Abstract
People diagnosed with Parkinson's disease (PD) frequently experience visual and non‐visual hallucinations often with comorbid psychosis, however, there is currently no gold standard tool for accurately assessing these symptoms. To address this problem, we designed a novel questionnaire to evaluate the presence of hallucinatory and psychotic symptoms in PD, as well as related symptoms, such as attentional dysfunction and sleep disturbance. We administered the 20‐item Psychosis and Hallucinations Questionnaire (PsycH‐Q) and three common questionnaire measures in a large cohort of 197 patients with idiopathic PD via a postal survey. We established concurrent validity, convergent validity, and internal consistency of the questionnaire and then assessed test‐retest reliability in a subcohort of 44 patients. PsycH‐Q was found to be a valid instrument when analogous items were compared across three other existing tools (Spearman's rho range: 0.34–0.64; P < 0.01). PsycH‐Q demonstrated a strong relationship between self‐reported hallucinations and psychosis and symptoms of the broader hallucinatory phenotype (Kendall's tau = 0.41; P < 0.01; positive predictive value = 0.97). PsycH‐Q also displayed a high level of internal consistency (Cronbach's alpha = 0.900; range, 0.696–0.923) and reproducibility (intraclass correlation coefficient = 0.928). PsycH‐Q is a simple, valid, self‐completed instrument that reliably identifies hallucinations and psychosis in PD and has the ability to characterize related patterns of attentional and sleep impairments. As such, PsycH‐Q is a highly valuable tool for use in both clinical and research settings.
Keywords: Parkinson's disease, visual misperceptions, visual hallucinations, questionnaire, attentional networks
The evolution of psychotic symptoms is common in Parkinson's disease (PD) and not surprisingly, the presence of visual hallucinations (VH) represents a strong predictor for the development of more florid psychosis,1 impaired quality of life,2 and frequently leads to nursing home placement.3, 4 Patients vary in their ability to self‐identify these symptoms, making accurate diagnosis and treatment problematic in both the clinical and research setting.5 Many patients are unable to understand the medical terminology associated with these symptoms, leading to potential under‐reporting. Indeed, a recent task force concluded that a novel questionnaire encompassing the broad phenomenology of the disorder would help to increase the sensitivity of the diagnosis of psychosis and VH in PD.5, 6 In addition, hallucinations in PD have consistently been shown to occur in the presence of broader phenotypic abnormalities, including problems with attention,7, 8, 9, 10 general cognition,8, 10 and sleep,11, 12, 13 thus highlighting the specific domains that can be targeted to better characterize the broader phenomenology of psychosis and hallucinations in PD.
In an attempt to solve these two related issues, we designed a novel self‐report questionnaire—the Psychosis and Hallucinations Questionnaire (PsycH‐Q). The questionnaire has been specifically written in “nonscientific” language with a focus on the phenomenological aspects of hallucinations and related phenomena, allowing for easier self‐identification of the symptoms associated with VH. Our aim was to evaluate the validity and reliability of the PsycH‐Q, by comparison with questionnaire measures that have previously been used to assess hallucinations in PD5, 6 in a large cohort of patients with idiopathic PD (iPD).
Methods
Questionnaire Development
PsycH‐Q is a 20‐item self‐report questionnaire that was derived through consultations with PD patients, caregivers, and a panel of experts (including two movement disorders specialists, three neuropsychologists, and a nurse specialist). The questionnaire was designed to assess for the presence of symptoms associated with the hallucinatory phenotypes in PD that fall into five categories: visual misperceptions, sensory misperceptions, disordered thought, attentional dysfunction, and sleep impairment (Fig. 1). These categories were further grouped into two sections: those directly assessing for hallucinations and psychosis (Section I: 13 questions in three sub‐scales); and those assessing for symptoms of a broader hallucinatory phenotype (Section II: 7 questions in two sub‐scales). A copy of the questionnaire is available from the authors upon request.
Figure 1.
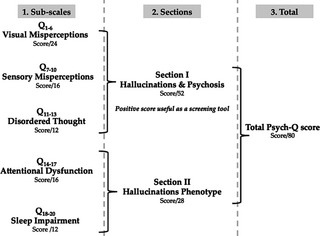
Psychosis and Hallucinations Questionnaire scoring structure. The scoring structure of PsycH‐Q comprised of subscales (left panel), sections (center panel), and total score (right panel).
The questionnaire was initially tested in a cohort of 62 patients with iPD who attended the PD Research Clinic at the Brain and Mind Research Institute (BMRI) in Sydney to assess the feasibility and comprehensibility of items. Every participant was able to complete the questionnaire in under 10 minutes without assistance from their caregiver, and interviews demonstrated that patients found the questions clear and the physical scoring system reasonable (i.e., shading in small circles was not problematic; see Appendix S1). Results from this pilot study showed there was a strong agreement between self‐reported hallucinations on PsycH‐Q and ratings on item 2 within Part I of the International Parkinson and Movement Disorder Society (MDS)‐sponsored revision of the UPDRS (MDS‐UPDRS)14 however, minor changes to the frequency and severity scales were incorporated to improve clarity.
Questionnaire Content
Section I of the PsycH‐Q identifies core hallucinatory and psychotic symptomatology as described in the National Institutes of Health diagnostic criteria15 and was designed for use as a screening tool. Section I is divided into three subscales: The first subscale assesses for visual hallucinatory phenomena, such as presence hallucinations (Q1)—reported as a stimulus moving past the patient in the peripheral field, passage hallucinations (Q2–3)—reported as a sense of “something” perceived in the peripheral field (out of the corner of the eye), and three frequently reported semantic categories of misperception, such as animals, objects, and people (Q4–6); the second sub‐scale assesses for the presence of misperceptions and hallucinations in other sensory modalities, including audition (Q7), touch (Q8), olfaction (Q9), and gustation (Q10); and the third sub‐scale contains three questions that probe for the presence of thought disorder and psychotic behavior (Q11–13). If the patient had positive answers on one or more of the first 10 questions of Section I, they were asked to respond to a series of dichotomous (Yes/No) sub‐questions that assessed whether they: (1) experienced the symptoms before sleep; (2) thought the experiences were real and/or could be convinced otherwise; (3) were frightened by the experiences; and (4) experienced the symptoms outside the past month.
Section II of the PsycH‐Q was designed as an exploratory tool for the assessment of deficits in attention and sleep, both of which have been implicated in the broader hallucinatory phenotype.10, 11, 16, 17 The first four questions of this section (Q14–17) assessed for dysfunction within externally directed attention, failure across which has been proposed as a mechanism underlying the pathophysiology of VHs in PD.19 Specifically, these questions probed for impaired function of the dorsal attention network (Q14 and Q16)—reported as the ability to maintain concentration and follow conversations16, 18; the ventral attention network (Q15)—reported as the ability to mentally multitask19; along with increased activity within the default mode network (Q17)—reported as the tendency to day dream.20, 21 The final subscale (Q18–20) contained three questions assessing the presence of sleep dysfunction, including questions that probed for the presence of vivid dreams (Q18), symptoms of rapid eye movement (REM) behavior disorder (Q19), and confusion upon waking (Q20), all of which have been implicated in the pathophysiology of Parkinsonian hallucinations.
Questionnaire Scoring
For each question, subjects rated the frequency of symptom occurrence using a 5‐point Likert scale22 ranging from 0 (“never”) to 4 (“daily”). Subjects were also asked to rate the severity of each positive symptom on a scale from 1 (“not at all distressing”) to 4 (“extremely distressing”). A positive score on Section I (Q1–13) was used as a screening tool for the presence of hallucinations and psychosis. The total score was calculated by summing the frequency score for Section I (maximum score: 52) and II (maximum score: 28) and ranged from 0 to 80 (Fig. 1). For convenience, the frequency and severity scores derived from the PsycH‐Q also allow calculation of a single composite score (frequency × severity). Responses to Section I sub‐questions were only used for descriptive purposes and excluded from the final score.
Participants
One hundred and ninety‐seven patients (115 men 82 women; mean disease duration: 7.4 ± 6.6 years) with iPD were recruited from the BMRI via a postal survey (see Fig. 2). Eligible participants were drawn from a larger cohort assessed consecutively as part of an ongoing study investigating heterogeneity and clinicopathological correlates in PD. All patients satisfied the United Kingdom PD Society Brain Bank criteria and were assessed while on their regular medication. Permission for the study was obtained from the local research ethics committee and all patients gave written informed consent, in accordance with the Declaration of Helsinki.
Figure 2.
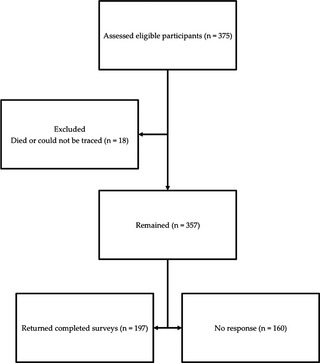
Flowchart of participant inclusion into the study. Of the 375 eligible participants, 18 were excluded because they had died or could not be traced. The final sample consisted of 197 returned surveys (response rate, 55.2%).
For reporting purposes, all patients were assessed on the MDS‐UPDRS,14 the Hoehn & Yahr scale (H&Y),23 the Mini Mental State Examination (MMSE),24 and the Beck Depression Inventory (BDI‐II)25 within 12 months of completing the questionnaire (mean duration: 4.1 ± 2.2 months; Table 1).
Table 1.
Demographics and performance scores for study participants (n = 197) based on PsycH‐Q responses
| Negative | Positive | P Value | |
|---|---|---|---|
| Demographics | |||
| Number | 111 (56%) | 86 (44%) | |
| Age, years | 68.6 ± 8.4 | 70.5 ± 8.5 | n.s. |
| Duration, years | 7.2 ± 7.0 | 7.7 ± 6.3 | n.s. |
| Education, years | 14.0 ± 2.9 | 14.1 ± 3.3 | n.s. |
| MDS‐UPDRS III | 30.3 ± 15.6 | 34.5 ± 18.0 | n.s. |
| H&Y | 2.2 ± 0.9 | 2.2 ± 0.9 | n.s. |
| LEDD, mg/day | 720.6 ± 527.8 | 749.6 ± 583.5 | n.s. |
| MMSE | 28.2 ± 2.5 | 27.2 ± 3.7 | n.s. |
| Neuropsychological | |||
| BDI‐II | 7.5 ± 6.6 | 11.3 ± 10.1 | ** |
| PDQ‐39 | 31.7 ± 26.6 | 45.0 ± 28.4 | ** |
| PsycH‐Q | |||
| Visual Misperceptions scorea | 0.0 ± 0 | 4.5 ± 5.0 | *** |
| Sensory Misperceptions scorea | 0.0 ± 0 | 1.4 ± 2.5 | *** |
| Thought Disorder scorea | 0.0 ± 0.0 | 0.5 ± 1.5 | *** |
| Attention Dysfunction scorea | 3.3 ± 3.8 | 6.8 ± 4.4 | *** |
| Sleep Impairment scorea | 1.5 ± 1.6 | 3.2 ± 2.5 | *** |
| Total severity scorea | 22.3 ± 3.0 | 28.5 ± 8.6 | *** |
Patients were split into two groups (Negative and Positive) depending on whether they reported positive symptoms on Section I of the PsycH‐Q.
Statistical significance (Bonferroni adjusted t‐test of means): **P < 0.01; ***P < 0.001; n.s., P > 0.05.
Kruskall‐Wallis test used for non‐parametric data.
LEDD, levo‐dopa equivalent daily dose.
Questionnaire Administration Procedure
The PsycH‐Q was mailed as part of a questionnaire pack sent in October 2013, accompanied by a cover letter detailing the general purpose and anticipated study outcomes, and a prepaid addressed envelope for return. The pack also included modified “self‐report” versions of the Neuropsychiatric Inventory Questionnaire (NPI‐Q),26 SCales for Outcomes in PArkinson's disease–Psychiatric Complications (SCOPA‐PC),27 and Parkinson Psychosis Questionnaire (PPQ).28 These three tools were selected for comparison because they are common questionnaire measures currently used in research and clinical settings for assessing hallucinations and related neuropsychiatric symptoms in PD that could be effectively modified, for the purpose of our experiment, to be self‐administered. Furthermore, the PPQ is designated as a “suggested” psychosis scale in PD.6 Specific instructions for answering the questionnaires by shading the corresponding circles were printed on the first page. The surveys were designed and processed using Form Return (EB Strada Holdings, Brisbane, QLD, Australia), which is an Optical Mark Recognition computer software application that automated the collection of handwritten data. Software output was manually verified for each subject.
Questionnaire Validity
As that there is no currently accepted gold standard tool that can accurately detect the presence or absence of VH in PD,5, 6 concurrent validity of Section I of the PsycH‐Q was evaluated by comparison with analogous sub‐scales of the NPI‐Q, SCOPA‐PC, and PPQ using bivariate Spearman's rank‐order correlations. Convergent validity was assessed for scores on Section I and II using a Kendall's tau correlation. The positive predictive value (PPV) of a positive screening test for core hallucinatory and psychotic symptoms (Section I) given a positive score on Section II was also evaluated.
Questionnaire Reliability
Internal consistency of the PsycH‐Q was assessed using Cronbach's alpha and item‐to‐total Spearman's rank‐order correlations. “Alpha if item omitted” tests were measured for each item's contribution to the sub‐scale and section of interest. Test‐retest reliability was computed using a two‐way mixed model for average measures to estimate the intraclass correlation coefficient (ICC) for a subgroup of 44 subjects (22%) who completed the test twice.
Results
PsycH‐Q Responses
Of the 197 patients enrolled in our study, 76 (39%) reported experiencing symptoms of visual misperceptions and/or hallucinations on the first PsycH‐Q subscale (Q1–6) and another 6 (3%) reported experiencing exclusively non‐visual misperceptions (Q7–10; Fig. 3). Overall, 86 subjects (44%) self‐reported hallucinatory or psychotic symptoms on Section I and only 19 (10%) had a total score of 0 on the PsycH‐Q. A total of 83 (42%) out of the 175 subjects (89%) who reported positive symptoms on Section II also reported positive symptoms on Section I.
Figure 3.
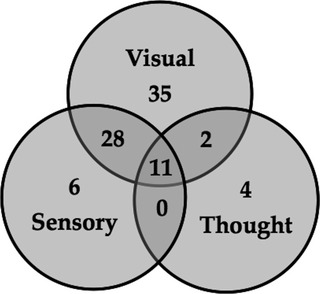
Relationships between self‐reported symptoms of hallucinations and psychosis. Positive responses on PsycH‐Q Section I (n = 86) as a function of symptom subscales: visual misperceptions (Q1–6; n = 76); sensory misperceptions (Q7–10; n = 45); and disordered thought (Q11–13; n = 17).
Table 2 shows the distribution of self‐rated positive symptoms of hallucinations and psychosis on the PsycH‐Q. Mean visual misperceptions score (frequency × severity) was 3.67 (range: 0–96), mean sensory misperceptions score was 1.10 (range: 0–28), mean disordered thought score was 0.62 (range: 0–48), mean attention dysfunction score was 11.65 (range: 0–64), and mean sleep impairment score was 4.47 (range: 0–48). Regarding the additional descriptive sub‐questions, of the 82 subjects who had positive scores for Q1–10, 9 (11%) experienced hallucinatory experiences when they were about to fall asleep, 18 (22%) thought they could be real and could be convinced otherwise, 9 (11%) were frightened by the experiences, and 45 (55%) had these experiences outside of the past month.
Table 2.
Number and percentage of patients with Parkinson's disease in the study that scored positively on each question of the Psychosis and Hallucinations Questionnaire
| Item | Category | N | % | |
|---|---|---|---|---|
| Section I | Q1 | Presence hallucinations | 42 | 21 |
| Q2 | Corner vision | 51 | 26 | |
| Q3 | Passage hallucinations | 29 | 15 | |
| Q4 | General misperception | 41 | 21 | |
| Q5 | Object misperception | 33 | 17 | |
| Q6 | Animal misperception | 36 | 18 | |
| Q7 | Auditory | 29 | 15 | |
| Q8 | Tactile | 16 | 8 | |
| Q9 | Olfactory | 18 | 9 | |
| Q10 | Gustatory | 6 | 3 | |
| Q11 | Persecutory delusions | 10 | 5 | |
| Q12 | Threatening delusions | 10 | 5 | |
| Q13 | Social delusions | 5 | 3 | |
| Section II | Q14 | Concentration | 124 | 63 |
| Q15 | Impaired multitasking | 121 | 61 | |
| Q16 | Following conversation | 93 | 47 | |
| Q17 | Day dreaming | 95 | 48 | |
| Q18 | Vivid dreams | 129 | 65 | |
| Q19 | REM behavior disorder | 89 | 45 | |
| Q20 | Confusion upon waking | 46 | 23 |
As shown in Table 1, even though subjects who reported positive symptoms on Section I were similar to those without positive symptoms on a number of demographics, such as age, disease duration, daily levodopa equivalent dose (LED), cognitive ability (as measured by MMSE) and motor severity (as measured by MDS‐UPDRS Part III and H&Y), they had more depressive symptoms (as measured by BDI‐II; t = 2.75, P < 0.01) and worse quality of life (as measured by PDQ‐39; t = 3.05, P < 0.01; Bonferroni's adjusted t‐test of means). Using a BDI‐II cut‐off of 14,29 21% (18 out of 86) patients with and 11% (12 out of 111) without psychosis and hallucinations met the screening criteria for depression.
Compared to responders, patients who did not return the questionnaire (n = 160) were equivalent in terms of disease duration, daily LED, severity of depressive symptoms, and education (P values not significant [n.s.]). However, the non‐responder cohort was significantly older (mean age: 72.9 [standard deviation (SD), 11.5] vs. 69.5 [SD, 8.5]; t = −3.11, P < 0.05), had worse quality of life (mean PDQ‐39: 51.4 [SD, 29.8] vs. 37.0 [SD, 28.0]; t = − 4.41, P < 0.001), more advanced disease (44% were H&Y stage ≥ 3.0 vs. 25%; t = −4.83, P < 0.001; mean MDS‐UPDRS III: 41.3 [SD, 19.2] vs. 32.1 [SD, 16.7]; t = −4.67, P < 0.001), and were more cognitively impaired (mean MMSE: 25.8 [SD, 5.0] vs. 27.8 [SD, 3.1]; t = 4.14, P < 0.001).
Questionnaire Validity
Correlations between Section I of the PsycH‐Q and NPI‐Q, PPQ, and SCOPA‐PC analogous sub‐scale scores are shown in Table 3. PsycH‐Q demonstrated strong concurrent validity (P < 0.01), when compared with existing questionnaire measures, showing that the instruments were measuring equivalent symptoms. The weakest correlation was between the sub‐scales of Delusions on SCOPA‐PC and Disordered Thought on PsycH‐Q (r = 0.34, P < 0.01). The strongest correlation was between the hallucinations sub‐scales of SCOPA‐PC and PsycH‐Q (r = 0.64, P < 0.01). Furthermore, the strong positive correlation (Kendall's tau = 0.405, P < 0.01) between total scores on Sections I and II suggests that PsycH‐Q has effective convergent validity. Based on the PPV of 0.97, the presence of Section I symptoms are estimated to increase the probability of positive Section II symptoms. These results suggest that the self‐report of hallucinatory symptoms strongly predicts the presence of dysfunctional attention and sleep in patients with PD.
Table 3.
Spearman's rank order coefficient (ρ) for correlations between for PsycH‐Q Section I sub‐scale scores (frequency × severity) and NPI‐Q (severity), PPQ, and SCOPA‐PC subscales in PD (n = 197), demonstrating concurrent validity of the PsycH‐Q
| PsycH‐Q | NPI‐Q | ρ | PPQ | ρ | SCOPA‐PC | ρ |
|---|---|---|---|---|---|---|
| Q1–10 | Hallucinations | 0.37** | Hallucinations | 0.58** | Hallucinations | 0.64** |
| Q11–13 | Delusions | 0.51** | Delusions | 0.38** | Ideations | 0.34** |
Statistical significance: **P < 0.01.
Questionnaire Reliability
The Cronbach's alpha for the 20‐item PsycH‐Q was 0.900 for frequency and 0.908 for severity, suggesting that all of the items on the questionnaire were measuring a similar construct. The frequency and severity scores were also highly correlated for each sub‐scale (α range: 0.657–0.947), suggesting that all items identified similar aspects of the same symptom. Moreover, only one item caused an improvement in Cronbach's alpha of > 0.1 if omitted (Q7: 0.720 could increase to 0.743). The alpha score only fell below the recommended level of 0.7 for group comparisons in the sleep impairment sub‐scale. Scores from the five PsycH‐Q sub‐scales had a high degree of internal consistency (α range: 0.696–0.923). Scores from the Visual Misperceptions sub‐scale were strongly correlated with scores from each of the other four sub‐scales (average Spearman's rho = 0.461, P < 0.01; range = 0.302–0.621). In addition, PsycH‐Q had strong test‐retest reliability (ICC = 0.928, 95% confidence interval: 0.869–0.961; average time between questionnaires: 2.2 months).
Discussion
This study showed that PsycH‐Q is a robust self‐report questionnaire that can accurately separate patients with PD into groups of hallucinators and non‐hallucinators. Based on results of the reliability and validity analyses, PsycH‐Q is able to consistently measure visual and non‐visual hallucinatory phenomenology. Additionally, results of the concurrent validity analyses highlight the utility of PsycH‐Q as a potential method for the earlier detection of hallucinatory and psychotic symptoms and hence timely intervention.30
PsycH‐Q fulfills a number of the major criteria set forth by a MDS Task Force commissioned to determine the current state of hallucinations questionnaires in PD.5, 6 For instance, PsycH‐Q contains a series of questions to probe the broad spectrum of hallucinatory symptoms in PD as well as the presence of attentional and sleep‐related symptoms that have been shown to form important aspects of the hallucinatory phenotype in PD. Indeed, PsycH‐Q was specifically designed to interrogate the prevalence of hallucinations and related phenomena in PD, distinguishing it from other commonly used questionnaires. Together, these results suggest that PsycH‐Q will assist in the ongoing interrogation of VH and related phenomena in PD.
There are several benefits of using the PsycH‐Q to estimate the prevalence and incidence of hallucinations in PD over existing tools. For example, many of the currently used questionnaires were not written specifically for PD and may lead to under‐reporting of hallucinatory symptoms,5, 6, 10, 11, 16 especially as patients lack recognition of the perceptual subtleties associated with the disorder. Indeed, PsycH‐Q items were designed to systematically probe for well‐known phenotypic traits of hallucinatory symptoms that patients with PD may experience. PsycH‐Q is also able to assess factors thought to be closely related to patient and caregiver outcomes, including specific attentional dysfunctions7, 31 that may impact on daily living. Ultimately, this could provide a better understanding of the frequency, perceived severity, and patient's degree of insight of this symptom of PD across the spectrum.
Consistent with a large study that used a semi‐structured interview to elucidate the presence of hallucinations in PD,32 we observed a moderate prevalence of corner of vision hallucinations (26%), as well as presence hallucinations (21%), ill‐formed misperceptions (21%), and misperceptions of animals (18%) and objects (17%; Table 2). Similar to previous studies,9, 33 we observed a moderate prevalence of non‐visual hallucinations (23%), of which 87% occurred in the presence of visual misperceptions (Fig. 3). However, in contrast to one previous study,32 our estimates of prevalence were based entirely on self‐report questionnaires. In addition, we were able to extend the clinical characterization of VH in PD patients with retained insight, which is an admitted requisite for the accurate completion of our tool. In contrast with a recent report that PD patients with delusional thinking are likely to suffer from concomitant hallucinations in sensory domains,9 few patients in our cohort (5% of participants) admitted to a fearful association with VH and we observed a low prevalence of thought disorder (9%). These differences are most likely due to the relatively smaller sample size in our study and lower likelihood that patients attending a research clinic have advanced psychosis.
The data we present here can be utilized to characterize the demographic features of VH in PD, especially in patients without significant cognitive impairment and in early stages of PD, which is a critical stage for commencing treatment interventions.30 In our sample, we did not observe any association between positive scores on Section I of the PsycH‐Q and age, disease duration, motor severity, or daily LED (Table 1). However, we observed significantly higher rates of depressive symptoms and impaired quality of life with worsening hallucinatory symptomatology, which is consistent with previous reports.2, 32 Our analyses showed that excluding data from demented individuals (n = 14) did not influence major outcomes. Future longitudinal studies and the assessment of patients with older age, more advanced disease, higher prevalence of dementia, and reduced insight will help to clarify these associations across the clinical spectrum. A future study validating the PsycH‐Q in a larger and more demographically diverse cohort would ensure its utility across socioeconomic and education levels.
PsycH‐Q can also aid in the clarification of the neurobiological mechanism underlying VH in PD. The combination of targeted questions probing visual misperceptions with questions interrogating related symptomology, such as sleep disturbance [11, 34] and attention network dysfunction [16], will allow for the prospective assessment of concomitant patterns of dysfunction associated with VH. Indeed, the results of our analysis suggest that there is a strong correlation between self‐reported visual misperceptions (Q1–6) and all other symptomatic categories assessed. Whilst the items in Section II should not be used as a screening tool for a presymptomatic state per se, it is possible that a substantial proportion of subjects scoring positively on Section II will convert into a hallucinatory phenotype over time—a prediction that can be directly tested with longitudinal PsycH‐Q assessments. In future studies, these symptomatic details can also be combined with structural and functional neuroimaging35 to help further clarify the pathophysiological mechanism of hallucinations.36
Together, these results highlight the utility of the PsycH‐Q to accurately assess a patient cohort for the presence or absence of hallucinatory phenomenology in PD, which has been identified as a critical gap in the literature.5, 6 Importantly, the sensitivity of the PsycH‐Q to changes in the frequency and severity of hallucinatory and psychotic symptoms in PD may provide a useful measure to augment the monitoring of treatment efficacy and disease progression in both clinical and research settings.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the first draft, B. Review and Critique.
J.M.S.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
J.M.Z.M.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
J.Q.: 1A, 2C, 3B
C.O.: 1A, 2C, 3B
Z.T.: 1A, 2C, 3B
G.M.H.: 1A, 2C, 3B
S.L.N.: 1A, 2C, 3B
S.J.G.L.: 1A, 1B, 2C, 3B
Disclosures
Funding Sources and Conflicts of Interest: The study was supported by Parkinson's NSW Seed Grant. The authors report no conflicts of interest.
Financial Disclosures for previous 12 months: The study was supported by Parkinson's NSW Seed Grant. Individually, the work was supported by the Australian Rotary Health (Scholarship to J.M.S.); the Australian Government (Endeavour Postgraduate Scholarship to J.M.Z.M.); and the National Health & Medical Research Council of Australia (630434 to G.M.H.; 1008117 to S.L.N.; 1003007 to S.J.G.L.). No other authors received funding in order to complete this project.
Supporting information
Appendix S1. The self‐administered “Psychosis and Hallucinations Questionnaire”.
Acknowledgment
The authors thank the patients involved in the study for their ongoing support.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Forsaa EB, Larsen JP, Wentzel‐Larsen T, et al. A 12‐year population‐based study of psychosis in Parkinson disease. Arch Neurol 2010;67:996–1001. [DOI] [PubMed] [Google Scholar]
- 2. Aarsland D, Larsen JP, Lim NG, Janvin C, Karisen K, Tandberg E, Cummings JL. Range of neuropsychiatric disturbances in patient with Parkinson's disease. J Neurol Neurosurg Psychiatry 1999;67:492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Klein C, Kömpf D, Pulkowski U, Moser A, Vieregge P. A study of visual hallucinations in patients with Parkinson's disease. J Neurol 1997;244:371–377. [DOI] [PubMed] [Google Scholar]
- 4. Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population‐based prospective study. J Am Geriatr Soc 2000;48:938–942. [DOI] [PubMed] [Google Scholar]
- 5. Goetz CG. Scales to evaluate psychosis in Parkinson's disease. Parkinsonism Relat Disord 2009;15:S38–S41. [DOI] [PubMed] [Google Scholar]
- 6. Fernandez HH, Aarsland D, Fenelon G, et al. Scales to assess psychosis in Parkinson's disease: critique and recommendations. Mov Disord 2008;23:484–500. [DOI] [PubMed] [Google Scholar]
- 7. Shine JM, Halliday GH, Carlos M, Naismith SL, Lewis SJG. Investigating visual misperceptions in Parkinson's disease: a novel behavioral paradigm. Mov Disord 2012;27:500–505. [DOI] [PubMed] [Google Scholar]
- 8. Ramírez‐Ruiz B, Junqué C, Martí M‐J, Valldeoriola F, Tolosa E. Neuropsychological deficits in Parkinson's disease patients with visual hallucinations. Mov Disord 2006;21:1483–1487. [DOI] [PubMed] [Google Scholar]
- 9. Fenelon G, Soulas T, Zenasni F, Cleret de Langavant L. The changing face of Parkinson's disease‐associated psychosis: a cross‐sectional study based on the new NINDS‐NIMH criteria. Mov Disord 2010;25:763–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barnes J, Boubert L. Executive functions are impaired in patients with Parkinson's disease with visual hallucinations. J Neurol Neurosurg Psychiatry 2008;79:190–192. [DOI] [PubMed] [Google Scholar]
- 11. Arnulf I, Bonnet AM, Damier P, Bejjani BP, Seilhean D, Derenne JP, Agid Y. Hallucinations, REM sleep, and Parkinson's disease: a medical hypothesis. Neurology 2000;55:281–288. [DOI] [PubMed] [Google Scholar]
- 12. Pappert EJ, Goetz CG, Niederman FG, Raman R, Leurgans S. Hallucinations, sleep fragmentation, and altered dream phenomena in Parkinson's disease. Mov Disord 1999;14:117–121. [DOI] [PubMed] [Google Scholar]
- 13. Manni R, Terzaghi M, Ratti P‐L, Repetto A, Zangaglia R, Pacchetti C. Hallucinations and REM sleep behaviour disorder in Parkinson's disease: dream imagery intrusions and other hypotheses. Conscious Cogn 2011;20:1021–1026. [DOI] [PubMed] [Google Scholar]
- 14. Goetz CG, Fahn S, Martinez‐Martin P, et al. Movement Disorder Society‐sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): process, format, and clinimetric testing plan. Mov Disord 2007;22:41–47. [DOI] [PubMed] [Google Scholar]
- 15. Ravina B, Marder K, Fernandez HH. Diagnostic criteria for psychosis in Parkinson's disease: report of an NINDS, NIMH work group. Mov Disord 2007;22:1061–1068. [DOI] [PubMed] [Google Scholar]
- 16. Shine JM, Halliday GM, Naismith SL, Lewis SJG. Visual misperceptions and hallucinations in Parkinson's disease: dysfunction of attentional control networks? Mov Disord 2011;26:2154–2159. [DOI] [PubMed] [Google Scholar]
- 17. Collerton D, Perry E. Dreaming and hallucinations – continuity or discontinuity? Perspectives from dementia with Lewy bodies. Conscious Cogn 2011;20:1016–1020. [DOI] [PubMed] [Google Scholar]
- 18. Ptak R, Schnider A. The dorsal attention network mediates orienting toward behaviorally relevant stimuli in spatial neglect. J Neurosci 2010;30:12557–12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corbetta M, Shulman GL. Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 2002;3:215–229. [DOI] [PubMed] [Google Scholar]
- 20. Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal‐directed cognition. Neuroimage 2010;53:303–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Spreng RN, Schacter DL. Default network modulation and large‐scale network interactivity in healthy young and old adults. Cereb Cortex 2012;22:2610–2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Likert R. A technique for the measurement of attitudes. Arch Psychol 1932;40:1–55. [Google Scholar]
- 23. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology 1998;50:318. [DOI] [PubMed] [Google Scholar]
- 24. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;2:189–198. [DOI] [PubMed] [Google Scholar]
- 25. Beck AT, Steer RA, Ball R, Ranieri W. Beck depression inventory (BDI). J Pers Assess 1996;67:588–597. [DOI] [PubMed] [Google Scholar]
- 26. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI‐Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000;12:233–239. [DOI] [PubMed] [Google Scholar]
- 27. Visser M, Verbaan D, van Rooden SM, Stiggelbout AM, Marinus J, Van Hilten JJ. Assessment of psychiatric complications in Parkinson's disease: the SCOPA‐PC. Mov Disord 2007;22:2221–2228. [DOI] [PubMed] [Google Scholar]
- 28. Brandstaedter D, Spieker S, Ulm G, et al. Development and evaluation of the Parkinson Psychosis Questionnaire. J Neurol 2005;252:1060–1066. [DOI] [PubMed] [Google Scholar]
- 29. Leentjens AF, Verhey FRJ, Luijckx G‐J, Troost J. The validity of the Beck Depression Inventory as a screening and diagnostic instrument for depression in patients with Parkinson's disease. Mov Disord 2000;15:1221–1224. [DOI] [PubMed] [Google Scholar]
- 30. Goetz CG, Fan W, Leurgans S. Antipsychotic medication treatment for mild hallucinations in Parkinson's disease: positive impact on long‐term worsening. Mov Disord 2008;23:1541–1545. [DOI] [PubMed] [Google Scholar]
- 31. Lewis SJG, Shine JM, Duffy S, Halliday G, Naismith SL. Anterior cingulate integrity: executive and neuropsychiatric features in Parkinson's disease. Mov Disord 2012;27:1262–1267. [DOI] [PubMed] [Google Scholar]
- 32. Fenelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain 2000;123:733–745. [DOI] [PubMed] [Google Scholar]
- 33. Papapetropoulos S, Katzen H, Schrag A, et al. A questionnaire‐based (UM‐PDHQ) study of hallucinations in Parkinson's disease. BMC Neurol 2008;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Onofrj M, Thomas A, D'Andreamatteo G, et al. Incidence of RBD and hallucination in patients affected by Parkinson's disease: 8‐year follow‐up. Neurol Sci 2002;23(suppl 2):S91–S94. [DOI] [PubMed] [Google Scholar]
- 35. Shine JM, Halliday GM, Gilat M, et al. The role of dysfunctional attentional control networks in visual misperceptions in Parkinson's disease. Hum Brain Mapp 2013;35:2206–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shine JM, O'Callaghan C, Halliday GM, Lewis SJG. Tricks of the mind: visual hallucinations as disorders of attention. Prog Neurobiol 2014;116:58–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. The self‐administered “Psychosis and Hallucinations Questionnaire”.


