nicR finR double mutant (Fig. 4B, C, D, and E). finR deletion weakened the induction of nicC and nicX operons in the presence of NA/6HNA (Fig. 4B and C), which might be the reason for the impaired growth of the finR mutant in minimal medium containing NA/6HNA. Furthermore, the expression of the nicA operon was induced by NA but not 6HNA in all tested strains, which is similar to the result from a previous report (15). These results suggest that finR plays a positive role and cooperates with NicR in the induction of nicC and nicX operons in the presence of NA/6HNA.
FinR and NicR bind nicX and nicC promoters in vitro.
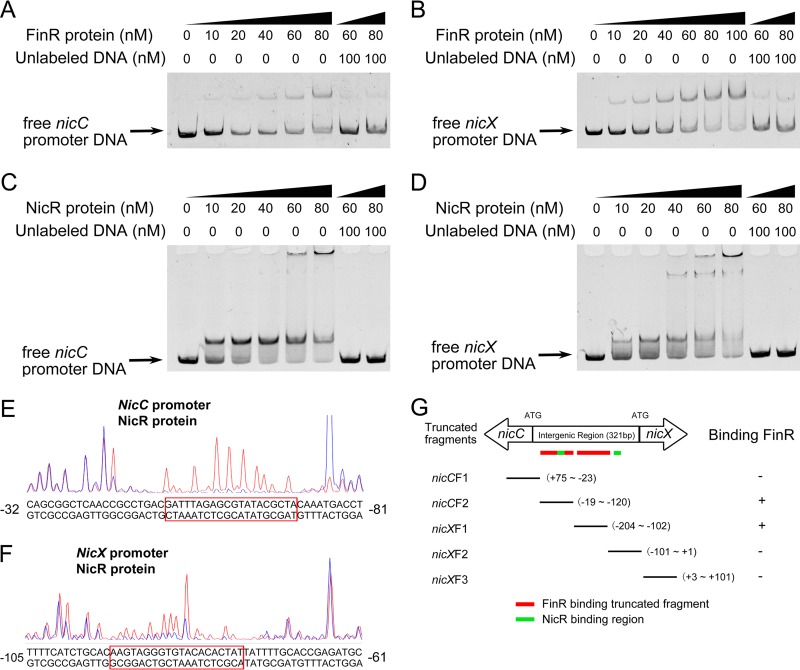
FinR is a DNA-binding transcriptional regulator and has been shown to bind to the promoter of the target genes to activate their transcription (8, 9). NicR is a repressor of nicC and nicX operons, but whether NicR directly binds to nicC and nicX promoters is unknown. To test whether FinR and NicR bind nicC and nicX promoters, we purified FinR/NicR and carried out an electrophoretic mobility shift assay (EMSA). Purified FinR/NicR was incubated with a fragment of DNA spanning −134 to +51 bp relative to the translational start site of nicC. The addition of FinR/NicR to the reaction mixtures caused a shift in the mobility of the nicC promoter DNA fragment. A competition experiment with unlabeled DNA fragments eliminated the shift (Fig. 5A and C). In the case of the nicX promoter (−181 to +43 bp relative to the translational start of nicX), there was also a shift in the mobility of the nicX operon promoter. Similarly, unlabeled DNA from the nicX promoter eliminated the shift (Fig. 5B and D). These results indicated there were specific interactions between the FinR or NicR protein and the nicC or nicX promoter DNA. To further determine the precise binding sites of FinR/NicR in the two promoters, we performed a DNase I footprinting assay. The data obtained indicated that the GATTTAGAGCGTATACGCTA sequence in the nicC promoter and the AAGTAGGGTGTACACACTAT sequence in the nicX promoter were protected by the NicR protein (Fig. 5E and F). In the case of FinR binding, we tried several times but failed to confirm the precise binding site of FinR to the nicC and nicX promoters.
FIG 5.
FinR and NicR proteins bind to nicX and nicC promoters. (A) Binding of FinR protein to nicC promoter and competition experiment with the unlabeled DNA fragment. The concentrations of the FinR protein and the amounts of unlabeled DNA used are indicated. (B) Binding of the FinR protein to the nicX promoter. (C) Binding of the NicR protein to the nicC promoter. (D) Binding of the NicR protein to the nicX promoter. (E) DNase I footprinting assay with fragments containing the nicC promoter in the presence (blue peaks) and absence (red peaks) of NicR. The protected nucleotides (relative to the translational start codon) are boxed. (F) DNase I footprinting assay with fragments containing the nicX promoter in the presence (blue peaks) and absence (red peaks) of NicR. (G) Binding of the truncated promoter fragments to FinR. Schematic diagram of the truncated fragments of nicC or nicX promoter DNA; a + or − on the right represents the fragment binding to FinR (+) or not (−). The coordinates represent the location relative to the ATG initiation codon. Red boxes represent the position of the truncated fragments bound by FinR in the intergenic region. Green boxes represent the position of the DNA sequences protected by NicR in the intergenic region.
To map the specific domains of the promoters that interacted with FinR, series of truncated fragments of the nicC and nicX promoters were amplified and used in the EMSA. As shown in Fig. 5G and Fig. S1 in the supplemental material, nicCF2 and nicXF1 DNA fragments produced strong band shifts with FinR on the gel, whereas no such retarded band was observed with the other three fragments, indicating that the binding sites are located between positions −19 and −120 on the nicC promoter and −102 and −204 on the nicX promoter relative to their translational start codons.
Oxidative stress showed no influence on the expression of nicX and nicC operons.
Since FinR was previously reported to be a redox-sensing transcriptional regulator and to induce fpr-1 expression under superoxide stress conditions, we wondered whether the genes controlled by FinR revealed in this study were regulated by oxidative stress. We tested the influence of two superoxide-generating agents (hydrogen peroxide and paraquat) on the expression of the 11 FinR-regulated genes in the wild-type strain using RT-qPCR. As shown in Fig. 6, except for the expression of fpr-1, which was apparently induced by paraquat as previously described (16), the expression of the other 10 genes, including nicC and nicX, showed no difference in the presence/absence of hydrogen peroxide and paraquat under our experimental conditions, indicating that the expression of these genes was not regulated by oxidative stress via FinR.
FIG 6.
Influence of oxidative stress (1 mM H2O2 or 1 mM paraquat [PQ]) on the expression of the 11 FinR-regulated genes in wild-type KT2440. The results are the averages from three independent assays. The data values represent the mean values with standard deviations. **, P ≤ 0.01.
DISCUSSION
NA is a carboxylic derivative of pyridine and is a widely distributed molecule that serves as the sole carbon and nitrogen source for some bacteria and fungi (17, 18). NA forms parts of biological molecules such as pyridine cofactors, e.g., NAD(P), and alkaloids, e.g., nicotine and anabasine, and it is essential (in the form of vitamin B3) for organisms that are not able to carry out its synthesis (17). The degradation of NA in P. putida depends on the expression of genes from the nic cluster (Fig. 2A). NicR is a repressor of nicC and nicX operons and 6HNA is the inducer (15). Here, we reveal that FinR is an activator of nicC and nicX operons. finR deletion leads to decreased expression of the two operons and thus results in impaired NA/6HNA utilization (Fig. 2B and C and Fig. 3). The results of a transcriptional assay show that finR deletion weakened the induction of nicC and nicX in the presence of NA/6HNA (Fig. 4B and C), indicating that FinR and NicR cooperate in the regulation of nicC and nicX operons. Furthermore, the expression of nicC and nicX operons was significantly increased in the finR nicR double mutant relative to that in the wild type, but to a lower level than that in the nicR mutant (Fig. 4A), indicating that the repressive function of NicR played a chief role in regulating the expression of the two operons, while a positive role of FinR was needed for full induction.
Apart from the nicC and nicX operons, the finR mutant exhibited increased expression levels of genes that encode enzymes related to histidine utilization, such as hutG (N-formylglutamate deformylase), hutI (imidazolonepropionase), hutH (histidine ammonia-lyase), hutU (urocanate hydratase), and hutF (formiminoglutamate deiminase) (Table 1). The results from previous studies revealed that the histidine utilization (hut) loci of Pseudomonas fluorescens and P. putida SBW25 conferred the ability to utilize histidine as a sole carbon and nitrogen source (19, 20). hut genes in P. putida are divided into three major transcriptional units: hutF, hutC (the repressor gene), and hutUHIG (see Fig. S2 in the supplemental material) (21). The three hut operons are negatively regulated by the hutC repressor, with urocanate (the first intermediate of the histidine degradation pathway) as the physiological inducer. CbrB, an enhancer binding protein for σ54 RNA polymerase, is required for bacterial histidine utilization, indicating positive control of hut gene expression by CbrB (20). Here, our results indicated that FinR was a repressor of hut cluster expression. However, more studies are required to demonstrate how FinR regulates the expression of hut genes and whether FinR influences bacterial histidine utilization via regulating the expression of the hut cluster. Furthermore, we also found that the expression of PP_4735, encoding an omega-amino acid pyruvate aminotransferase that catalyzes the reversible transamination of various sulfur-containing ω-amino acids and ω-amino carboxylic acids with pyruvate to yield the corresponding aldehydic acids and l-alanine, was increased in the finR mutant.
A transcriptomic analysis indicated that 26 operons were differentially expressed more than 4-fold in the finR mutant. However, an RT-qPCR analysis showed that only 15 of them were differentially expressed in the mutant strain. We infer that two reasons exist for this obvious discrepancy between the transcriptomic and RT-qPCR results obtained in this study. The most important reason is that the three RNA copies of the wild-type strain (or finR mutant) used for the transcriptomic assay were extracted from one single sample, while the RNAs used for the RT-qPCR assay were extracted from several individual samples; thus, some discrepancy between RNAs for the two techniques surely existed, and we consider the RT-qPCR result more precise under this condition. Second, the different principles and sensitivities of the two techniques may also lead to some discrepancy in the final expression results.
FinR is a member of the LysR family of transcriptional regulators, which often use palindromic DNA sequences as a binding box to modulate the expression of the target genes (1), but no consensus sequence for the FinR binding box on target gene promoters has been identified so far. The results from EMSAs and site-directed mutagenesis methods in a previous study of P. aeruginosa revealed that FinR bound specifically to the palindromic sequence TATCCATATTCTGGATA in the fpr-1 promoter (9–12). We tried several times to confirm the precise binding sites of FinR on nicC and nicX promoters by using a DNase I footprinting assay, but all attempts failed to reveal clear DNA protection. To narrow the range of binding sites, we performed EMSAs with truncated promoter fragments and found that the binding sites are located within a 100-bp region for both promoters (Fig. 5G and Fig. S1 in the supplemental material). Further studies on interactions between FinR and target promoters are needed to reveal the precise binding sequences.
In conclusion, we investigated the effect of finR deletion on the transcriptomic profile of P. putida KT2440 and found 11 operons that are likely regulated by FinR. We further proved that FinR plays a positive role in the expression of nicC and nicX operons and bacterial nicotinic acid utility. These results deepened our knowledge of FinR function and nicotinic acid degradation in P. putida KT2400.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this work are listed in Table S2 in the supplemental material. The P. putida KT2440 and E. coli strains were routinely cultured at 28°C and 37°C, respectively, in Luria-Bertani (LB) broth, which was solidified with 1.5% agar when necessary. For the NA/6HNA degradation assay, P. putida strains were grown at 28°C in M9 minimal medium supplemented with 5 mM NA or 6HNA as the carbon source. Antibiotics were used, when required, at the following concentrations: kanamycin, 50 mg · liter−1; chloramphenicol, 25 mg · liter−1; gentamicin, 20 mg · liter−1 for E. coli or 40 mg · liter−1 for P. putida.
Plasmid and strain construction.
All DNA manipulations were performed according to standard protocols (22). All cloning steps involving PCR were verified by commercial sequencing (Tsingke). Plasmids were transferred to E. coli and P. putida strains by transformation, biparental mating, or electroporation. Isogenic mutants of finR or nicR, promoter-lacZ reporter plasmids, and complementary plasmids were constructed according to a previously described method (23). The primers used for plasmid and strain construction are listed in Table 2. The strains and constructs are available upon request.
TABLE 2.
Primers used for plasmid construction and RT-qPCR in this study
aThe restriction site regions of the primers are underlined.
Assays for β-galactosidase activity.
β-Galactosidase activity was measured as described previously (24). Overnight cultures were inoculated (1:100 dilution) in LB medium and grown for 1.5 h; cultures were diluted 1:1 three times (every half hour) before the start of sample collection. These steps were performed to ensure proper dilution of β-galactosidase that accumulated after overnight growth. Experiments were repeated at least three times with two technical repeats per sample, and data are given in Miller units.
Library preparation, RNA sequencing, and data analyses.
Transcriptome libraries were constructed as previously described (25, 26). The total RNA sample extracted from exponentially growing cells incubated in LB medium was depleted of rRNA with the Ribo-Zero kit for Gram-negative bacteria (Illumina). cDNA libraries were prepared with the TruSeq stranded mRNA sample preparation kit (Illumina) according to the low sample LS protocol. Libraries were validated with a DNA 1000 chip on the Agilent 2100 Bioanalyzer, and the concentration was measured using a Qubit 2.0 fluorometer (Invitrogen, Life Technologies). The concentration of each library was normalized to 10 nM in TE buffer (10 mM Tris-Cl [pH 7.0], 1 mM EDTA), and cDNA libraries were pooled for sequencing on the Illumina HiSeq 2000 platform at Beckman Coulter Genomics. The transcriptome libraries were single-end sequenced with 100-bp reads.
The RNA-seq data were trimmed using Trimmomatic (27) and analyzed with the open source software Rockhopper (version 2.0.3) with the default settings, choosing reverse complement reads and strand-specific analysis (28). The reads were mapped to the sequenced reference P. putida KT2440 genome (GenBank accession no. NC_002947.3). Using SAMtools (29), the mapped files were merged and the identification of novel transcripts was performed by visual inspection with Integrative Genomics Viewer (30), as Rockhopper detects many false positives. The differential gene expression analysis was carried out with the Web server T-REx (31) using the reads per kilobase per million (RPKM) values generated in the Rockhopper analysis, in which the finR mutant was compared to the wild-type control. Differential expression of genes was considered significant with a log2 fold change of ≥1 and an adjusted P value of ≤0.05. The Basic Local Alignment Search Tool (BLAST) with search criteria including a query of >80%, identity of >60%, and E value of <10−6 was used in sequence homology searches.
RNA extraction, preparation of cDNA, and RT-qPCR.
Total RNA from exponentially growing cells was extracted with total RNA extraction reagent (Vazyme) as recommended by the manufacturer. Reverse transcription reactions to generate the corresponding cDNA were performed with 1 μg of RNA using a PrimeScript RT reagent kit (TaKaRa RR047A). RpoD was used as an internal control for normalization. The primers used for RT-qPCR are listed in Table 2. The results were analyzed by means of the comparative threshold cycle method to determine the relative expression of each gene in the mutants with respect to that in the wild type. Three individual replicates were performed with three independent cultures grown on different days. Standard errors were calculated from these independent replicates.
Expression and purification of His-tagged FinR/NicR.
An E. coli BL21 strain carrying the FinR/NicR expression vector was grown overnight in LB broth with kanamycin, diluted 1:100, and grown for 4 h at 37°C. The expression of His-tagged FinR/NicR protein was induced with the addition of 0.4 mM IPTG (isopropyl-β-d-thiogalactopyranoside), followed by an incubation at 16°C overnight. Cultures were harvested by centrifugation at 6,000 × g for 10 min and resuspended in lysing buffer (10 mM Tris-Cl [pH 8.0], 300 mM KCl, and 10% glycerol). The cells were lysed in a JNBIO pressure cell breaking apparatus, and the extract was clarified by centrifugation at 15,000 × g for 5 min. The soluble fraction was filtered through a 0.22-μm-pore-size filter. The crude extract was loaded on a NiSO4 column and then collected from the column in elution buffer (10 mM Tris-Cl [pH 8.0], 300 mM KCl, 10% glycerol, and 200 mM imidazole). The protein concentration was determined with a bicinchoninic acid (BCA) assay.
Electrophoretic mobility shift assay.
The EMSA was performed as previously described (32). The fragments of the nicC and nicX promoters were generated by PCR using 6-FAM (6-carboxyfluorescein phosphoramidite)-tagged primers. Equal amounts of each of these labeled DNA fragments (50 ng) were added to binding reactions with various amounts of FinR/NicR in the binding buffer (10 mM Tris [pH 7.8], 8 mM magnesium acetate, 50 mM KCl, 5% glycerol, 250 ng · ml−1 bovine serum albumin [BSA]; 20 μl total reaction volume) in each gel. FinR/NicR was incubated with DNA for 25 min at room temperature. All reaction mixtures were loaded onto a 5% acrylamide gel containing 10 mM Tris-Cl (pH 8.0), 400 mM glycine, and 5 mM EDTA and electrophoresed at 100 V at room temperature for 1 to 1.5 h. The gels were dried and exposed to a phosphorimaging screen.
DNase I footprinting assay.
A DNase I footprinting assay was performed as described previously (33) with slight modifications. The 6-FAM-labeled promoter DNA sequences of nicC and nicX were PCR-amplified from genomic DNA using 5′-end 6-FAM-labeled primers and purified by 6% PAGE. In a 600-μl reaction system, 1,000 ng of labeled DNA fragment was bound to 1,000 μg FinR/NicR (bovine serum albumin was used instead of FinR/NicR in the control experiment) in buffer containing 10 mM Tris-HCl (pH 7.8), 10 mM MgCl2, 1 mM CaCl2, 0.4 mM dithiothreitol, 100 mM KCl, and 5% glycerol and incubated for 20 min at 25°C. After binding, 0.03 U of RNase-free DNase I (Roche, Basel, Switzerland) was added and allowed to react for 5 min at 25°C. The reaction was stopped and precipitated with ethanol. Samples were analyzed in a 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA), and the electropherograms were aligned with GeneMapper v3.5 (Applied Biosystems).
Statistical analysis.
For analyses of the significance of differences in gene expression, Student's t tests were used for comparison of two groups of data. A P value of ≤0.05 was considered significantly statistically different; a P value of ≤0.01 was considered extremely significantly statistically different.
Accession number(s).
RNA-seq data have been deposited in the SRA database under accession numbers SRP139421, SRP139424, SRP139442, SRP139452, SRP139482, and SRP139486.
Supplementary Material
ACKNOWLEDGMENTS
The research was financially supported by the National Key Research and Development Program of China (2016YFD0800206) and the National Natural Science Foundation of China (41571230).
We have no competing interests.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01210-18.
REFERENCES
- 1.Maddocks SE, Oyston PC. 2008. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology 154:3609–3623. doi: 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- 2.Kovacikova G, Skorupski K. 1999. A Vibrio cholerae LysR homolog, AphB, cooperates with AphA at the tcpPH promoter to activate expression of the ToxR virulence cascade. J Bacteriol 181:4250–4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao H, Krishnan G, Goumnerov B, Tsongalis J, Tompkins R, Rahme LG. 2001. A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc Natl Acad Sci U S A 98:14613–14618. doi: 10.1073/pnas.251465298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang JP, Zhang WM, Chao HJ, Zhou NY. 2017. PnpM, a LysR-type transcriptional regulator activates the hydroquinone pathway in para-nitrophenol degradation in Pseudomonas sp. strain WBC-3. Front Microbiol 8:1714. doi: 10.3389/fmicb.2017.01714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhari SS, Thomas VC, Sadykov MR, Bose JL, Ahn DJ, Zimmerman MC, Bayles KW. 2016. The LysR-type transcriptional regulator, CidR, regulates stationary-phase cell death in Staphylococcus aureus. Mol Microbiol 101:942–953. doi: 10.1111/mmi.13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Z, Takeuchi M, Sato T. 2007. The LysR-type transcriptional regulator YofA controls cell division through the regulation of expression of ftsW in Bacillus subtilis. J Bacteriol 189:5642–5651. doi: 10.1128/JB.00467-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrillo N, Ceccarelli EA. 2003. Open questions in ferredoxin-NADP+ reductase catalytic mechanism. Eur J Biochem 270:1900–1915. doi: 10.1046/j.1432-1033.2003.03566.x. [DOI] [PubMed] [Google Scholar]
- 8.Yeom S, Yeom J, Park W. 2010. Molecular characterization of FinR, a novel redox-sensing transcriptional regulator in Pseudomonas putida KT2440. Microbiology 156:1487–1496. doi: 10.1099/mic.0.034181-0. [DOI] [PubMed] [Google Scholar]
- 9.Boonma S, Romsang A, Duang-Nkern J, Atichartpongkul S, Trinachartvanit W, Vattanaviboon P, Mongkolsuk S. 2017. The FinR-regulated essential gene fprA, encoding ferredoxin NADP+ reductase: roles in superoxide-mediated stress protection and virulence of Pseudomonas aeruginosa. PLoS One 12:e0172071. doi: 10.1371/journal.pone.0172071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y, Peña-Llopis S, Kang YS, Shin HD, Demple B, Madsen EL, Jeon CO, Park W. 2006. Expression analysis of the fpr (ferredoxin-NADP+ reductase) gene in Pseudomonas putida KT2440. Biochem Biophys Res Commun 339:1246–1254. doi: 10.1016/j.bbrc.2005.11.135. [DOI] [PubMed] [Google Scholar]
- 11.Bianchi V, Haggård-Ljungquist E, Pontis E, Reichard P. 1995. Interruption of the ferredoxin (flavodoxin) NADP+ oxidoreductase gene of Escherichia coli does not affect anaerobic growth but increases sensitivity to paraquat. J Bacteriol 177:4528–4531. doi: 10.1128/jb.177.15.4528-4531.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pomposiello PJ, Demple B. 2000. Identification of SoxS-regulated genes in Salmonella enterica serovar Typhimurium. J Bacteriol 182:23–29. doi: 10.1128/JB.182.1.23-29.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green J, Paget MS. 2004. Bacterial redox sensors. Nat Rev Microbiol 2:954–966. doi: 10.1038/nrmicro1022. [DOI] [PubMed] [Google Scholar]
- 14.Jiménez JI, Canales Á, Jiménez-Barbero J, Ginalski K, Rychlewski L, García JL, Díaz E. 2008. Deciphering the genetic determinants for aerobic nicotinic acid degradation: the nic cluster from Pseudomonas putida KT2440. Proc Natl Acad Sci U S A 105:11329–11334. doi: 10.1073/pnas.0802273105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiménez JI, Juárez JF, García JL, Díaz E. 2011. A finely tuned regulatory circuit of the nicotinic acid degradation pathway in Pseudomonas putida. Environ Microbiol 13:1718–1732. doi: 10.1111/j.1462-2920.2011.02471.x. [DOI] [PubMed] [Google Scholar]
- 16.Yeom J, Lee Y, Park W. 2012. ATP-dependent RecG helicase is required for the transcriptional regulator OxyR function in Pseudomonas species. J Biol Chem 287:24492–24504. doi: 10.1074/jbc.M112.356964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaiser JP, Feng Y, Bollag JM. 1996. Microbial metabolism of pyridine, quinoline, acridine, and their derivatives under aerobic and anaerobic conditions. Microbiol Rev 60:483–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fetzner S. 1998. Bacterial degradation of pyridine, indole, quinoline, and their derivatives under different redox conditions. Appl Microbiol Biotechnol 49:237–250. doi: 10.1007/s002530051164. [DOI] [Google Scholar]
- 19.Zhang XX, George A, Bailey MJ, Rainey PB. 2006. The histidine utilization (hut) genes of Pseudomonas fluorescens SBW25 are active on plant surfaces, but are not required for competitive colonization of sugar beet seedlings. Microbiology 152:1867–1875. doi: 10.1099/mic.0.28731-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang XX, Rainey PB. 2007. Genetic analysis of the histidine utilization (hut) genes in Pseudomonas fluorescens SBW25. Genetics 176:2165–2176. doi: 10.1534/genetics.107.075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu L, Phillips AT. 1988. Organization and multiple regulation of histidine utilization genes in Pseudomonas putida. J Bacteriol 170:4272–4279. doi: 10.1128/jb.170.9.4272-4279.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch EF, Maniatis T. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 23.Xiao Y, Nie H, Liu H, Luo X, Chen W, Huang Q. 2016. C-di-GMP regulates the expression of lapA and bcs operons via FleQ in Pseudomonas putida KT2440. Environ Microbiol Rep 8:659–666. doi: 10.1111/1758-2229.12419. [DOI] [PubMed] [Google Scholar]
- 24.Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 25.Gómez-Lozano M, Marvig RL, Molin S, Long KS. 2012. Genome-wide identification of novel small RNAs in Pseudomonas aeruginosa. Environ Microbiol 14:2006–2016. doi: 10.1111/j.1462-2920.2012.02759.x. [DOI] [PubMed] [Google Scholar]
- 26.Bojanovič K, D'Arrigo I, Long KS. 2017. Global transcriptional responses to osmotic, oxidative, and imipenem stress conditions in Pseudomonas putida. Appl Environ Microbiol 83:e03236-16. doi: 10.1128/AEM.03236-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClure R, Balasubramanian D, Sun Y, Bobrovskyy M, Sumby P, Genco CA, Vanderpool CK, Tjaden B. 2013. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res 41:338–345. doi: 10.1093/nar/gkt444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorvaldsdóttir H, Robinson JT, Mesirov JP. 2013. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform 14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Jong A, Meulen SVD, Kuipers OP, Kok J. 2015. T-REx: transcriptome analysis webserver for RNA-seq expression data. BMC Genomics 16:663. doi: 10.1186/s12864-015-1834-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hickman JW, Harwood CS. 2008. Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol Microbiol 69:376–389. doi: 10.1111/j.1365-2958.2008.06281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abella M, Rodriguez S, Paytubi S, Campoy S, White MF, Barbé J. 2007. The Sulfolobus solfataricus radA paralogue sso0777 is DNA damage inducible and positively regulated by the Sta1 protein. Nucleic Acids Res 35:6788–6797. doi: 10.1093/nar/gkm782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.