Abstract
Because the musculoskeletal anatomy of the trunk is the framework for the behaviors of locomotion, ventilation, and body support in lepidosaurs, comparative study of trunk anatomy in this group is critical for unraveling the selective pressures leading to extant diversity in axial form and function among vertebrates. This work uses gross dissection and computed tomography to describe the muscular and skeletal anatomy of the trunk of varanid lizards (Varanidae, Anguimorpha). Gross muscle dissections were conducted to investigate the axial muscular anatomy of Varanus exanthematicus, Varanus giganteus, Varanus rosenbergi, and Varanus panoptes. Computed tomography scans of these and additional varanid lizards from the Varanus and Odatria subgenera were conducted to investigate rib and vertebral number and gross morphology. The number of vertebrae differs between species, with 27–35 presacral and 47–137 postsacral vertebrae. Although the number of floating and abdominal ribs in varanids is variable, most species examined have three to four cervical ribs and three true ribs. Attachment and insertion points of the epaxial and hypaxial musculature are detailed. The body wall has four main hypaxial layers, from superficial to deep: oliquus externus, intercostalis externi, intercostalis internii, and transversus. Varanids differ from other investigated lepidosaurs in having supracostalis dorsus brevis (epaxial) and levator costae (hypaxial), which independently connect each rib to the vertebral column. Although more basic muscle descriptions of the body wall in reptiles are needed, comparisons with the condition in the green iguana (Iguana iguana) can be made.
Keywords: epaxial, hypaxial, lepidosaur, musculoskeletal, varanidae
Introduction
Every tetrapod has a trunk made up of vertebrae, ribs, and multiple, overlapping muscle layers. The arrangement of these bones and muscles, however, differs greatly between species, allowing for highly derived body forms such as snakes and frogs (Koob & Long, 2000; Kardong, 2006; Young & Kardong, 2010; Bonnan, 2016). Because these bones and muscles serve as the vertebrate chassis, diversity in this anatomy represents multiple solutions to the biomechanical problems of locomotion (Gans et al. 1978; Carrier, 1996; Reilly & Delancey, 1997; Hoff & Wassersug, 2000; O'Reilly et al. 2000; Schilling, 2011), support (Janis & Keller, 2001; Daeschler et al. 2006), and ventilation (Carrier, 1996; Brainerd, 1999). Analysis of this diversity is therefore a useful lens to reveal the differing biomechanical and evolutionary pressures that led to the diverse vertebrate body shapes we observe today (Carrier, 1987; Burke et al. 1995; Koob & Long, 2000; Wiens & Slingluff, 2001; Ward & Brainerd, 2007). Although cataloguing this diversity is a logical first step, axial musculature remains relatively undescribed in many groups of terrestrial vertebrates. This work seeks to characterize the musculoskeletal anatomy of the trunk of monitor lizards (Varanidae), a group of Anguimorph lizards living in Africa, Asia, and Australia consisting of 53 species (Fitch et al. 2006). Varanids are diverse lepidosaurs with a conserved body plan (Pianka, 1995) that has long been of interest to comparative anatomists (Wolf, 1933) and physiologists (Bennett, 1973).
The axial anatomy of varanid lizards was first described by Nishi (1938), who examined Varanus varius, a large arboreal and terrestrial monitor from Australia. The axial musculature of reptiles was later reviewed by Gasc (1981), who examined Varanus griseus, Varanus rudicollis, and Varanus niloticus. Ritter (1995) described the gross anatomy of most of the epaxial muscles of Varanus salvator, and compared them to Carrier's work (1989; 1990) on the Green iguana (Iguana iguana). More recently, Tsuihiji (2007) conducted a comparative study focused on the attachment and insertion points of the longissmus, iliocostalis, and hypaxial muscles in Diapsida, examining Varanus exanthematicus and Varanus salvadori. Al‐Hassawi (2007) reviewed the musculature of the neck in lizards but did not address the trunk region. Finally, Moritz & Schilling (2013) used histochemistry to investigate the fiber types of the perivertebral musculature in many lizards, including the savannah monitor (V. exanthematicus).
The vertebral and rib morphology of reptiles was thoroughly reviewed by Hoffstetter & Gasc (1969). The study of the axial skeleton also has a long history in chameleons, starting with Siebenrock (1893). Recent, detailed anatomical analysis of the axial skeleton has been undertaken in this group by Čerňanský et al. (2014) and in Cordyliformes generally (Čerňanský, 2016). A comparative study of the axial skeleton in the neck region was carried out in lizards by Al‐Hassawi (2007). Greer (1989) contributed quantitative data on the number and type of vertebrae in many Australian varanid species. Finally, the osteology of two Anguimorphs has been well described by Conrad, including Shinosaurus (Conrad, 2006a) and a basal Anguimorph lizard from the Eocene of Wyoming (Conrad, 2006b).
Although there is a history of comparative research on lizard musculature, few authors have comprehensively described the arrangement of muscles in specific taxa. Furthermore, disagreements exist in the literature about the topology and naming of certain muscles, and different studies include or ignore certain muscles. This work seeks to fill in the gaps in previous research by comprehensively describing the hypaxial muscles in V. exanthematicus including insertion and attachment points of each muscle and comparing the musculoskeletal morphology among Australian varanids, which vary considerably in habitat (Thompson et al. 2008), metabolic rate (Clemente et al. 2009), body size (Pianka, 1995), and overall body proportions (Thompson et al. 2008). Although few studies of this kind exist, comparisons between varanids and the green iguana (I. iguana) are possible. This comparison is valuable because I. iguana and V. exanthematicus are phylogenetically well‐separated (Wiens et al. 2012; Pyron et al. 2013; Reeder et al. 2015; Zheng & Wiens, 2016). Furthermore, varanids are active foragers with high metabolic rates (Clemente et al. 2009) that use buccal pumping to overcome ventilatory‐locomotor constraints (Wang et al. 1997; Owerkowicz et al. 1999), whereas green iguanas are herbivores that are constrained from simultaneous breathing and walking (Wang et al. 1997) and sustained exercise (Farmer & Hicks, 2000). Although some descriptions of the axial musculature of snakes exist (Mosauer, 1935; Pregill, 1977; Penning, 2018), comparisons between varanids and snakes are problematic due to the extreme specializations for limblessness in snakes (Gasc, 1981).
Methods
Six individuals of V. exanthematicus were dissected and examined to confirm previous published findings and describe the muscular anatomy of this species in more detail. One individual each of Varanus giganteus, Varanus panoptes, and Varanus rosenbergi were also dissected.
Computed tomography data were used to investigate the skeletal anatomy of additional available varanid species. Skeletal anatomical findings were confirmed through gross dissection in V. exanthematicus only (n = 6). The number of individuals used in each skeletal analysis are indicated in Tables 1 and 2. Animals used in this study were made available from previous studies or loaned from museum collections. Computed tomography data were also used to prepare the rendering of the skeleton used in the illustrations. To render an image that could readily display all of the axial muscles simultaneosly, the sternum and sternal rib elements were combined from different digital viewpoints than the rest of the skeleton.
Table 1.
Number of vertebrae in varanids
| Varanus sp. | Presacral | Postsacral | ||||
|---|---|---|---|---|---|---|
| Range | Mean | n | Range | Mean | n | |
| V. acanthurus a | 28–29 | 28.3 | 12 | 81–95 | 87.0 | 5 |
| V. albigularis b | 27 | – | 1 | – | – | – |
| V. baritji b | 27 | – | 1 | – | – | – |
| V. brevicauda a | 31–35 | 32.3 | 7 | 47–66.5 | 56.5 | 3 |
| V. bushi b | 30 | – | 1 | – | – | – |
| V. caudolineatus b | 28 | –‐ | 1 | – | – | – |
| V. eremius a | 29 | 29.0 | 4 | 110 | – | 1 |
| V. exanthematicus b | 28 | – | 1 | – | – | – |
| V. giganteus a | 30 | – | 1 | 130 | – | 1 |
| V. gilleni a | 29–30 | 29.3 | 16 | 70–82 | 75.2 | 6 |
| V. gouldii a | 29–30 | 29.2 | 5 | 109–115 | 112.3 | 3 |
| V. hammersleyensis b | 29 | – | 1 | – | – | – |
| V. indicus a | 29 | 29.0 | 5 | 119–128 | 124.0 | 3 |
| V. jobiensis b | 29 | – | 1 | – | – | – |
| V. keithornei b> | 29 | – | 1 | – | – | – |
| V. komodoensis b | 28 | – | 1 | – | – | – |
| V. mertensi a | 29 | 29.0 | 3 | 114–118 | 116 | 2 |
| V. mitchelli a | 30–31 | 30.3 | 4 | 122–123 | 122.3 | 3 |
| V. niloticus b | 29 | – | 1 | – | – | – |
| V. pilbarensis a | 29 | – | 1 | 102+ | – | 1 |
| V. panoptes a | 29 | 29.0 | 2 | 123 | – | 1 |
| V. prasinus b | 29 | – | 1 | – | – | – |
| V. primordius a | 29–30 | 29.3 | 4 | 74–81 | 77.5 | 2 |
| V. rosenbergi b | 29 | 29 | 2 | – | – | – |
| V. semiremex a | 29–32 | 30.5 | 2 | 100 | – | 1 |
| V. spenceri a | 29 | – | 1 | – | – | – |
| V. storri a | 27–28 | 27.2 | 9 | 73–78 | 75.5 | 5 |
| V. timorensis a | 29–31 | 29.6 | 13 | 102–107 | 104.6 | 5 |
| V. tristis a | 29 | 29.0 | 9 | 105–117 | 112.3 | 6 |
| V. varius a | 29 | 29.0 | 9 | 137–143 | 139.4 | 8 |
aGreer (1989). bPresent study.
Table 2.
Number of different types of ribs in extant varanid lizards
| Varanus sp. | Cervical | True | Floating | ‘Abdominal’ |
|---|---|---|---|---|
| V. acanthurus (n = 1) | 4 | 3 | 12 | 3 |
| V. albigularis (n = 1) | 4 | 3.5a | 7.5a | 5 |
| V. baritji (n = 1) | 4 | 3 | 11 | 3 |
| V. brevicauda/sparnus (n = 2) | 3 | 3 | 15–21b | 3 |
| V. bushi (n = 1) | 4 | 3 | 14 | 3 |
| V. caudolineatus (n = 1) | 4 | 3.5a | 11.5 | 3 |
| V. eremius (n = 1) | 4 | 3 | 15 | 4 |
| V. exanthematicus (n = 3) | 4 | 3 | 8–10 | 3–5 |
| V. giganteus (n = 3) | 3 | 3 | 11 | 4 |
| V. gilleni (n = 1) | 4 | 3 | 12 | 3 |
| V. gouldii (n = 2) | 3 | 3 | 12 | 3 |
| V. hammersleyensis (n = 1) | 5 | 3 | 11 | 5 |
| V. indicus (n = 1) | 3 | 3 | 12 | 3 |
| V. jobiensis (n = 1) | 3 | 3 | 11 | 3 |
| V. keithornei (n = 1) | 3 | 3 | 12 | 3 |
| V. komodoensis (n = 1) | 4 | 3 | 11 | 4 |
| V. mertensi (n = 1) | 4 | 3 | 11 | 5 |
| V. mitchelli (n = 1) | 3 | 3 | 12 | 4 |
| V. niloticus (n = 1) | 3 | 4 | 9 | 5 |
| V. panoptes (n = 3) | 4 | 3 | 11–12 | 4 |
| V. prasinus (n = 1) | 3 | 3 | 12 | 3 |
| V. rosenbergi (n = 2) | 4 | 3 | 11 | 4 |
| V. semiremex (n = 1) | 3 | 3 | 11 | 4 |
| V. spenceri (n = 2) | 4 | 3 | 11 | 4 |
| V. storri (n = 1) | 4 | 3 | 11 | 4 |
| V. varius (n = 1) | 4 | 3 | 11 | 4 |
In these animals, the fourth sternal rib is mostly ossified but does not fully connect to the sternum.
Varanus brevicauda was split into V. brevicauda and V. sparnus (Doughty, 2014), which has a more elongate body and is found in the northeastern part of the species complex range. The individual (Queensland Museum J52766) with 21 floating ribs, labeled V. brevicauda, was collected before the taxonomic split in the range of V. sparnus, east of Broome, Western Australia, and is thus probably V. sparnus.
Results
Vertebrae and ribs
There are 28 presacral vertebrae in V. exanthematicus and the sixth vertebra is the first to bear ribs (Fig. 1). The number of presacral vertebrae ranges between 27 and 35 among observed varanids (Table 1). All observed vertebrae were procoelous. In V. exanthematicus, the first four ribs are cervical ribs, consisting of a single bony portion. The first two ribs are significantly smaller than the third and fourth. Caudally, three bipartite true ribs consist of a bony vertebral portion and a sternal portion made of calcified cartilage that articulates with the sternum (Fig. 2). The vertebral and sternal portions are joined by a flexible intercostal joint, but the sternal portions are also flexible at its most laterally extending point, such that there are functionally two intercostal joints among the true ribs.
Figure 1.
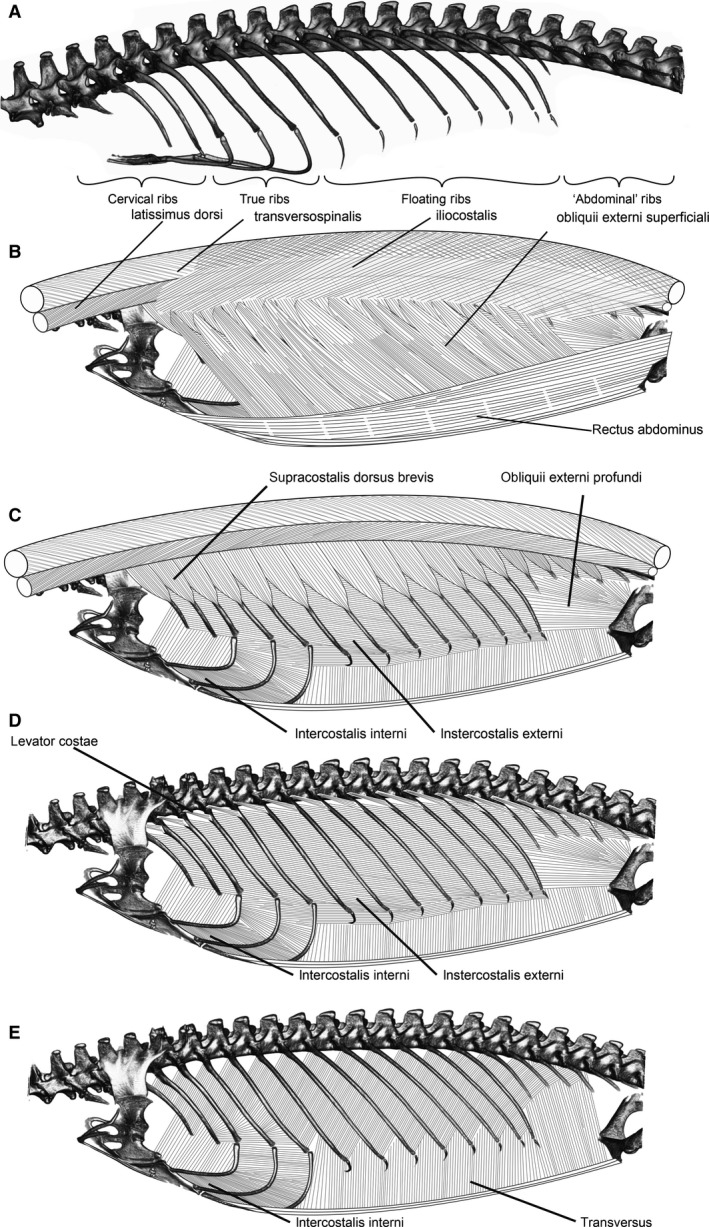
Axial skeleton and successive muscle layers in Varanus exanthematicus. (A) Axial skeleton showing the vertebral column, sternum, and ribs. The pectoral girdle has been removed for clarity. (B–E) Layers of hypaxial and epaxial layers moving from superficial to deep. For details of attachment and origin points for each muscle, see text. To render an image that could readily display all of the axial muscles simultaneously, the sternum and sternal rib elements were combined from different digital viewpoints than the rest of the skeleton.
Figure 2.
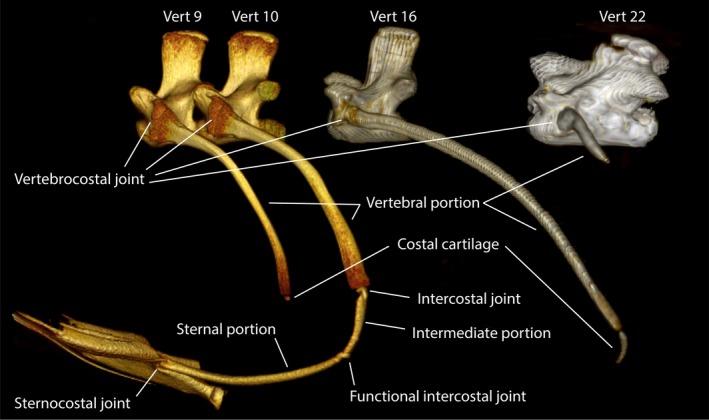
Rib morphology in Varanus exanthematicus. The cervical ribs (e.g. on vert 9) have a bony vertebral portion and sometimes a stubby costal cartilage. The true ribs (e.g. on vert 10) have a bony vertebral portion and a sternal portion of calcified cartilage that reach the sternum. The floating ribs (e.g. on vert 16) have a bony vertebral portion and a costal cartilage. The caudal floating ‘abdominal’ ribs (e.g. on vert 22) are much shorter than the preceding floating ribs and usually do not have any associated costal cartilage. The vertebral portion of the true ribs corresponds to the ancestral vertebrocostal rib and the sternal portion to the fusion of the ancestral intercostal and sternocostal ribs. See text for details.
Further caudally lie a range of floating ribs that bear no connection to the sternum. The number and anatomy of these ribs in V. exanthematicus is variable between individuals. There are 8–10 longer floating ribs, with 3–5 further floating ribs (sometimes referred to as abdominal ribs) which are comparatively much shorter. Variation in rib numbers among varanids is summarized in Table 2.
Among the longer, cranial group of floating ribs, at least the first six (sometimes all) are bipartite, consisting of a bony vertebral portions and short costal cartilages. The next 3–4 floating ribs are usually bipartite, but in one animal they were occasionally tripartite, with a mobile joint dividing the dorsal portion into two parts, both proximal to the costal cartilage. The final 3–5 floating ribs are much shorter and consist solely of a bony vertebral portion. All of the intercostal joints among the floating ribs are relatively flexible, allowing the rib segments to bend between approximately 80° and 0° angles.
Musculature
Because no substantial differences in the insertion or attachment points or topologies among either the epaxial or hypaxial muscles among the four species examined were observed, the following descriptions, based on dissections of V. exanthematicus, apply generally to all species examined (Fig. 1).
Epaxial musculature
The anatomy of many epaxial muscles (longissimus dorsi, transversopinalis, and iliocostalis), including details of insertion and origin tendons, have been described in detail previously (Gasc, 1981; Ritter, 1995; Tsuihiji, 2007) and will not be described here.
Supracostalis dorsus brevis
Supracostalis dorsus brevis lies superficial to the intercostal muscles and is strongly developed in V. exanthematicus. The muscle is segmentally divided into triangularly shaped sheets that originate from the aponeurosis that divides the longissimus from iliocostalis and insert along the dorsal aspect of each dorsal rib between one‐ or two‐thirds of the way down its length. Among the tripartite floating ribs (ribs 13–15), the supracostalis inserts onto the rib precisely at the distal edge of the bony, vertebral rib portion, immediately proximal to the intercostal joint.
Hypaxial musculature
Obliqus externus
The obliqus externus is well‐developed in V. exanthematicus, consisting of two portions: superficialis and profundus. The superficialis covers the lateral body wall. Ventrally, it lies medial to the rectus abdominus lateralis and originates at the margin between the rectus abdominus lateralis and the rectus abdominus ventralis. Fibers of the superficialis insert onto the lateral aspect of the dorsal ribs approximately one‐third of the way down their length by means of short tendons continuous with those of the supercostalis dorsus brevis muscle. Wide sheets of muscle course caudoventrally to their origin at the margin of the rectus abdominus.
Fibers of the profundus originate on the pubes and course cranially and craniodorsally, connecting the pelvic girdle to the caudal aspects of the 11th dorsal (8th floating) rib and the ventral and caudal aspects of the following dorsal ribs (12–16th).
Obliqus interni
This muscle is not present in varanids.
Intercostales externi
Fibers of the intercostales interni run obliquely, originating from the cranial aspect of each rib and inserting onto the caudal aspect of the next most cranial rib. The muscle occupies the intercostal space, extending dorsally to the top of the caudal aspect of each vertebral rib segment and ventrally to cover fully the costal cartilages of the floating ribs and approximately the dorsal third of each sternal portions of the true ribs. Between dorsal rib segments and from floating rib to floating rib, fibers course dorsocranially at an angle of approximately 15° above the long axis of the body.
Between floating rib sternal segments and from the sternal segment of the last true rib to the sternal segment of the first floating rib, fibers course cranially parallel to the long axis of the body. Between sternal rib segments, the fibers course parallel to the long axis of the body.
Intercostales interni
Fibers of the intercostales interni muscles run from rib to rib, extending from the cranial aspect of each dorsal rib to the sternocostal joints in the region of the true ribs, and to the ventral aspect of each floating rib. Between the dorsal ribs, they course caudodorsally at approximately a 60° angle above the long axis of the body and parallel to the long axis of the body between the sternal segments of the true ribs.
Levator costae
The levator costae is a small muscle, deep to the longissimus dorsi and external intercostal, that connects each rib to its articulating vertebrae. Fibers originate from the dorsocaudal aspect of the diapophysis, fanning out to insert on the dorsal edge of the cranial aspect of the next vertebral rib segment.
Transversus
The transversus, the deepest and thinnest of the body wall muscles, courses dorsally to insert along the cranial aspect of the ventral quarter of the vertebral portion of each bipartite dorsal rib. Fibers caudal to the bipartite ribs insert upon the lumbo‐dorsal fascia, covering the short, unipartite caudal ribs. Fibers originate from medial fascia deep to the sternum and from the fascia at the lateral margin of the rectus abdominus caudal to the sternum.
Rectus abdominus
The rectus abdominus, consisting of the main, ventral muscle belly and the superficial rectus abdmonius lateralis (see Bhullar, 2009) covers the ventral and much of the lateral aspects of the abdomen. Fibers originate from the entire cranial aspect of the pubis and they insert on the ventral aspect of the sternum, blending with fibers of the posterior portion of the pectoralis.
Discussion
The ancestral state likely possessed three rib portions or segment: the bony vertebrocostal, a cartilaginous intercostal, and a cartilaginous sternocostal (Hoffstetter & Gasc, 1969). The sternal rib portions of varanids are made of the ancestral intercostal and sternocostal ribs (Hoffstetter & Gasc, 1969), so the functional joint at the lateral‐most projecting point of the sternal portions probably represent an incomplete fusion (Fig. 2) of these ancestral rib portions. It seems likely, therefore, that the additional rib portion seen among the floating ribs in one individual is a variant dorsal rib portion representing the vertebrocostal rib only, instead of an incomplete fusion of the vertebrocostal and intercostal ribs. Hoffstetter & Gasc (1969) report that the articulation between cervical ribs and their vertebrae is often doubled, such that these cranial ribs are bicapitate. To me it seems that these ribs instead articulate with the vertebrae along one broad region shaped like an ellipse running dorsoventrally.
The numbers of vertebrae and ribs are fairly variable among varanids. The variation in postsacral vertebral number is probably underestimated without Varanus kingorum, which has an extremely long tail (Cogger, 2014). Varanus eremius and brevicauda brevicauda are closely related (Vidal et al. 2012), small, terrestrial (Clemente et al. 2009) monitors that both have an unusually high number of floating ribs. Fewer floating ribs are found in the African ‘niloticus group’ (Vidal et al. 2012) (Varanus albigularis, V. exanthematicus, Varanus niloticus) than in the Australian monitors, although these species may have more true ribs.
Varanus exanthematicus has two muscles not found in Iguana iguana: levator costae and supracostalis dorus brevis. These muscles are both positioned (especially the supracostalis dorsus brevis) to lift the rib cage or, because the origins are on the vertebrae instead of other ribs, each rib independently relative to the vertebral column. Supracostalis dorsus brevis is positioned to effect caliper motion of the ribs (rotation around a dorsoventral axis) and may be responsible for the dorsoventral flattening often seen in monitors during basking (Bartholomew & Tucker, 1964), combat (Carpenter et al. 1976), and defensive (Johnson, 1976; Thompson, 1995) or threat displays (Murphy & Lamoreaux, 1978). Finally, because both muscles attach at the same part of the rib, the supracostalis dorsus brevis may facilitate the lateral bending role (Ritter, 1996) of the external oblique. If contraction of the supracostalis brevis holds the ribs in place relative to the vertebral column, the external oblique could better accomplish lateral bending by pulling on the ribs. This muscle was first named by Nishi (1938) but was ignored by Gasc (1981), Ritter (1995), and Tsuihiji (2007). Perhaps this muscle does not occur in the species examined by Gasc and Ritter, but it was present in every individual dissected in this study.
My dissections support the argument of Ritter (1995) and Tsuihiji (2007) that Gasc's (1981) claim that the iliocostalis fibers in varanids are transversely differentiated is erroneous. The muscle labeled iliocostalis in Gasc (1981) is probably instead superficialis dorsalis brevis, although Tsuihiji (2007) argues that it is likely a slip of the intercostalis externi. The iliocostalis is very thin and adheres to the skin in varanids, and Gasc may have missed it.
The only muscles active during exhalation in I. iguana are the transversus and retrahentes costarum (Carrier, 1989). Because retrahentes costarum is not present in V. exanthematicus, another muscle is probably involved in addition to transversus. Fibers of the external intercostal are more horizontally oriented in V. exanthematicus (15° above the horizontal) externals than I. iguana (30–40° above the horizontal; Carrier, 1989). Perhaps the flatter orientation of the external intercostal in V. exanthematicus allows this muscle to be used in exhalation. The straps of external intercostal that run between the cartilaginous sternal segments and the sternal rib portions may be especially suited for this purpose. There are other muscles in V. exanthematicus positioned to effect inhalation, such as the supracostalis brevis or the levator costae.
Concluding remarks
Because lepidosaurs locomote and ventilate with their hypaxial and epaxial musculature, diversity in the form of these muscles represents diversity in approaches to solving these fundamental biomechanical challenges. Future work integrating differences in posture and musculoskeletal anatomy will be critical to understanding the selective pressures and evolutionary pathways that led to the remarkably diverse vertebrate chassis.
Author contributions
R. Cieri conceived the study, collected and analyzed the data, and wrote the manuscript.
Acknowledgements
The author is indebted to Lynn Hartzler for donated specimens of V. exanthematicus and to the following for assistance in accessing museum specimens and in collecting: Andrew Amey, Gavin Bedford, Brian Bush, Christopher Clemente, Patrick Couper, Taylor Dick, Paul Doughty, Suzanne Munns, David Rhind, Jojo Schultz, Graham Thompson, Scott Thompson, Nicholas Wu, and Stephen Zozaya. This manuscript was improved greatly by helpful comments from Elizabeth Brainerd, David Carrier, Adam Huttenlocker, Jeremy Klingler, Mark Nielsen, and two anonymous reviewers. The author acknowledges the facilities, and the scientific and technical assistance of the National Imaging Facility at the Centre for Advanced Imaging, The University of Queensland. This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. 1747505 and an international travel allowance through the Graduate Research Opportunites Worldwide (GROW). Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. The author declares no conflict of interest.
References
- Al‐Hassawi AMA (2007) A Comparative Study of the Neck Region in Lizards. Victora, BC: Trafford Publishing. [Google Scholar]
- Bartholomew GA, Tucker V (1964) Size, body temperature, thermal conductance, oxygen consumption, and heart rate in Australian varanid lizards. Physiol Zool 37, 341–354. [Google Scholar]
- Bennett AF (1973) Blood physiology and oxygen transport during activity in two lizards, Varanus gouldii and Sauromalus hispidus . Comp Biochem Physiol A Comp Physiol 46, 673–690. [DOI] [PubMed] [Google Scholar]
- Bhullar B‐AS (2009) A reevaluation of the unusual abdominal musculature of squamate reptiles (Reptilia: Squamata). Anat Rec 292, 1154–1161. [DOI] [PubMed] [Google Scholar]
- Bonnan MF (2016) The Bare Bones: An Unconventional Evolutionary History of The Skeleton. Bloomington: Indiana University Press. [Google Scholar]
- Brainerd EL (1999) New perspectives on the evolution of lung ventilation mechanisms in vertebrates. Exp Biol Online 4, 1–28. [Google Scholar]
- Burke AC, Nelson CE, Morgan BA, et al. (1995) Hox genes and the evolution of vertebrate axial morphology. Development 121, 333–346. [DOI] [PubMed] [Google Scholar]
- Carrier DR (1990) Activity of the hypaxial muscles during walking in the lizard Iguana iguana. J Exp Biol 152, 453–470. [DOI] [PubMed] [Google Scholar]
- Carpenter CC, Gillingham JC, Murphy JB, et al. (1976) A further analysis of the combat ritual of the Pygmy mulga monitor, Varanus gilleni (Reptilia: Varanidae). Herpetologica 32, 35–40. [Google Scholar]
- Carrier DR (1987) The evolution of locomotor stamina in tetrapods: circumventing a mechanical constraint. Paleobiology 13, 326–341. [Google Scholar]
- Carrier DR (1989) Ventilatory action of the hypaxial muscles of the lizard Iguana iguana: a function of slow muscle. J Exp Biol 457, 435–457. [DOI] [PubMed] [Google Scholar]
- Carrier DR (1996) Function of the intercostal muscles in trotting dogs: ventilation or locomotion? J Exp Biol 199, 1455–1465. [DOI] [PubMed] [Google Scholar]
- Čerňanský A (2016) From lizard body form to serpentiform morphology: the atlas‐axis complex in African cordyliformes and their relatives. J Morphol 277, 512–536. [DOI] [PubMed] [Google Scholar]
- Čerňanský A, Boistel R, Fernandez V, et al. (2014) The atlas‐axis complex in chamaeleonids (Squamata: Chamaeleonidae), with description of a new anatomical structure of the skull. Anat Rec 297, 369–396. [DOI] [PubMed] [Google Scholar]
- Clemente CJ, Withers PC, Thompson GG (2009) Metabolic rate and endurance capacity in Australian varanid lizards (Squamata: Varanidae: Varanus). Biol J Linn Soc 97, 664–676. [Google Scholar]
- Cogger H (2014) Reptiles and Amphibians of Australia, 7th edn Melbourne: Csiro Publishing. [Google Scholar]
- Conrad JL (2006a) Postcranial skeleton of Shinisaurus crocodilurus (Squamata: Anguimorpha). J Morphol 267, 759–775. [DOI] [PubMed] [Google Scholar]
- Conrad JL (2006b) An eocene shinisaurid (Reptilia, Squamata) from Wyoming, U.S.A. J Vertebr Paleontol 26, 113–126. [Google Scholar]
- Daeschler EB, Shubin NH, Jenkins FA (2006) A Devonian tetrapod‐like fish and the evolution of the tetrapod body plan. Nature 440, 757–763. [DOI] [PubMed] [Google Scholar]
- Doughty P, Kealley L, Fitch A, Donnellan SC (2014) A new diminutive species of Varanus from the Dampier Peninsula, western Kimberley region, Western Australia. Rec. West. Aust. Museum 29, 128–140. [Google Scholar]
- Farmer CG, Hicks JW (2000) Circulatory impairment induced by exercise in the lizard Iguana iguana . J Exp Biol 203, 2691–2697. [DOI] [PubMed] [Google Scholar]
- Fitch AJ, Goodman AE, Donnellan SC (2006) A molecular phylogeny of the Australian monitor lizards (Squamata: Varanidae) inferred from mitochondrial DNA sequences. Aust J Zool 54, 253. [Google Scholar]
- Gans C, Dessauer H, Baic D (1978) Axial differences in the musculature of uropeltid snakes: the freight‐train approach to burrowing. Science 199, 189–192. [DOI] [PubMed] [Google Scholar]
- Gasc J‐P (1981) Axial musculature. Biol Reptil 11, 355–435. [Google Scholar]
- Greer AE (1989) The Biology and Evolution of Australian Lizards. Chipping Norton, NSW: Surrey Beatty & Sons. [Google Scholar]
- Hoff KvS , Wassersug RJ (2000) Tadpole locomotion: axial movement and tail functions in a largely vertebraeless vertebrate. Am Zool 40, 62–076. [Google Scholar]
- Hoffstetter R, Gasc J‐P (1969) Vertebrae and ribs of modern reptiles In: Biology of the Reptilia Volume 1 ‐ Morphology A (ed. Gans C.), pp. 201–310. London: Academic Press. [Google Scholar]
- Janis CM, Keller J (2001) Modes of ventilation in early tetrapods: costal aspiration as a key feature of amniotes. Acta Palaeontol Pol 46(2), 137–170. [Google Scholar]
- Johnson CR (1976) Some behavioural observations on wild and captive sand monitors, Varanus gouldii (Sauria: Varanidae). Zool J Linn Soc 59, 377–380. [Google Scholar]
- Kardong KV (2006) Vertebrates: Comparative Anatomy, Function, Evolution, 4th edn New York: McGraw‐Hill. [Google Scholar]
- Koob TJ, Long JH Jr (2000) The vertebrate body axis: evolution and mechanical function. Am Zool 40, 1–18. [Google Scholar]
- Moritz S, Schilling N (2013) Fiber‐type composition in the perivertebral musculature of lizards: Implications for the evolution of the diapsid trunk muscles. J Morphol 274, 294–306. [DOI] [PubMed] [Google Scholar]
- Mosauer W (1935) The myology of the trunk region of snakes and its significance for ophidian taxonomy and phylogeny. Publ Univ Calif Los Angeles Biol Sci 1, 81–120. [Google Scholar]
- Murphy JB, Lamoreaux WE (1978) Threatening behavior in Mertens’ water monitor Varanus mertensi (Sauria: Varanidae). Herpetologica 34, 202–205. [Google Scholar]
- Nishi S (1938) Muskeln des Rumpfes In: Handbuch der vergleichenden Anatmoie der Wirbeltiere (eds Bolk L, Goppert E, Kallius E, Lubosch W.), pp. 351–446. Berlin: Urban and Schwarzenberg. [Google Scholar]
- O'Reilly J, Summers A, Ritter D (2000) The evolution of the functional role of trunk muscles during locomotion in adult amphibians. Am Zool 135, 123–135. [Google Scholar]
- Owerkowicz T, Farmer CG, Hicks JW, et al. (1999) Contribution of gular pumping to lung ventilation in monitor lizards. Science 284, 1661–1663. [DOI] [PubMed] [Google Scholar]
- Penning DA (2018) Quantitative axial myology in two constricting snakes: Lampropeltis holbrooki and Pantherophis obsoletus . J Anat 232, 1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pianka ER (1995) Evolution of body size: Varanid Lizards as a model system. Am Nat 146, 398. [Google Scholar]
- Pregill GK (1977) Axial myology of the racer Coluber constrictor with emphasis on the neck region. Trans San Diego Soc Nat Hist 18, 185–206. [Google Scholar]
- Pyron RA, Burbrink FT, Wiens JJ (2013) A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol Biol 13, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder TW, Townsend TM, Mulcahy DG, et al. (2015) Integrated analyses resolve conflicts over squamate reptile phylogeny and reveal unexpected placements for fossil taxa. PLoS ONE 10, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly S, Delancey M (1997) Sprawling locomotion in the lizard Sceloporus clarkii: the effects of speed on gait, hindlimb kinematics, and axial bending during walking. J Zool 243, 417–433. [Google Scholar]
- Ritter D (1995) Epaxial muscle function during locomotion in a lizard (Varanus salvator) and the proposal of a key innovation in the vertebrate axial musculoskeletal system. J Exp Biol 198, 2477–2490. [DOI] [PubMed] [Google Scholar]
- Ritter D (1996) Axial muscle function during lizard locomotion. J Exp Biol 199, 2499–2510. [DOI] [PubMed] [Google Scholar]
- Schilling N (2011) Evolution of the axial system in craniates: morphology and function of the perivertebral musculature. Front Zool 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenrock F (1983) Das Skelet von Brookesia superciliaris Kuhl. Sitzungsberichte d.kais Adad d Wiss math‐naturw 102, 71–118. [Google Scholar]
- Thompson GG (1995) Foraging patterns and behaviours, body postures and movement speed for goannas, Varanus gouldii (Reptilia: Varanidae), in a semi‐urban environment. J R Soc West Aust 78, 107–114. [Google Scholar]
- Thompson GG, Clemente CJ, Withers PC, et al. (2008) Is body shape of varanid lizards linked with retreat choice? Aust J Zool 56, 351. [Google Scholar]
- Tsuihiji T (2007) Homologies of the longissimus, iliocostalis, and hypaxial muscles in the anterior presacral region of extant diapsida. J Morphol 268, 986–1020. [DOI] [PubMed] [Google Scholar]
- Vidal N, Marin J, Sassi J, et al. (2012) Molecular evidence for an Asian origin of monitor lizards followed by tertiary dispersals to Africa and Australasia. Biol Let 8, 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Carrier DR, Hicks JW (1997) Ventilation and gas exchange in lizards during treadmill exercise. J Exp Biol 200, 2629–2639. [DOI] [PubMed] [Google Scholar]
- Ward AB, Brainerd EL (2007) Evolution of axial patterning in elongate fishes. Biol J Linn Soc 90, 97–116. [Google Scholar]
- Wiens JJ, Slingluff JL (2001) How lizard turn into snakes: a phylogenetic analysis of body‐from evolution in anguid lizard. Evolution 55, 2303–2318. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Hutter CR, Mulcahy DG, et al. (2012) Resolving the phylogeny of lizards and snakes (Squamata) with extensive sampling of genes and species. Biol Lett 8, 1043–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S (1933) Zur Kenntnis von Bau und Funktion der Reptilienlunge. Zollog Jahrb 57, 139–190. [Google Scholar]
- Young BA, Kardong KV (2010) The functional morphology of hooding in cobras. J Exp Biol 213, 1521–1528. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Wiens JJ (2016) Combining phylogenomic and supermatrix approaches, and a time‐calibrated phylogeny for squamate reptiles (lizards and snakes) based on 52 genes and 4162 species. Mol Phylogenet Evol 94, 537–547. [DOI] [PubMed] [Google Scholar]


