Abstract
Flash glucose monitoring - an alternative to traditional self-monitoring of blood glucose (SMBG) - prevents hypoglycaemic events without impacting glycated haemoglobin (HbA1c).21 Given the potential benefits, this study assessed the cost-effectiveness of using flash monitoring versus SMBG alone in patients with type 1 diabetes (T1D) receiving intensive insulin treatment in Sweden. Methods: This study used the IQVIA CORE Diabetes Model (IQVIA CDM, v9.0) to simulate the impact of flash monitoring versus SMBG over 50 years from the Swedish societal perspective. Trial data informed cohort data, intervention effects, and resource utilisation; literature and Tåndvards-Läkemedelförmånsverket (TLV) sources informed utilities and costs. Scenario analyses explored the effect of key base case assumptions. Results: In base case analysis, direct medical costs for flash monitor use were SEK1,222,333 versus SEK989,051 for SMBG use. Flash monitoring led to 0.80 additional quality-adjusted life years (QALYs; 13.26 versus 12.46 SMBG) for an incremental cost effectiveness ratio (ICER) of SEK291,130/QALY. ICERs for all scenarios remained under SEK400,000/QALY. Conclusion: Hypoglycaemia and health utility benefits due to flash glucose monitoring may translate into economic value compared to SMBG. With robust results across scenario analyses, flash monitoring may be considered cost-effective in a Swedish population of T1D intensive insulin users.
Keywords: Type 1 diabetes, glucose monitoring, cost, cost analysis, economic analysis
The prevalence of diabetes continues to increase globally,1 and is projected to remain of importance to Sweden’s public health,2 given the higher risk people with diabetes face regarding disabling and/or life-threatening health problems and mortality.1,3 A recent estimate puts national prevalence at 7.0% (type 1 diabetes [T1D] and type 2 diabetes [T2D]); approximately 10% have T1D.1,4 Sweden is currently ranked number two internationally for incidence of T1D among children at 43.2 cases/100,000 in the population.1
Sweden faces a growing burden in diabetes treatment and management. Although this figure is for T1D and T2D combined, Sweden spends nearly US$8,000 per patient annually.1 A recent projection indicates that 940,000 patients will be affected by the year 2050.2 In addition to the direct costs of managing diabetes, healthcare costs are incurred in the management of short-term hypoglycaemic events and longer-term macro- and microvascular complications associated with poor glycaemic control.5,6 Indirect costs associated with lost productivity likewise impact the overall cost burden.1,7
All patients with T1D require daily insulin,1 yet while insulin usage helps reduce glycated haemoglobin (HbA1c), it is associated with complications such as increased hypoglycaemic events.8 Frequent glucose monitoring allows patients to manage their blood glucose levels,1,9 with self-monitoring of blood glucose (SMBG) as the current standard of care. However, many people fail to adhere to recommended testing.10–13 Impediments to SMBG adherence include discomfort associated with obtaining a blood sample, inconvenience of carrying testing kits, perceived social stigma, needle phobia and difficulty interpreting results.14–18 Additionally, SMBG provides limited data on glucose levels and variability, as it measures a single time point.19 This can create difficulties for clinicians to recommend changes to therapy. Although continuous monitoring may provide additional information, it is expensive and therefore not universally reimbursed and utilised.18,20
Flash glucose monitoring is an alternative to traditional blood glucose monitoring. In this approach, a sensor worn by the patient permits continuous monitoring of interstitial glucose. A reader can then be passed over the sensor at any time to obtain a current reading, trend arrow and data from the past 8 hours. FreeStyle Libre (Abbott Diabetes Care, Witney, UK), a flash glucose monitoring system, was recently tested in intensively insulin-treated T1D population in the IMPACT trial.
The IMPACT trial21 was a 6-month, multicentre randomised controlled trial of the flash monitoring system versus SMBG in adults with well-controlled T1D (HbA1c <7.5% [58 mmol/mol]) using multiple daily injection insulin therapy or continuous subcutaneous insulin infusion and testing glucose levels at least 10 times/week. The study found a 25.8% reduction in favour of flash monitoring in number of hypoglycaemic events (<70 mg/dL [3.9 mmol/L]) without changing HbA1c.21 Given potential clinical benefits of intervention, this study sought to assess the value of using flash monitoring instead of SMBG alone through cost-effectiveness evaluation in patients with T1D receiving intensive insulin treatment in Sweden.
Research design and methods
The present study was performed using the IQVIA Core Diabetes Model v9.0 (IQVIA CDM).
IQVIA Core Diabetes Model
The IQVIA CDM is a non-product specific internet application to assess long-term health outcomes and economic consequences of interventions for T1D or T2D. The underlying mathematical engine consists of a series of diabetes-complication sub-modules that combine Markov techniques with Monte Carlo simulation and run simultaneously to capture comorbidities and outcomes associated with treatments of interest. These submodules are permitted to interact and each patient profile is updated at the end of each 1-year cycle to account for events across all submodules. The model captures differences in life expectancy, quality adjusted life years (QALYs), costs, cumulative incidences of complications due to adverse event-related intervention effects as well as HbA1c levels and other physiological parameters that affect risks of major diabetes complications. The model has been published previously in detail and has been extensively validated against clinical and epidemiological studies.22,23
In the model, changes in physiological parameters (e.g. HbA1c, blood pressure, weight, lipid parameters) are entered and translated into long-term microvascular and macrovascular complications based on specific risk equations derived from landmark studies like the Diabetes Control and Complications Trial and its extension Epidemiology of Diabetes Interventions and Complications in T1D.24–32 Most important for this analysis, however, is the hypoglycaemia module that considers three severity levels as well as diurnal versus nocturnal status. Severe hypoglycaemic events may require third-party medical assistance (SHE2s) or third-party non-medical assistance (SHE1s). The model also considers non-severe hypoglycaemic events (NSHEs).
Analyses took a Swedish payer perspective, evaluating costs and effects over a 50-year horizon, intended to capture a lifetime (e.g. <1% of the full cohort will remain alive) from the age at model entry (approximately 43.7 years). Swedish non-specific mortality information derived from WHO was included.33 Costs and effects were discounted at 3%. All analyses were run with 1,000 patients for 1,000 iterations.
Model inputs
Baseline characteristics
The model cohort was designed to represent the IMPACT trial population (Table 1).24,34–40 Some cohort characteristics were unavailable from IMPACT; published sources were used to supplement IMPACT cohort data with T1D population estimates.
Table 1: Cohort characteristics.
| Default value | Source | |
|---|---|---|
| Demographics | ||
| Start age (years, mean [SD]) | 43.7 (13.9) | Abbotts Diabetes Care, 201636 |
| Duration of diabetes (years, mean [SD]) | 22.0 (12.0) | Hayes et al., 201337 |
| Male (%) | 56.9% | Hayes et al., 201337 |
| Baseline risk factors | ||
| HbA1c (%, mean [SD]) | 6.78 (0.58) | Hayes et al., 201337 |
| Systolic blood pressure (mmHg, mean [SD]) | 126.0 (15.0) | Hayes et al., 201337 |
| Total cholesterol (mg/dL, mean [SD]) | 193.0 (38.0) | Hayes et al., 201337 |
| HDL (mg/dL, mean [SD]) | 72.0 (20.0) | Hayes et al., 201337 |
| LDL (mg/dL, mean [SD]) | 106.0 (33.0) | Hayes et al., 201337 |
| Triglycerides (mg/dL, mean [SD]) | 76.0 (45.0) | Hayes et al., 201337 |
| BMI (mean [SD]) | 25.0 (3.6) | Hayes et al., 201337 |
| eGFR (mean [SD]) | 91.7 (20.1) | Nathan et al., 201424 |
| Haemoglobin (mean, [SD]) | 14.5 (0.0) | Hayes et al., 201337 |
| WBC (mean [SD]) | 6.8 (0.0) | Paterson et al., 200738 |
| Heart rate (bpm, mean [SD]) | 68.0 (11.0) | Paterson et al., 200738 |
| Proportion smoker (%) | 14.0% | Hayes et al., 201337 |
| Cigarettes/day | 1 | Hayes et al., 201337 |
| Alcohol consumption (oz/week) | 1.58 | Hayes et al., 201337 |
| Racial characteristics (%) | ||
| White | 99.60% | Hayes et al., 201337 |
| Black | 0.40% | Hayes et al., 201337 |
| Hispanic | 0.00% | Hayes et al., 201337 |
| Native American | 0.00% | Hayes et al., 201337 |
| Asian/Pacific Islander | 0.00% | Hayes et al., 201337 |
| Utilities | ||
| Baseline | 0.7850 | Clarke et al., 200234 |
| SHE2 (daytime) | -0.055 | Evans et al., 201339 |
| SHE2 (nocturnal) | -0.057 | Evans et al., 201339 |
| SHE1 | -0.0183 | Marrett et al., 201040 |
| NSHE (calculated using flash monitoring event rate) | -0.002 | Lauridsen et al., 201435 |
| NSHE (calculated using SMBG event rate) | -0.00163 | Lauridsen et al., 201435 |
BMI = body mass index; bpm = beats per minute; eGFR = estimated glomerular filtration rate; HbAlc = glycated haemoglobin; HDL = high-density lipoprotein; LDL = low-density lipoprotein; NSHE = non-severe hypoglycaemic event; SD = standard deviation; SHE1 = severe hypoglycaemic event that may require third-party non-medical assistance; SHE2 = severe hypoglycaemic event that maybe require third-party medical assistance; SMBG = self-monitored blood glucose; WBC = white blood cells.
Intervention effects
Intervention effects for T1D were based on data from IMPACT and captured in Table 2.21,36,41,42 No significant differences were seen in the evolution of HbA1c between the two treatment arms in the IMPACT study, so an HbA1c increase of 0.12% (standard deviation [SD] 0.45%); 1.32 mmol/mol [SD 4.95]) compared to baseline was included for both flash monitoring and SMBG. NSHEs were populated using data from the IMPACT trial.36 The NSHE rate for the SMBG treatment arm was based on baseline symptomatic hypoglycaemia event data as a proxy for events below 70 mg/dL.43 The NSHE rate for the flash monitor arm was calculated by adjusting the baseline rate by percentage difference in events between SMBG and flash glucose monitoring over the study period (25.5% decrease in daytime and 33.2% decrease in nocturnal events).
Table 2: Treatment effects.
| Flash monitoring | SMBG | Source | |
|---|---|---|---|
| Physiological parameters | |||
| Change from baseline HbA1c (mean [SD]) | 0.12% (0.45%) | 0.12% (0.45%) | Bolinder et al., 201621 |
| Hypoglycaemic events - T1D | |||
| NSHEs (/100 PYs) | 4897.10 | 6760.00 | Abbott Diabetes Care, 201636 |
| SHE1 events (/100 PYs) | 282.24 | 282.24 | UK Hypoglycaemia Study Group, 200741 |
| SHE2 events (/100 PYs) | 37.76 | 37.76 | UK Hypoglycaemia Study Group, 200741 |
| Proportion of events that are nocturnal | 25.00% | 27.00% | Abbott Diabetes Care, 201636 |
| Other | |||
| Utility increment related to flash monitoring (mean [95% CI]) | 0.03 (0.023, 0.038) | 0.00 | Matza et al.42 |
CI = confidence interval; HbAlc = glycated haemoglobin; NSHE = non-severe hypoglycaemic event; PY = patient-year; SD = standard deviation; SHE1 = severe hypoglycaemic event that may require third-party non-medical assistance; SHE2 = severe hypoglycaemic event that maybe require third-party medical assistance; SMBG = self-monitored blood glucose; T1D = type 1 diabetes.
Few SHEs were observed in IMPACT as the trial was not designed to assess severe events. Therefore, data from the UK Hypoglycaemia Study Group41 was used to inform the total rate of severe events (320/100 patient-years [PYs]); these were used as a proxy to align with ‘significant’ hypoglycaemic events as noted in current guidance (<55mg/dL).43 The proportion of severe events that are SHE2s was derived from the literature (11.8%),27 resulting in 282.24 SHE1 events/100 PYs and 37.76 SHE2 events/100 PYs. These values were assumed equivalent across both treatment arms.
Intervention-related resource utilisation
Intervention-related resource use was based on reported values from IMPACT. Patients using flash monitoring required 182.5 test strips/year, 267.4 lancets/year and 45.8 units of insulin/day. It was also assumed that flash monitoring patients require 26 sensors/year and an extra physician visit in year 1. Patients with SMBG used 1,971 test strips/year, 657.6 lancets/year and 38.4 units of insulin/day.
Unit costs
Table 344–48 shows key cost inputs for the analyses, including intervention-related unit costs, total intervention costs, and costs for key acute events; Appendix 1 shows the full list of costs used in the analyses. Intervention-specific consumables reflect lowest-cost items available from Tandvards-Lakemedelformansverket (TLV).44 Costs were inflated as needed to 2016 using the consumer price index for Sweden from the Organization for Economic Co-operation and Development.49
Table 3: Key cost inputs.
| Default value (SEK) | Source | |
|---|---|---|
| Intervention: Unit Costs | ||
| Sensor | 526.78 | Abbott Diabetes Care |
| Reader (reimbursed every 2 years) | 599.00 | Abbott Diabetes Care |
| Flash monitoring test strip | 3.22 | Abbott Diabetes Care |
| Test strip | 2.34 | Tandvards-Och Lakemedelsformansverket, 201744 |
| Lancet | 0.30 | Tandvards-Och Lakemedelsformansverket, 201744 |
| Insulin (per unit) | 0.36 | Tandvards-Och Lakemedelsformansverket, 201744 |
| Physician visit | 1,426.59 | Skane, Sodra regionvardnamnden, 201445 |
| T1D Intervention costs | ||
| Annual flash monitoring cost (year 1) | 22,142.7 | Calculated |
| Annual flash monitoring cost (year 2+) | 20,716.1 | Calculated |
| Annual SMBG cost (year 1+) | 9,891.46 | Calculated |
| Direct costs for key acute events | ||
| SHE2 | 5,036.14 | Jonsson et al., 200646 Anderson et al., 200247 The Diabetes Control and Complications Trial Research Group, 199148 |
| SHE1 | 0.00 | Assumption |
| NSHE | 0.00 | Assumption |
NSHE = non-severe hypoglycaemic event; SHE1 = severe hypoglycaemic event that may require third-party non-medical assistance; SHE2 = severe hypoglycaemic event that maybe require third-party medical assistance; SMBG = self-monitored blood glucose; T1D = type 1 diabetes.
Utilities
Utilities and disutilities (Table 1;24,34–40 Appendix 2) are derived from published literature, with the baseline diabetes utility34 and complication-related values obtained from T2D populations,50 as it is not expected that quality of life for complications would differ based on diabetes type. For hypoglycaemia, literature shows that patients experience relatively high disutilities for initial non-severe events; yet, the disutility per event diminishes as frequency increases.35 Therefore, the IQVIA CDM automated incorporation of the Lauridsen approach to estimate NSHE disutility was utilised. A treatment-related utility benefit of 0.030 was applied to the flash monitor arm based on a recent time trade off (TTO) study.27 We explored this concept further with sensitivity analyses.
Analyses
The base case analysis compared flash monitor use against SMBG use, reflecting the intervention effects summarised in Table 2.21,36,41,42 In addition to the base case, scenario analyses (summarised in Table 4) explored the impact of key model assumptions. Note that due to the comprehensive nature of the IQVIA CDM, parameters are too numerous to perform systematic univariate sensitivity analyses; however, scenarios tested key inputs, assumptions, and alternate data sources to provide evidence-based clarity on sensitivity to each of these components.
Table 4: Summary of modelled scenarios and results.
| Analysis | Description | Flash monitoring | SMBG | Incremental | ICER/LY (SEK) | ICER/QALY (SEK) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Costs (SEK) | LYs | QALYs | Costs (SEK) | LYs | QALYs | Costs (SEK) | LYs | QALYs | ||||
| Base case | Flash monitoring versus SMBG, using default inputs and assumptions | 1,222,333 | 21.10 | 13.26 | 989,051 | 21.10 | 12.46 | 233,283 | 0.000 | 0.801 | NA | 291,130 |
| SA 1 | Alternate SMBG resource use assumption, year 1: for SMBG, incorporate extra observed resource use from the clinical trial. Remove the cost of severe hypoglycaemic events to avoid double counting | 1,181,555 | 21.10 | 13.26 | 948,907 | 21.10 | 12.46 | 232,648 | 0.000 | 0.801 | NA | 290,338 |
| SA 2 | Alternate SMBG resource use assumption, all years: for SMBG, incorporate extra observed resource use from the clinical trial. Remove the cost of severe hypoglycaemic events to avoid double counting | 1,141,246 | 21.10 | 13.26 | 962,283 | 21.10 | 12.46 | 178,963 | 0.000 | 0.801 | NA | 273,644 |
| SA 3 | Real world scan frequency with flash monitoring (-0.58% impact on HbA1c, versus 0% impact for SMBG) | 1,112,006 | 21.39 | 13.60 | 970,024 | 21.16 | 12.53 | 141,982 | 0.229 | 1.071 | 620,009 | 132,557 |
| SA 4 | Reduction in SHEs: 55% reduction in SHEs based on sensor data for <40mg/dL | 1,195,837 | 21.10 | 14.12 | 989,051 | 21.10 | 12.46 | 206,786 | 0.000 | 1.660 | NA | 124,705 |
| SA5 | Alternate NSHE data source:UK Hypo Study Group (2,900/100 patient-years for SMBG; 2,100/100 patient-years for flash monitoring) | 1,222,333 | 21.10 | 13.58 | 989,051 | 21.10 | 12.82 | 233,283 | 0.000 | 0.760 | NA | 305,784 |
| SA 6 | Flash monitoring treatment utility: vary treatment-related utility benefit of flash monitoring using the 95% CI (lower value 0.023) | 1,222,333 | 21.10 | 13.11 | 989,051 | 21.10 | 12.46 | 233,283 | 0.000 | 0.65 | NA | 358,180 |
| Flash monitoring treatment utility: vary treatment-related utility benefit of flash monitoring using the 95% CI (upper value 0.038) | 1,222,333 | 21.10 | 13.44 | 989,051 | 21.10 | 12.46 | 233,283 | 0.000 | 0.977 | NA | 239,830 | |
| SA 7 | Discount rate: investigate the impact of 0% discounting in lieu of base case 3% | 2,476,061 | 36.02 | 22.29 | 2,075,401 | 36.02 | 20.91 | 400,661 | 0.000 | 1.38 | NA | 290,439 |
| Discount rate: investigate the impact of 5% discounting in lieu of base case 3% | 832,167 | 15.88 | 10.06 | 656,947 | 15.88 | 9.46 | 175,220 | 0.000 | 0.60 | NA | 291,644 | |
| SA 8 | Time horizon: explore shorter time horizon, 5 years | 151,249 | 4.56 | 2.97 | 100,443 | 4.56 | 2.80 | 50,806 | 0.000 | 0.17 | NA | 297,460 |
| Time horizon: explore shorter time horizon, 10 years | 301,242 | 8.43 | 5.49 | 208,352 | 8.43 | 5.49 | 92,890 | 0.000 | 0.32 | NA | 293,770 |
CI = confidence interval; HbAlc = glycated haemoglobin; ICER = incremental cost effectiveness ratio; LY = life year; NSHE = non-severe hypoglycaemic event; QALY = quality-adjusted life years; SA = sensitivity analysis; SEK = Swedish kroner; SHE = severe hypoglycaemic event; SMBG = self-monitored blood glucose.
Two scenarios test the impact of incorporating resource utilisation observed in the trials. In these scenarios, trial-based use of resources such as ambulances, emergency room visits and hospitalisation were implemented. Based on the hypothesis that some of these differences may have been driven by hypoglycaemic events, assigned hypoglycaemic event costs were removed from the model. In the first scenario, these resources were assumed to apply in year 1 only (as in the trial), and the second analysis explored continued differences over the model horizon.
Another scenario explored the potential impact on cost-effectiveness due to real-world intervention effect, rather than trial-based values. Specifically, cross-sectional data from over 50,000 flash monitoring readers shows an average of 16 scans/day,51 which can be compared to a population average for patients with T1D of 5-6 tests/day.52 Miller et al. have shown an association in patients with T1D between number of daily blood glucose tests and HbA1c levels,52 which is replicated in flash monitor user data. Therefore, exploratory analysis, based on assuming that high scan frequency leads to HbA1c improvements, evaluated the potential impact of a higher scan rate on cost-effectiveness. Data revealed that patients who scan 16 times/day versus those who only scan 5-6 times/day have 0.58% lower HbA1c. For the purposes of this exploration, this value was applied as a potential decrease in HbA1c due to flash monitoring, compared to 0% decrease for SMBG.
Additional scenarios explored the impact of varying the flash monitoring utility benefit (95% confidence interval [CI] 0.023, 0.038), an alternate set of data (UK Hypo Study Group)41 to inform NSHE rates (2,900/100 PYs for SBMG; 2,100/100 PYs for flash monitoring when applying the IMPACT-based reduction due to flash monitoring), 50% decrease in SHEs based on observed decrease in sensor measurements under 40 mg/dL, and varied discount rates and time horizons.
Results
Disaggregated results, including total costs and QALYs per strategy, are reported in Table 4. In the base case analysis, total direct medical costs for flash monitor use were SEK1,222,333 compared to SEK989,051 for SMBG use in T1D. These costs aligned with an equivalent life expectancy of 21.1 life-years (LYs) due to non-differential survival effect, but with a total of 0.80 additional QALYs for flash monitor patients (13.26 versus 12.46 SMBG). The corresponding incremental cost-effectiveness ratio (ICER) is SEK291,130/QALY. Patients with T1D with flash monitoring experience 687 fewer NSHEs than those with SMBG; the cost per NSHE averted was SEK339.56.
Key scenarios evaluated to test the impact of assumptions or input values are reported in Table 4 and Figure 1. The most favourable ICERs - more than 50% lower than base case - reflect improved intervention effects. In the first case, reducing severe hypoglycaemia by 55% according to sensor-based reductions in 40 mg/dL events led to an ICER ofSEK124,705/QALY. Potential HbA1c improvement led to another similarly low ICER (SEK132,557/QALY); this ICER was based on exploring the potential relationship between high daily scan frequency found to occur in real-world use of the flash monitor and HbA1c level. Conversely, the highest ICER was associated with the lower 95% CI for health state utility benefit due to flash monitor use, which impacts quality-adjusted survival. Although the model is thus shown to be most sensitive to intervention effects on HbA1c, severe hypoglycaemia and monitoring-related health utility, all ICERs remained under SEK400,000/QALY.
Figure 1: Scenario analyses: ICER (SEK/QALY).
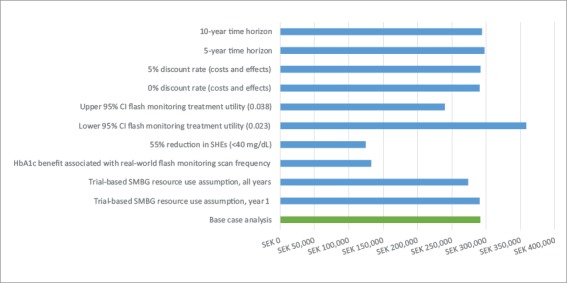
CI = confidence interval; HbAlc = glycated haemoglobin; ICER = incremental cost effectiveness ratio; QALY = quality-adjusted life years; SEK = Swedish kroner;SHE = severe hypoglycaemic event; SMBG = self monitoring of blood glucose.
Discussion
This is the first cost-effectiveness analysis to evaluate a flash monitoring system. Results demonstrate that use of flash monitoring is associated with a modest impact on diabetes-related costs and can be considered cost-effective compared to current standard of care (SMBG) in intensive insulin users with T1D. In clinical trials, use of the system has been shown to reduce the number of hypoglycaemic events without raising HbA1c across populations and may increase awareness of a patient’s glucose profile, potentially improving management of their condition. Although no survival difference is expected based on default intervention effects, meaningful QALY differences accrue over a lifetime horizon. These differences are due to offsetting hypoglycaemic events, and to a greater extent, to nearly eliminating the finger-pricking associated with SMBG, which has been shown to translate to a health utility improvement.51
When exploring additional scenarios, results are generally robust to evidence-based alternate assumptions. Model results may be sensitive to additional clinical benefits (e.g. HbA1c improvement or reduction in SHEs) as well as decrease in health utility associated with flash monitoring (which impacts QALY outcomes). However, ICERs remaining under willingness-to-pay ranges for Sweden for all scenarios. Although Sweden does not publish an explicit cost-effectiveness threshold, interventions have been accepted with an average ICER of €36,000/QALY (which is approximately SEK400,000/QALY) and publications note an ‘informal threshold’ of SEK500,000.53,54 Treatments for more severe conditions have been considered acceptable up to the much higher amount of €90,000/ QALY (SEK827,000 /QALY).54,55
A number of limitations for this study must be acknowledged. The analysis assumes that NSHEs are not associated with the occurrence of other more severe events like severe hypoglycaemia, myocardial infarction and mortality. However, in the Predictable Results and
Experience in Diabetes through Intensification and Control to Target: An International Variability Evaluation (PREDICTIVE) study, a high frequency of NSHEs was significantly associated with the occurrence of SHEs.56 The base case analysis also does not assume an explicit link between hypoglycaemic unawareness and downstream increased risk of SHEs. Given the extent of previously unrecognised hypoglycaemia detected by flash monitoring in the IMPACT trial, use of the flash monitoring system to better understand personal glucose trends may constitute an intervention to potentially help avoid hypoglycaemic unawareness and thereby reduce the risk of SHEs. Thus, reduction in SHEs was explored in scenario analysis.
Additionally, the analysis may not capture all health outcomes. For example, the model captures risk of cardiovascular disease based on HbA1c, lipid levels, blood pressure, comorbidities and body mass index. However, recent evidence suggests that people experiencing hypoglycaemic events may be at increased risk of cardiovascular disease.57–58 Although baseline characteristics in those T2D studies differ from our analysis, it raises the possibility that this analysis underestimates the value of offsetting hypoglycaemic events.
The main clinical data and patient characteristics for each analysis are taken from a 6-month trial of patients with well-controlled T1D, and may not exactly represent real-world effects of the flash monitor system or represent the patient population using flash monitoring in the real world. However, there were no protocol-mandated monitoring or adjustments to therapy, and default intervention effects may underestimate total benefit. This is based on recent evidence suggesting substantial clinical benefit to flash monitoring in real-world use; in addition to the cross-sectional real-world data that underpins this study’s HbA1c improvement scenario analysis,51 a recent meta-analysis has shown a similar level of HbA1c improvement (0.56% overall), with greater improvements in patients with higher baseline HbA1c levels upon initiating flash glucose monitoring use.59
Our analysis simplified the treatment pathway faced by patients by assuming glucose monitoring and insulin use do not change over time. In the absence of data, typical modelling practice is to assume no difference associated with treatment; therefore, any insulin change applying to both strategies equally would not alter the conclusions of this study.
Additionally, current utility values may not exactly reflect the quality-of-life impact of using flash monitoring. The intervention-associated utility benefit, derived from a time trade-off study, assumed that flash monitoring offsets the need for SMBG blood tests performed on average three times/day.42 However, guidelines recommend testing 6–10 times/day, and IMPACT resource utilisation indicates that during the trial, SMBG users tested 5.4 times/day versus 0.5 times/day for flash monitoring users. This greater difference in test frequency may translate into a larger utility benefit. Conversely, the time trade-off study measured utilities for patients based on the assumption of three tests versus no tests; therefore, minimal concurrent testing may lower the cited benefit. Sensitivity analyses applying the 95% CI from the time trade-off study showed that using the lower estimate of utility gain still results in a cost-effective result. The disutility associated with NSHEs is assumed to reflect the diminishing effect of each event as they become more frequent, as has been shown in recent research.35 However, the average value per event applying this technique is much smaller than that used in prior economic analyses,60 and therefore the ICERs in this study are likely to be more conservative but also more realistic relative to other published values.
Despite the limitations, this study facilitates understanding of the potential economic value of using flash monitoring versus SMBG in T1D intensive insulin users. This analysis shows that improved hypoglycaemia outcomes and health utility benefit associated with flash monitoring may translate into economic value compared to SMBG, with incremental costs per QALY under acceptable willingness-to-pay thresholds. As results remained robust across scenario analyses, flash monitoring may be considered cost-effective in a Swedish population among T1D intensive insulin users.
Funding Statement
Support: This work was supported by Abbott Diabetes Care.
References
- 1.IDF Diabetes Atlas. 8th edn. Brussels, Belgium: International Diabetes Federation; 2017. International Diabetes Federation. [Google Scholar]
- 2.Andersson A, Ahlbom A, Carlsson S. Diabetes prevalence in Sweden at present, and projections for year 2050. PLOSOne. 2015;10:e0143084. doi: 10.1371/journal.pone.0143084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:138–88. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 4.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:481–97. doi: 10.1016/j.ecl.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilmer TP. O’Connor PJ, Rush WA, et al. Predictors of health care costs in adults with diabetes. Diabetes Care. 2005;28:59–64. doi: 10.2337/diacare.28.1.59. [DOI] [PubMed] [Google Scholar]
- 6.Menzin J, Korn JR, Cohen J. et al. Relationship between glycaemic control and diabetes-related hospital costs in patients with type 1 or type 2 diabetes mellitus. J Manag Care Pharm. 2010;16:264–75. doi: 10.18553/jmcp.2010.16.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hex N, Bartlett C, Wright D. et al. Estimating the current and future costs of type 1 and type 2 diabetes in the UK, including direct health costs and indirect societal and productivity costs. Diabet Med. 2012;29:855–62. doi: 10.1111/j.1464-5491.2012.03698.x. [DOI] [PubMed] [Google Scholar]
- 8.The Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S. et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 9.Schütt M, Kern W, Krause U. et al. Is the frequency of selfmonitoring of blood glucose related to long-term metabolic control? Multicenter analysis including 24,500 patients from 191 centers in Germany and Austria. Exp Clin Endocrinol Diabetes. 2006;114:384–8. doi: 10.1055/s-2006-924152. [DOI] [PubMed] [Google Scholar]
- 10.Schnell O, Alawi H, Battelino T. et al. Consensus statement on self-monitoring of blood glucose in diabetes: A European perspective. Diabetes, Stoffwechsel und Herz. 2009;18:3–7. [Google Scholar]
- 11.National Institute for Health and Care Excellence. Type 1 diabetes in adults: diagnosis and management. 2015. Available at: www.nice.org.uk/guidance/ng17 (accessed 2 August 2018). [PubMed]
- 12.National Institute for Health and Care Excellence. Diabetes (type 1 and type 2) in children and young people: diagnosis and management. 2015. Available at: www.nice.org.uk/guidance/ng18 (accessed at 2 August 2018). [PubMed]
- 13.Lee WC, Smith E, Chubb B, Wolden ML. Frequency of blood glucose testing among insulin-treated diabetes mellitus patients in the United Kingdom. J Med Econ. 2014;17:167–75. doi: 10.3111/13696998.2013.873722. [DOI] [PubMed] [Google Scholar]
- 14.Wagner J, Malchoff C, Abbott G. Invasiveness as a barrier to self-monitoring of blood glucose in diabetes. Diabetes Technol Ther. 2005;7:612–19. doi: 10.1089/dia.2005.7.612. [DOI] [PubMed] [Google Scholar]
- 15.Hortensius J, Kars MC, Wierenga WS. et al. Perspectives of patients with type 1 or insulin-treated type 2 diabetes on self-monitoring of blood glucose: a qualitative study. BMC Public Health. 2012;12:167. doi: 10.1186/1471-2458-12-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincze G, Barner JC, Lopez D. Factors associated with adherence to self-monitoring of blood glucose among persons with diabetes. Diabetes Educ. 2004;30:112–25. doi: 10.1177/014572170403000119. [DOI] [PubMed] [Google Scholar]
- 17.Fisher WA, Kohut T, Schachner H, Stenger P. Understanding self-monitoring of blood glucose among individuals with type 1 and type 2 diabetes: an information-motivation-behavioral skills analysis. Diabetes Educ. 2011;37:85–94. doi: 10.1177/0145721710391479. [DOI] [PubMed] [Google Scholar]
- 18.Langendam M, Luijf YM, Hooft L. et al. Continuous glucose monitoring systems for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD008101.pub2. 1:CD008101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nardacci EA, Bode BW, Hirsch IB. Individualizing care for the many: the evolving role of professional continuous glucose monitoring systems in clinical practice. Diabetes Educ. 2010;36:4S–19S. doi: 10.1177/0145721710362798. [DOI] [PubMed] [Google Scholar]
- 20.Heinemann L, Franc S, Phillip M. et al. Reimbursement for continuous glucose monitoring: a European view. J Diabetes Sci Technol. 2012;6:1498–502. doi: 10.1177/193229681200600631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolinder J, Antuna R, Geelhoed-Duijvestijn P. et al. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre,-non-masked, randomised controlled trial. Lancet. 2016;388:2254–63. doi: 10.1016/S0140-6736(16)31535-5. [DOI] [PubMed] [Google Scholar]
- 22.Palmer AJ, Roze S, Valentine WJ. et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and costeffectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20:S5–S26. doi: 10.1185/030079904X1980. [DOI] [PubMed] [Google Scholar]
- 23.McEwan P, Foos V, Palmer JL. et al. Validation of the IMS CORE Diabetes Model. Value Health. 2014;17:714–24. doi: 10.1016/j.jval.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Nathan DM. DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9–16. doi: 10.2337/dc13-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gubitosi-Klug RA. DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: summary and future directions. Diabetes Care. 2014;37:44–9. doi: 10.2337/dc13-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lachin JM, Orchard TJ, Nathan DM. DCCT/EDIC Research Group. Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:39–43. doi: 10.2337/dc13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foos V, Varol N, Curtis BH. et al. Economic impact of severe and non-severe hypoglycaemia in patients with Type 1 and Type 2 diabetes in the United States. J Med Econ. 2015;18:420–32. doi: 10.3111/13696998.2015.1006730. [DOI] [PubMed] [Google Scholar]
- 28.De Boer IH. DCCT/EDIC Research Group. Kidney disease and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:24–30. doi: 10.2337/dc13-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Boer IH, Rue TC, Cleary PA. et al. Long-term outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. 2011;171:412–20. doi: 10.1001/archinternmed.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin CL, Albers JW, Pop-Busui R. DCCT/EDIC Research Group. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:31–8. doi: 10.2337/dc13-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White NH, Sun W, Cleary PA. et al. Prolonged Effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the diabetes control and complications trial. Arch Ophthalmol. 2008;126:1707–15. doi: 10.1001/archopht.126.12.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Albers JW, Herman WH, Pop-Busui R. et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33:1090–6. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.World Health Organization. Mortality Database. 2014. Available at: www.who.int/healthinfo/mortality_data/en/ (accessed 31 July 2014)
- 34.Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62) Med Decis Making. 2002;22:340–9. doi: 10.1177/0272989X0202200412. [DOI] [PubMed] [Google Scholar]
- 35.Lauridsen JT, Lønborg J, Gundgaard J, Jensen HH.. diminishing marginal disutility of hypoglycaemic events: results from a time trade-off survey in five countries. Qual Life Res. 2014;23:2645–50. doi: 10.1007/s11136-014-0712-x. [DOI] [PubMed] [Google Scholar]
- 36.Abbott Diabetes Care. IMPACT Trial data on file. 2016.
- 37.Hayes AJ, Leal J, Gray AM. et al. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia. 2013;56:1925–33. doi: 10.1007/s00125-013-2940-y. [DOI] [PubMed] [Google Scholar]
- 38.Paterson AD, Rutledge BN, Cleary PA. et al. The effect of intensive diabetes treatment on resting heart rate in type 1 diabetes: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Diabetes Care. 2007;30:2107–12. doi: 10.2337/dc06-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Evans M, Khunti K, Mamdani M. et al. Health-related quality of life associated with daytime and nocturnal hypoglycaemic events: a time trade-off survey in five countries. Health Qual Life Outcomes. 2013;11:90. doi: 10.1186/1477-7525-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marrett E, Radican L, Davies MJ, Zhang Q. Assessment of severity and frequency of self-reported hypoglycaemia on quality of life in patients with type 2 diabetes treated with oral antihyperglycaemic agents: a survey study. BMC Res Notes. 2011;4:251. doi: 10.1186/1756-0500-4-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.UK Hypoglycaemia Study Group. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–7. doi: 10.1007/s00125-007-0599-y. [DOI] [PubMed] [Google Scholar]
- 42.Matza LS, Stewart KD, Davies EW. et al. Health state utilities associated with glucose monitoring devices. Value Health. 2017;20:507–11. doi: 10.1016/j.jval.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 43.American Diabetes Association. Standards of medical care in diabetes – 2017. Journal of Clinical and Applied Research and Education. 2017;40:S1–134. [Google Scholar]
- 44.Tandvårds-Och Läkemedelsförmånsverket (TLV). Sök i database [Drug database, in Swedish]. 2017. Available at: www.tlv.se/beslut/sok/lakemedel/ (accessed 3 August 2018)
- 45.Skåne, Södra regionvårdnämnden. Regionala priser och ersattningar for sodra sjukvardsregionen 2014. Available at: www.skane.se/Upload/Webbplatser/Sodra%20regionvardsnamnden/prislista/2014/helaprislistan2014.pdf (accessed 2015)
- 46.Jonsson L, Bolinder B, Lundkvist J. Cost of hypoglycaemia in patients with Type 2 diabetes in Sweden. Value Health. 2006;9:193–8. doi: 10.1111/j.1524-4733.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- 47.Anderson S, Høgskilde PD, Wetterslev J. et al. Appropriateness of leaving emergency medical service treated hypoglycaemic patients at home: a retrospective study. Acta Anaesthesiol Scand. 2002;46:464–8. doi: 10.1034/j.1399-6576.2002.460424.x. [DOI] [PubMed] [Google Scholar]
- 48.The Diabetes Control and Complications Trial Research Group. Epidemiology of severe hypoglycaemia in the Diabetes Control and Complications Trial. Am J Med. 1991;90:450–9. [PubMed] [Google Scholar]
- 49.Organization for Economic Co-operation and Development (OECD). Consumer price index (all items) for Sweden. Available at: www.scb.se/hitta-statistik/sverige-i-siffror/samhalletsekonomi/prisernas-utveckling (accessed 30 September 2015).
- 50.Beaudet A, Clegg J, Thuresson PO. et al. Review of utilities for economic modeling in Type 2 diabetes. Value Health. 2014;17:462–70. doi: 10.1016/j.jval.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Dunn TC, Xu Y, Hayter G, Ajjan RA. Real-world flash glucose monitoring patterns and associations between selfmonitoring frequency and glycaemic measures: A European analysis of over 60 million glucose tests. Diabetes Res Clin Pract. 2018;137:37–46. doi: 10.1016/j.diabres.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 52.Miller KM, Beck RW, Bergenstal RM. et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36:2009–14. doi: 10.2337/dc12-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bolin K, Sandin R, Koltowska-Häggström M. et al. The costeffectiveness of growth hormone replacement therapy (Genotropin®) in hypopituitary adults in Sweden. Cost Eff Resour Alloc. 2013;11:24. doi: 10.1186/1478-7547-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Persson U. Value & Valuation of Health Technologies The Swedish Experience. 2010. www.swisshta.ch/index.php/Internationale_Erfahrungen.html?file=tl_files/SwissHTA/documents/Workshop_11_2010/Praesentationen/Praesentation_Persson_20101005.pdf Available at: (Accessed 30 June 2015) [Google Scholar]
- 55.European Central Bank. Euro to Swedish Kroner exchange rate. 2015. Available at: www.ecb.europa.eu/stats/policy_and_exchange_rates/euro_reference_exchange_rates/html/eurofxref-graph-sek.en.html (accessed August 2018)
- 56.Sreenan S, Andersen M, Thorsted BL. et al. Increased risk of severe hypoglycaemic events with increasing frequency of non-severe hypoglycaemic events in patients with type 1 and type 2 diabetes. Diabetes Ther. 2014;5:447–58. doi: 10.1007/s13300-014-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsu P-F, Sung S-H, Cheng H-M. et al. Association of clinical symptomatic hypoglycaemia with cardiovascular events and total mortality in type 2 diabetes: a nationwide populationbased study. Diabetes Care. 2013;36:894–900. doi: 10.2337/dc12-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goto A, Aran OA, Goto M. et al. Severe hypoglycaemia and cardiovascular disease: a systematic review and meta-analysis with bias analysis. BMJ. 2013;347:f4533. doi: 10.1136/bmj.f4533. [DOI] [PubMed] [Google Scholar]
- 59.Siebold A, Ells S, Schlaeger C. et al. A meta-analysis of real world observational studies on the impact of flash glucose monitoring on glycemic control as measured by HbA1c. Presented at: American Diabetes Association (ADA) 78th Scientific Sessions, Orlando, Florida, USA, 22–26 June 2018 [Google Scholar]
- 60.Currie CJ, Morgan CL, Poole CD. et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22:1523–34. doi: 10.1185/030079906X115757. [DOI] [PubMed] [Google Scholar]


