Abstract
Dopaminergic medications are used as first‐line treatment for Parkinson's disease (PD). In 1999, a case series was published describing 9 patients who took dopamine agonists (pramipexole or ropinirole) and experienced sudden irresistible sleep attacks. Sleep attacks have subsequently been reported with other dopaminergic medications, including levodopa. Because these symptoms might not be rare and can affect health‐related quality of life, we set out to review the prevalence and clinical characteristics of sleep attacks in patients with PD on dopaminergic medications. We conducted a systematic literature review using the terms parkinson* AND dopamine* AND narcolep* OR sleep attack in multiple databases (PubMed, Embase, and PsycINFO). The systematic literature review yielded 23 relevant articles, including nine case reports or case series and 14 original studies. According to the pooled data from the five studies reporting prevalence figures (n = 10,084), sleep attacks occur in 13.0% of patients with PD on dopaminergic medications. Our analysis failed to show significant differences in the Epworth Sleepiness scores between patients with and without sleep attacks (mean difference: 2.92; 95% confidence interval: −0.47–6.31). The I2 value of 76% indicated high heterogeneity among the studies. Sleep attacks are not a rare occurrence in patients with PD on dopamine agonist treatment. We found conflicting results on whether sleep attacks in PD resemble narcolepsy. The pathophysiology of these symptoms might be related to dopamine D2 and D4 receptor gene polymorphisms. The most effective management strategies were dose reduction and discontinuation of the offending drugs.
Keywords: Parkinson's disease, dopamine, medication, nonmotor symptoms, sleep attacks
The progressive loss of neurons of the SN in patients with Parkinson's disease (PD) is known to result in clinically relevant depletion of dopamine in the caudate nucleus and putamen.1 Dopaminergic medications are therefore considered as first‐line pharmacotherapy, especially nonergot dopamine agonists (pramipexole, ropinirole, and rotigotine).2 Over the last few years, there has been increased research interest for the nonmotor symptoms in patients with PD, as well as their relationship with dopaminergic treatment. Frucht et al.3 first described a case series of 9 patients who took pramipexole or ropinirole and experienced sudden irresistible sleep attacks causing motor accidents. Subsequently, sleep attacks have been reported with other dopaminergic medications and a direct causal link has been postulated.4, 5 Sleep attacks are defined as clinical events characterized by overwhelming sleepiness either without warning or with slow prodrome drowsiness.5 It is controversial whether sleep attacks represent side effects of dopaminergic medications, because researchers pointed out that temporal association and dose‐effect relationship have not consistently been reported, and sleep attacks can also occur in patients with PD who are not on dopamine agonists.6 In fact, although patients on dopaminergic medications are warned to exercise caution when driving and operating machinery, available data are insufficient to warrant a driving ban.5
Sleep disorders in the context of PD have been widely investigated over the last few years; however, little attention has been paid to the relationship between sleep attacks and dopaminergic medications. A review published in 2002 showed that 6.6% of patients taking dopamine agonists had sleep events, including an unspecified proportion of sleep attacks.5 The first aim of the present review was to provide an estimate of the prevalence of sleep attacks among patients with PD on dopaminergic medications. Our second aim was to identify studies focusing on the pathophysiology and clinical presentation (including risk factors) of sleep attacks in patients with PD. Last, we set out to evaluate the available evidence on the assessment and management strategies.
Materials and Methods
We conducted a systematic literature search according to the principles developed by the PRISMA guidelines,7 using the terms parkinson* AND dopamine* AND narcolep* OR sleep attack in multiple scientific databases (PubMed, Embase, and PsycINFO). We limited our search to articles published in the English language since the first report of sleep attacks in patients with PD (1999). This search yielded a total of 137 documents, as shown in Figure 1.
Figure 1.
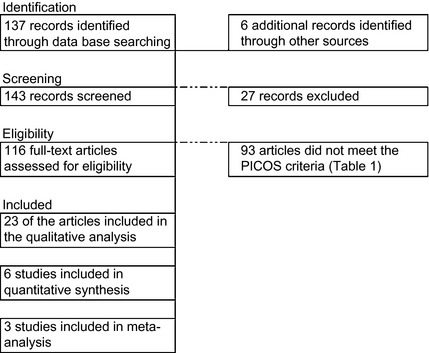
Flow chart illustrating the study selection process for the systematic literature review. PICOS, Participants, Intervention, Comparator, Outcome, Study Design.
The titles and abstracts were examined for all documents and duplicate articles were excluded. Clinical entities described as “irresistible daytime sleepiness,” “sudden onset of sleep,” “narcolepsy‐like” attacks were included as alternative descriptions of sleep attacks in the context of PD. Additional articles were retrieved from the bibliographies of the relevant articles and hand searches of relevant journals, including Brain, Movement Disorders, Neurology, Annals of Neurology, Parkinson's Disease, and Parkinsonism & Related Disorders. The eligibility criteria applied to the study selection process are shown in Table 1.
Table 1.
PICOS statement for the systematic literature search
| P | Human subjects with PD and sleep attacks |
| I | Dopaminergic medications |
| C | Placebo or any other comparators |
| O | Prevalence, pathophysiology, clinical characteristics, investigations, management |
| S | Case reports, case series, cohort studies, case‐control studies, randomized, controlled trials |
PICOS, Participants, Intervention, Comparator, Outcome, Study Design.
Data were extracted directly from the published articles, and only absolute measures were included in this review. Extracted data were sufficient to conduct a meta‐analysis with forest plot and random effect model on studies that reported the Epworth Sleepiness (ES) score among patients with PD taking dopaminergic medications and experiencing sleep attacks. All statistical analyses were performed using Review Manager 5 (Version 5.0.21; The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). A funnel plot was used to check for publication bias. A count of the number of studies with significant and nonsignificant findings was done to check for publication bias among the original studies included.
Results
From the retrieved documents, 23 full‐text articles were included in this systematic review. Table 2 presents the findings of nine case reports or case series, whereas Table 3 summarizes the results of 14 original studies.
Table 2.
Case reports and case series of sleep attacks in patients with PD on dopaminergic medications (n = 39)
| Study | Age | Gender | Duration of PD (Years) | Dopaminergic Medications When Sleep Attacks Began (Daily Dose; Duration in Months) | Concurrent Medications | Somnolence | Other Signs and Symptoms | Management | Country |
|---|---|---|---|---|---|---|---|---|---|
| Frucht et al.3 | 54 | NA | 13 |
Carbidopa/l‐dopa (NA; NA) Pramipexole (3 mg; 1) |
Trihexyphenidyl Selegiline | No | None | Stopped pramipexole | United States |
| Frucht et al.3 | 66 | NA | 2.5 |
Pramipexole (2 mg; 5) Pergolide (NA; NA) |
Cimetidine Bupropion Lamotrigine Clonazepam |
No | None | Stopped pramipexole | United States |
| Frucht et al.3 | 83 | NA | 3 |
Pramipexole (4.5 mg; NA) Carbidopa/l‐dopa (NA; NA) |
Selegiline | No | Visual hallucinations | Stopped pramipexole | United States |
| Frucht et al.3 | 55 | NA | 5 |
Pramipexole (4.5 mg; 4) Carbidopa/l‐dopa (NA; NA) |
Selegiline | No | Increased libido | Stopped pramipexole | United States |
| Frucht et al.3 | 75 | NA | 10 |
Pramipexole (4 mg; 12) Carbidopa/l‐dopa (NA; NA) |
None | No | None | Stopped pramipexole | United States |
| Frucht et al.3 | 58 | NA | 6 |
Pramipexole (2 mg; 14) Carbidopa/l‐dopa (NA; NA) |
Paroxetine | Yes | None | Stopped pramipexole | United States |
| Frucht et al.3 | 77 | NA | 7 |
Pramipexole (2 mg; 8) Carbidopa/l‐dopa (NA; NA) |
Cimetidine Selegiline |
Yes | Auditory hallucinations | Stopped pramipexole | United States |
| Frucht et al.3 | 54 | NA | 5 | Pramipexole (1 mg; 4) | Tranylcypromine | Yes | None | Stopped pramipexole | United States |
| Frucht et al.3 | NA | NA | NA | Ropinirole (16 mg; 1) | Tranylcypromine | Yes | None | Stopped ropinirole | United States |
| Schapira39 | 61 | Male | NA | Pergolide (5 mg; NA) |
Selegiline Aspirin |
Yes | None | Reduced the dose of pergolide to 3 mg/day | United Kingdom |
| Schapira39 | 57 | Female | NA | Pergolide (4.5 mg; NA) | None | No | None | Reduced the dose of pergolide to 3 mg/day | United Kingdom |
| Ferreira et al.40 | 72 | Male | 15 |
l‐dopa (800 mg; NA) Bromocriptine (30 mg; 48) |
NA | Yes | None | NA | France |
| Ferreira et al.40 | 55 | Female | 5 |
Pergolide (4 mg; NA) l‐dopa (600 mg; NA) |
Selegiline | Yes | None | Stopped pergolide occasionally | France |
| Ferreira et al.40 | 69 | Male | 2 |
l‐dopa (250 mg; NA) Piribedil (150 mg; NA) |
Atenolol Diacerein |
Yes | None | NA | France |
| Ryan et al.41 | 66 | Female | 9 |
Pramipexole (4 mg; NA) switched to Ropinirole (2.25 mg; NA) |
Selegiline Benztropine Indapamide Atenolol |
Yes | None | Switched to carbidopa/ l‐dopa (75/750 mg/day) | United States |
| Ryan et al.41 | 55 | Male | 9 |
Ropinirole (12 mg; NA) Carbidopa/l‐dopa (87.5/875 mg; NA) |
Selegiline | NA | None | Switched to pergolide (6 mg/day) | United States |
| Hauser et al.42 | 50 | Female | 1 | Pramipexole (4.5 mg; NA) |
Selegiline Sertraline Zolpidem Estradiol |
Yes | Prodrome (yawning, drowsiness) | Improved on lower dose | United States |
| Hauser et al.42 | 46 | Female | 3 |
Pramipexole (4.5 mg; NA) l‐dopa (300 mg; NA) |
Trihexyphenidyl Fosinopril Nifedipine |
Yes | Prodrome (yawning, tearing) | Resolved on discontinuation | United States |
| Hauser et al.42 | 66 | Male | 2 | Pramipexole (4.5 mg; NA) |
Selegiline Verapamil Atenolol Aspirin |
Yes | Prodrome (yawning, blinking) | Improved on lower dose | United States |
| Hauser et al.42 | 59 | Male | 1 | Pramipexole (4.5 mg; NA) |
Selegiline Enlapril |
No | NA | Persisted with discontinuation | United States |
| Hauser et al.42 | 70 | Male | 1 | Pramipexole (4.5 mg; NA) |
Propranolol Terazosin |
Yes | None | Resolved with amantadine | United States |
| Hauser et al.42 | 64 | Female | 2 | Pramipexole (4.5 mg; NA) |
Fluvastatin Aspirin |
Yes | None | Resolved on discontinuation | United States |
| Hauser et al.42 | 67 | Male | 4 |
Pramipexole (4.5 mg; NA) l‐dopa (50 mg; NA) |
Selegiline Benztropine |
Yes | None | Persisted on medication | United States |
| Hauser et al.42 | 44 | Female | 2 | Pramipexole (4.5 mg; NA) | Selegiline | Yes | NA | Resolved on discontinuation | United States |
| Hauser et al.42 | 55 | Male | 8 |
Pramipexole (4.5 mg; NA) l‐dopa (250 mg; NA) |
Selegiline Amantadine Aspirin |
Yes | None | Persisted on medication | United States |
| Hauser et al.42 | 74 | Male | 9 |
Pramipexole (0.75 mg; NA) l‐dopa (750 mg; NA) |
None | Yes | NA | Persisted with discontinuation | United States |
| Hauser et al.42 | 44 | Male | 4 |
Pramipexole (4.5 mg; NA) l‐dopa (400 mg; NA) |
Sertraline Nizatidine |
Yes | None | Improved on lower dose | United States |
| Hauser et al.42 | 73 | Male | 3 | Pramipexole (4.5 mg; NA) |
Sertraline Clozepate Lisinopril |
No | None | Improved on lower dose | United States |
| Hauser et al.42 | 65 | Male | 9 |
Pramipexole (0.75 mg; NA) l‐dopa (700 mg; NA) |
Selegiline Alprazolam |
NA | NA | NA | United States |
| Hauser et al.42 | 71 | Male | 3 | Pramipexole (4.5 mg; NA) | NA | NA | NA | NA | United States |
| Schafer and Greulich27 |
NA (n = 4) |
NA | NA | Dopamine agonists (NA; NA) | NA | No | Night sleep was significantly longer, less fragmented, and showed normal portions of slow‐wave sleep and REM sleep | NA | Germany |
| Schafer and Greulich27 |
NA (n = 2) |
NA | NA |
Ropinirole (NA; NA) l‐dopa (NA; NA) |
NA | NA | Night sleep was significantly longer, less fragmented and showed normal portions of slow wave sleep and REM sleep | NA | Germany |
| Ulivelli et al.23 | 44 | Female | NA |
l‐dopa (600 mg; NA) Pergolide (3 mg; 4) |
NA | No | Sleep episodes shared similarities with narcolepsy in both behavioral and EEG findings. | Reduced the dose of pergolide to 1.5 mg/day | Italy |
| Hirayama et al.43 | 73 | Female | 13 |
Pergolide (1.5 mg; NA) l‐dopa (NA; 13) |
NA | NA | Head waggling | Reduced the dose of pergolide and l‐dopa | Japan |
| Garcia Ruiz44 | 61 | Male | 2 | Rotigotine (8 mg; 6) | NA | Yes | None | Switched to l‐dopa (300 mg/day) daily | Spain |
NA, not available.
Table 3.
Original studies on patients with PD with sleep attacks when taking dopaminergic medications
| Study | Participants | Methods | Relevant Findings | Country | Significant Finding |
|---|---|---|---|---|---|
| Pal et al.45 | Three groups of patients with PD taking (1) pramipexole (n = 19), (2) cabergoline (n = 22), and (3) l‐dopa monotherapy (n = 14) | Interview and ES scale |
No patients reported sleep attacks. No significant difference in ES score among groups |
United Kingdom | No |
| Happe et al.37 | 56 patients with PD and 59 age‐matched controls with sleep disorders and depression | Interview using Sleep Disorders Questionnaire | Patients with PD had significantly higher values in narcolepsy scores | Austria | Yes |
| Hobson et al.36 | 420 drivers from 638 consecutive highly functional patients with PD without dementia (of whom 80.5% on dopaminergic medications) | Prospective survey in 18 clinics using ES scale and Inappropriate Sleep Composite Score |
3.8% had experienced SOS while driving after the diagnosis of PD. An ES score of 7 or more had a sensitivity of 75% and a specificity of 50% in predicting sleep attacks. An Inappropriate Sleep Composite Score of 1 had a sensitivity of 52% and a specificity of 82% in predicting sleep attacks. |
Canada | Yes |
| Arnulf et al.29 | 54 consecutive patients with PD treated with l‐dopa referred for sleepiness (of whom 50.0% were concurrently taking dopamine agonists) | ES scale, nighttime PSG and daytime MSL test |
39% patients met the PSG criterion used to define narcolepsy (i.e., 2 or more sleep‐onset REM periods during the 5 MSL test). This group of patients had shorter MSL, but no significant difference of ES score, nighttime sleep, daily dose of l‐dopa, or use and dose of dopamine agonists, compared to the other patients. Weak correlation between the severity of sleepiness and ES score (R = −0.34) and daily dose of l‐dopa (r = 0.30). No significant correlation between the severity of sleepiness and dopamine agonist treatment |
France | No |
| Paus et al.4 | 2,952 patients with PD from two German counties (of whom 97.5% were on dopaminergic medications) | Specific questionnaire |
6.0% of patients reported having sleep attacks with or without warning. The prevalence of sleep attacks was 2.9% in l‐dopa, 5.3% in dopamine agonist monotherapy, 7.3% in l‐dopa and dopamine monotherapy, and 9.2% in l‐dopa and multiple dopamine agonist users |
Germany | Yes |
| Schlesinger and Ravin14 | 70 consecutive patients with PD on dopamine agonists | Interview |
34.3% of patients experienced IDS. 50% of the patients on pramipexole, 15.4% of the patients on pergolide, and 23.1% of the patients on ropinirole experienced IDS. Patients with IDS were significantly younger. Daytime somnolence and early arousals were risk factors, whereas daytime napping and benzodiazepines were protective factors. IDS improved by reviewing the dosing schedule, changing the amount of agonist per dose, discontinuing the agonist, or accommodating the sleepiness. |
United States | Yes |
| Paus et al.21 | 102 patients with PD and sleep attacks were matched to 102 patients with PD without sleep attacks and identical type of dopaminergic treatment. | Polymorphisms were genotyped by polymerase chain reaction. | Significant association between sleep attacks without warning signs and the dopamine receptor D4*2 (short) allele, but not with dopamine D2 and D3 receptor and serotonin transporter gene | Germany | Yes |
| Manni et al.34 | 22 nondemented, adult patients with PD | Structured sleep interview, ES scale and 24‐hour ambulatory PSG |
32% of patients reported sleep attacks. Patients with sleep attacks had higher ES scores and a higher proportion of microsleeps and intentional naps. |
Italy | Yes |
| Korner et al.32 |
6,620 patients with PD (of whom 97% were on dopaminergic medications) |
Specific questionnaire |
16.4% of patients reported SOS in active situations. The administration of dopaminergic drugs was identified as a risk factor for SOS (dopamine agonists in patients <70 years old with disease duration up to 7 years). Other risk factors included older age, male sex, longer disease duration, and other sleep disturbances. |
Germany | Yes |
| Rissling et al.20 | 137 patients with PD and SOS and 137 patients with PD without SOS matched per use of dopamine agonists, disease duration, sex, and age | Association study on dopamine D2, D3, and D4 receptor gene polymorphisms |
Significant association between the dopamine D2 receptor gene polymorphism, Taq IA, and sudden onset of sleep in PD; no significant association between the dopamine D3 and D4 receptor genes |
Germany | Yes |
| Rissling et al.24 | 132 patients with PD and SOS and 132 patients with PD without SOS matched per use of dopamine agonists, disease duration, sex, and age | Polymerase chain reaction protocols or direct sequencing | Significant association between the variant allele T of (‐909T/C) preprohypocretin polymorphism and SOS in PD | Germany | Yes |
| Moller et al.35 | 10 patients with PD and sleep attacks matched with 10 patients with PD without any daytime sleepiness, matched to dopaminergic medications | MSL test | No significant difference of sleep latency between the two groups | Germany | No |
| Asai et al.38 | 25 patients with PD, including 9 with excessive daytime sleepiness and 4 with sleep attacks | Levels of orexin in samples of spinal CSF | Switching treatment to pergolide (selective dopamine D1/D2 agonist) significantly increased CSF‐orexin levels and completely resolved sleep attacks in the 4 patients with PD. | Japan | Yes |
| Christine et al.31 | 3 patients (on l‐dopa) with a prior diagnosis of narcolepsy from a registry of 1152 consecutive patients with PD | Medical records review |
Narcolepsy rate (0.26%) was 5 times higher than expected (0.05%). The 3 patients had treatment with amphetamine before the diagnosis of PD. |
United States | Yes |
IDS, irresistible daytime sleepiness; MSL, multiple sleep latency; SOS, sudden onset of sleep.
Table 4 summarizes the findings of the five studies that reported on the prevalence of sleep attacks among patients with PD on dopaminergic medications.
Table 4.
Studies reporting the prevalence of sleep attacks among patients with PD on dopaminergic medications
| Study | Number of Patients in the Study | Number of Patients With Possible “Sleep Attacks” | Percentage With Possible “Sleep Attacks” | Number of Patients on Dopaminergic Medications | Percentage of Patients on Dopaminergic Medications |
|---|---|---|---|---|---|
| Hobson et al.36 | 420 | 16 | 3.8 | 338 | 80.5 |
| Paus et al.4 | 2,952 | 177 | 6.0 | 2,878 | 97.5 |
| Schlesinger and Ravin14 | 70 | 24 | 34.3 | 70 | 100.0 |
| Manni et al.34 | 22 | 7 | 31.8 | 22 | 100.0 |
| Korner et al.32 | 6,620 | 1,083 | 16.4 | 6,421 | 97.0 |
| Total | 10,084 | 1,307 | 13.0 | 9,729 | 96.5 |
The overall prevalence of sleep attacks was estimated to be 4.8%. As shown in Figure 2, meta‐analysis on ES scores failed to show a significant difference between patients with PD on dopamine agonists with and without sleep attacks (2.92; 95% confidence interval (CI): −0.47–6.31).
Figure 2.
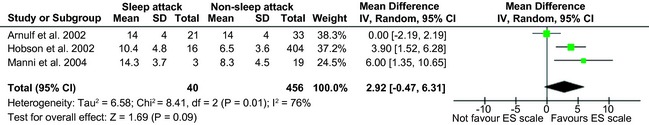
Forest plot of the studies reporting ES scores. SD, standard deviation.
The I2 value of 76% indicated high heterogeneity among the studies. The symmetry of the funnel plot suggested nonsignificant publication bias on the studies that reported ES scores (Fig. 3).
Figure 3.
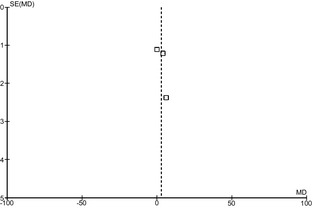
Funnel plot of the studies reporting ES scores.
Of the 14 original studies included in the present review, 11 showed significant findings, suggesting a possible publication bias (Table 3).
Discussion
Over the last few years, there has been increasing interest in the nonmotor aspects of PD,8 with particular attention to the behavioral symptoms.9, 10 Within the literature focusing on sleep disturbances in the context of PD,11, 12, 13 relatively little research has been conducted on sleep attacks since their first description in patients with PD on dopaminergic medication in 1999. We conducted the first systematic literature review on this topic and found that, according to the available evidence, these symptoms are not rare, with a prevalence of 13.0%. Moreover, sleep attacks in patients with PD on dopaminergic medication have been reported in different countries, including the United States, Germany, France, United Kingdom, Italy, Japan, Canada, and Spain, and do not appear to be limited to the use of any particular class of dopaminergic agents. However, sleep attacks seem to be most prevalent in patients taking levodopa and multiple dopamine agonists (9.2%), followed by l‐dopa and a single‐dopamine agonist (7.3%), dopamine agonists alone (5.3%), and l‐dopa alone (2.9%).4 Among dopamine agonists, sleep attacks were most prevalent with the use of pramipexole (50%), followed by ropinirole (23.1%) and pergolide (15.4%).14 This finding would be in line with the previously reported specific activity profile of pramipexole.15, 16
The reviewed literature clearly shows that dopaminergic drugs may increase sleepiness in patients with PD, in line with the known roles of dopamine neurotransmission and in sleep modulation. Dopamine‐containing neurons involved in the regulation of sleep and waking arise in the ventral tegmental area and the SNc. These structures are strongly interconnected with the dorsal raphe nucleus, the pedunculo‐pontine and laterodorsal tegmental nuclei, the locus coeruleus, the lateral and posterior hypothalamus, the basal forebrain, and the thalamus. Different dopamine receptors have been shown to play specific roles in sleep modulation, because agents with D1 or D2 receptor‐ blocking properties increase non‐REM (rapid eye movement) sleep and decrease wakefulness, whereas D3 receptor agonists induce somnolence and sleep in both laboratory animals and humans.17 Although some studies have linked excessive daytime sleepiness to the peak dose of dopamine and to the resulting autonomic imbalance, namely, hypotension,18 it is still controversial whether or not sedation occurs at the peak of the effect of the dopaminergic drugs.6, 19 Moreover, there are several reports suggesting that excessive daytime sleepiness in PD is a multifactorial phenomenon, linked to the degenerative process itself.
When sleep attacks were first reported in patients with PD, a pathophysiological mechanism was proposed whereby dopaminergic medications increase the amount of central dopamine, resulting in down‐regulation of the dopaminergic input to the reticular activating system and development of sleep attacks.3 One genetic study showed a significant association between the dopamine D2 receptor gene polymorphism, Taq IA, and sudden onset of sleep in patients with PD.20 Another genetic study showed a significant association between sleep attacks without warning signs and the dopamine receptor D4*2 (short) allele.21 This pathophysiological mechanism would be consistent with dysregulation of multiple dopaminergic pathways within the basal ganglia, with wide‐ranging implications for nonmotor symptoms reported by patients with PD.16, 22
In the first case series of sleep attacks in patients with PD, it was suggested that these symptoms resemble narcolepsy.3 A case report showed that narcolepsy and pergolide‐induced sleep attacks shared similar behavioral and electroencephalographic characteristics.23 A genetic study showed a significant association between the variant allele T of (‐909T/C) preprohypocretin polymorphism and sudden onset of sleep in patients with PD.24 Preprohypocretin is a precursor of hypocretin, and deficient hypocretin neurotransmission in the lateral hypothalamus is a probable cause of narcolepsy, suggesting that narcolepsy and sleep attacks in the context of PD may share some genetic factors. The link between hypocretin‐1 (orexin‐A) neuron degeneration and sleep attacks in PD deserves further investigation, because it has been reported that dopamine receptor stimulation can modify neuronal activity of these neurons, suggesting that there may be a link among neurodegeneration and dopaminergic therapy. Moreover, the relative preservation of hypocretin‐1 (orexin‐A) neurons in PD may explain why these patients present only some features of narcolepsy, mirroring narcolepsy without cataplexy in which cerebrospinal fluid (CSF) hypocretin‐1 levels are not constantly reduced.25 Locus coeruleus pathology has also been linked with REM sleep behavior disorder in the context of PD.26 However, sleep‐onset REM phases, typically observed in narcolepsy, were not observed in 6 patients with PD with sleep attacks when taking dopamine agonists.27 Moreover, modafanil, which is a first‐line medication for narcolepsy, was shown to be ineffective for sleep disorders in the context of PD,28 thus further questioning the similarity between sleep attacks in patients with PD and narcolepsy. Homann et al.6 argued that sleep attacks in patients with PD might not be a side effect of dopaminergic medication. First, the occurrence of sleep attacks seems to lack a temporal association with the peak level of the drugs. A survey showed that 47% of the patients with PD had sleep attacks at any time of the day.4 Second, there is the possibility that sleep attacks lack a genuine dose‐effect relationship with the dopaminergic treatment. The observed correlation between higher doses of medications and sleep attacks4 could, in fact, be an “epiphenomenon” of the fact that more‐advanced disease progression requires higher doses of medication.6 This possibility is supported by the results of another study, which failed to identify any significant difference in daily dose of l‐dopa or dopamine agonists in patients with PD and polysomnography (PSG) findings consistent with narcolepsy, compared to patients with PD alone.29 Third, Homann et al.6 reported on a case in which sleep attacks occurred in the absence of pharmacotherapy, suggesting that the pathological process of PD itself, rather than dopaminergic medications, might cause sleep attacks. This hypothesis is supported by the observation that sleep attacks have not been reported in patients with restless leg syndrome treated with dopamine agonists. However, a few years later, a case series of piribedil‐induced sleep attacks in 7 patients without PD was published.30 In addition, a case series reported on 3 patients on l‐dopa who had been treated with amphetamines for narcolepsy before developing PD.31
With regard to the clinical characteristics of patients with PD reporting sleep attacks, the reviewed studies suggest that these symptoms can occur in a wide range of age groups (44–83 years old), disease duration (2.5–13.0 years), and treatment duration (1–48 months). Sleep attacks do not appear to be more prevalent in one gender, and the presence of additional signs and symptoms shows inconsistencies among the patients with these symptoms. Approximately half of the patients reported somnolence preceding sleep attacks. Sudden onset of sleep was strongly associated with use of dopamine agonists in patients younger than 70 years and with disease duration inferior to 7 years.32 Other risk factors include older age, male sex, longer disease duration, and previous report of sleep disturbances. Daytime somnolence and early arousals appear to be risk factors for irresistible daytime sleepiness, whereas daytime napping and use of benzodiazepines could have protective roles.14 A recent study exploring the relationships among excessive daytime sleepiness, nighttime sleep quality, and cognitive impairment in PD found that daytime sleepiness, but not nighttime sleep problems, were associated with cognitive impairment in patients with PD, especially in the setting of dementia and specific cognitive deficits. The association between cognition and daytime sleepiness deserves further investigations, given the fluctuating arousal states observed in cases of PD with cortical Lewy body involvement.33
In terms of assessment, a few standardized tests have been used to predict the risk of sleep attacks in patients with PD taking dopaminergic medications. Three studies (n = 496) used the ES score and were included in our meta‐analysis, which failed to show a significant difference between patients with PD on dopaminergic medication with and without sleep attacks. It is possible that the use of ES scale alone was insufficient to identify patients at risk of developing sleep attacks. PSG and multiple sleep latency tests have been used in the reviewed studies to diagnose sleep attacks in patients with PD on dopaminergic medications. Twenty‐one of the fifty‐four patients treated with l‐dopa met the PSG criterion used to define narcolepsy: two or more sleep‐onset REM periods during five multiple sleep‐onset latency tests.29 This group of patients had significantly shorter sleep latencies, compared to the other patients. However, only a weak correlation was found between the severity of sleepiness and ES score (R = −0.34). Patients with PD and sleep attacks were also reported to have a higher proportion of microsleeps and intentional naps.34 However, a study on 10 patients with PD and sleep attacks did not show any significant difference in sleep latency, compared to 10 patients without any daytime sleepiness matched by dopaminergic medication.35 Other instruments have been used in the reviewed literature. For example, an Inappropriate Sleep Composite Score of 1 had a sensitivity of 52% and specificity of 82% for the identification of sleep attacks,36 and patients with PD reported significantly higher values in the narcolepsy score of the Sleep Disorders Questionnaire.37 No single test showed both good sensitivity and specificity, and the use of multiple tests is recommended for the assessment of patients with PD at risk of having sleep attacks.
With regard to management, the reviewed studies showed that dose reduction or discontinuation of the offending drug were the two most effective strategies to resolve sleep attacks. Similarly, according to an interview of 70 consecutive patients with PD on dopamine agonists, irresistible daytime sleepiness improved after changing the dosing schedule, reviewing the amount of agonist per dose, discontinuing the agonist, or accommodating the sleepiness.14 Moreover, switching treatment to pergolide (a selective dopamine D1/D2 agonist) completely resolved sleep attacks in 4 patients with PD previously treated with other dopaminergic medications.38 These findings suggest that specific dopamine receptor profiles could be associated with sleep attacks in patients with PD, in addition to the polymorphisms initially identified by human genetic studies.20, 21
Our systematic literature review has limitations. First, by limiting our search to articles published in the English language, we might have omitted some important articles. Second, in the absence of agreed diagnostic criteria for sleep attacks in the context of PD, in the present review, we have followed the classification of sleep events proposed by Homann et al.5 This could have led to the involuntary omission of relevant patients with ambiguous description of sleep events and to the inclusion of patients misdiagnosed with sleep attacks according to nonstandardized interviews and self‐report questionnaires open to responder bias. Third, most reviewed studies identified patterns of association, rather than causal relationships, between sleep disturbances and PD. Last, the present review might be prone to publication bias, because most of the included studies reported positive results.
Conclusions
The overall results of our systematic literature review provide a prevalence figure of 13.0% for sleep attacks in patients with PD taking dopaminergic medications. The presence of sleep attacks could be associated with specific dopamine receptor profiles, with initial findings from human genetic studies highlighting the role of dopamine D2 and D4 receptor gene polymorphism. We found conflicting results on whether sleep attacks in the context of PD resemble narcolepsy, with some studies showing similar genetic, behavioral, and electroencephalographic characteristics and others highlighting significant differences in REM phases and treatment strategies. It has also been hypothesized that the presence of sleep attacks could be related to the pathological process of PD, rather than the dopaminergic treatment. The demographic and clinical characteristics of patients with sleep attacks showed a wide variation across the reviewed studies. Further research is needed in order to explore the possible correlations between dopamine plasma levels, autonomic parameters, and excessive daytime sleepiness. Likewise, future studies should assess the prevalence of sleep attacks in patients with different clinical forms of PD (e.g., akinesia/rigidity vs. tremor) and in patients who are not on dopaminergic drugs. Although the ES scale, PSG, and multiple sleep latency tests have been used to investigate sleep attacks, no single test was shown to be sufficient to correctly identify these symptoms in isolation. Our meta‐analysis on studies using the ES scale showed no significant difference between patients with and without sleep attacks. Further case‐control trials and prospective cohort studies are needed to shed some light on the causal relationships between sleep disturbances and PD. More prospective trials should also be conducted to find out which types of investigations have good sensitivity and specificity (>80%) in order to correctly identify patients with sleep attacks. Finally, randomized, controlled trials should be carried out to investigate whether the identified management options (e.g., dose reduction and discontinuation of the offending drugs) are safe and effective strategies to improve patients’ health‐related quality of life.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
E.Y.H.Y.: 1A, 1B, 1C, 2A, 2B, 3A
A.E.C.: 1B, 1C, 2C, 3B
Disclosures
Funding Sources and Conflicts of Interest: This study did not receive any specific funding. The authors have no financial disclosures or conflicts of interest.
Financial Disclosures for previous 12 months: The authors declare that there are no disclosures to report.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Chen JJ, Nelson MV, Swope DM. Parkinson's disease In: Dipiro JT, ed. Pharmacotherapy: A Pathophysiologic Approach. New York: The McGraw‐Hill Companies, Inc.; 2008:977–988. [Google Scholar]
- 2. Grosset DG, Macphee GJ, Nairn M; Guideline Development Group . Diagnosis and pharmacological management of Parkinson's disease: summary of SIGN guidelines. BMJ 2010;340:b5614. [DOI] [PubMed] [Google Scholar]
- 3. Frucht S, Rogers JD, Greene PE, Gordon MF, Fahn S. Falling asleep at the wheel: motor vehicle mishaps in persons taking pramipexole and ropinirole. Neurology 1999;52:1908–1910. [DOI] [PubMed] [Google Scholar]
- 4. Paus S, Brecht HM, Koster J, Seeger G, Klockgether T, Wullner U. Sleep attacks, daytime sleepiness, and dopamine agonists in Parkinson's disease. Mov Disord 2003;18:659–667. [DOI] [PubMed] [Google Scholar]
- 5. Homann CN, Wenzel K, Suppan K, Ivanic G, Kriechbaum N, Crevenna R, Ott E. Sleep attacks in patients taking dopamine agonists: review. BMJ 2002;324:1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Homann CN, Homann B, Ott E, Park KB. Sleep attacks may not be a side effect of dopaminergic medication. Mov Disord 2003;18:1569–1570. [DOI] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chaudhari U, Romano P, Mulcahy LD, Dooley LT, Baker DG, Gottlieb AB. Efficacy and safety of infliximab monotherapy for plaque‐type psoriasis: a randomised trial. Lancet 2001;357:1842–1847. [DOI] [PubMed] [Google Scholar]
- 9. Barns Neurauter MP, Rickards H, Cavanna AE. The prevalence and clinical characteristics of pathological gambling in Parkinson's disease: an evidence‐based review. Funct Neurol 2010;25:9–13. [PubMed] [Google Scholar]
- 10. Spencer AH, Rickards H, Fasano A, Cavanna AE. The prevalence and clinical characteristics of punding in Parkinson's disease. Mov Disord 2011;26:578–586. [DOI] [PubMed] [Google Scholar]
- 11. Louter M, Aarden WC, Lion J, Bloem BR, Overeem S. Recognition and diagnosis of sleep disorders in Parkinson's disease. J Neurol 2012;259:2031–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maass A, Reichmann H. Sleep and non‐motor symptoms in Parkinson's disease. J Neural Transm 2013;120:565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raggi A, Bella R, Pennisi G, Neri W, Ferri R. Sleep disorders in Parkinson's disease: a narrative review of the literature. Rev Neurosci 2013;24:279–291. [DOI] [PubMed] [Google Scholar]
- 14. Schlesinger I, Ravin PD. Dopamine agonists induce episodes of irresistible daytime sleepiness. Eur Neurol 2003;49:30–33. [DOI] [PubMed] [Google Scholar]
- 15. Piedad JC, Cavanna AE. Dyskinesias and treatment with pramipexole in patients with Parkinson's disease. Parkinsons Dis 2012;2012:473769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cavanna AE. The behavioural neurology of basal ganglia disorders. Behav Neurol 2013;26:217–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Monti JM, Monti D. The involvement of dopamine in the modulation of sleep and waking. Sleep Med Rev 2007;11:113–133. [DOI] [PubMed] [Google Scholar]
- 18. Montastruc JL, Brefel‐Courbon C, Senard JM, et al. Sleep attacks and antiparkinsonian drugs: a pilot prospective pharmacoepidemiologic study. Clin Neuropharmacol 2001;24:181–183. [DOI] [PubMed] [Google Scholar]
- 19. Monaca C, Duhamel A, Jacquesson JM, et al. Vigilance troubles in Parkinson's disease: a subjective and objective polysomnographic study. Sleep Med 2006;7:448–453. [DOI] [PubMed] [Google Scholar]
- 20. Rissling I, Geller F, Bandmann O, et al. Dopamine receptor gene polymorphisms in Parkinson's disease patients reporting “sleep attacks”. Mov Disord 2004;19:1279–1284. [DOI] [PubMed] [Google Scholar]
- 21. Paus S, Seeger G, Brecht HM, et al. Association study of dopamine D2, D3, D4 receptor and serotonin transporter gene polymorphisms with sleep attacks in Parkinson's disease. Mov Disord 2004;19:705–707. [DOI] [PubMed] [Google Scholar]
- 22. Ward P, Seri And S, Cavanna AE. Functional neuroanatomy and behavioural correlates of the basal ganglia: evidence from lesion studies. Behav Neurol 2013;26:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ulivelli M, Rossi S, Lombardi C, et al. Polysomnographic characterization of pergolide‐induced sleep attacks in idiopathic PD. Neurology 2002;58:462–465. [DOI] [PubMed] [Google Scholar]
- 24. Rissling I, Korner Y, Geller F, Stiasny‐Kolster K, Oertel WH, Moller JC. Preprohypocretin polymorphisms in Parkinson disease patients reporting “sleep attacks”. Sleep 2005;28:871–875. [DOI] [PubMed] [Google Scholar]
- 25. Wienecke M, Werth E, Poryazova R, et al. Progressive dopamine and hypocretin deficiencies in Parkinson's disease: is there an impact on sleep and wakefulness? J Sleep Res 2012;21:710–717. [DOI] [PubMed] [Google Scholar]
- 26. García‐Lorenzo D, Longo‐Dos Santos C, Ewenczyk C, et al. The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson's disease. Brain 2013;136:2120–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schafer D, Greulich W. Effects of parkinsonian medication on sleep. J Neurol 2000;247(suppl 4):IV/24–IV/27. [DOI] [PubMed] [Google Scholar]
- 28. Ondo WG, Fayle R, Atassi F, Jankovic J. Modafinil for daytime somnolence in Parkinson's disease: double blind, placebo controlled parallel trial. J Neurol Neurosurg Psychiatry 2005;76:1636–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arnulf I, Konofal E, Merino‐Andreu M, et al. Parkinson's disease and sleepiness: an integral part of PD. Neurology 2002;58:1019–1024. [DOI] [PubMed] [Google Scholar]
- 30. Gouraud A, Millaret A, Descotes J, Vial T; French Association of Regional Pharmacovigilance Centres . Piribedil‐induced sleep attacks in patients without Parkinson disease: a case series. Clin Neuropharmacol 2011;34:104–107. [DOI] [PubMed] [Google Scholar]
- 31. Christine CW, Marks WJ Jr, Ostrem JL. Development of Parkinson's disease in patients with Narcolepsy. J Neural Transm 2012;119:697–699. [DOI] [PubMed] [Google Scholar]
- 32. Korner Y, Meindorfner C, Moller JC, et al. Predictors of sudden onset of sleep in Parkinson's disease. Mov Disord 2004;19:1298–1305. [DOI] [PubMed] [Google Scholar]
- 33. Goldman JG, Ghode RA, Ouyang B, Bernard B, Goetz CG, Stebbins GT. Dissociations among daytime sleepiness, nighttime sleep, and cognitive status in Parkinson's disease. Parkinsonism Relat Disord 2013;19:806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Manni R, Terzaghi M, Sartori I, Mancini F, Pacchetti C. Dopamine agonists and sleepiness in PD: review of the literature and personal findings. Sleep Med 2004;5:189–193. [DOI] [PubMed] [Google Scholar]
- 35. Moller JC, Rethfeldt M, Korner Y, et al. Daytime sleep latency in medication‐matched Parkinsonian patients with and without sudden onset of sleep. Mov Disord 2005;20:1620–1622. [DOI] [PubMed] [Google Scholar]
- 36. Hobson DE, Lang AE, Martin WR, Razmy A, Rivest J, Fleming J. Excessive daytime sleepiness and sudden‐onset sleep in Parkinson disease: a survey by the Canadian Movement Disorders Group. JAMA 2002;287:455–463. [DOI] [PubMed] [Google Scholar]
- 37. Happe S, Schrodl B, Faltl M, Muller C, Auff E, Zeitlhofer J. Sleep disorders and depression in patients with Parkinson's disease. Acta Neurol Scand 2001;104:275–280. [DOI] [PubMed] [Google Scholar]
- 38. Asai H, Hirano M, Furiya Y, et al. Cerebrospinal fluid‐orexin levels and sleep attacks in four patients with Parkinson's disease. Clin Neurol Neurosurg 2009;111:341–344. [DOI] [PubMed] [Google Scholar]
- 39. Schapira AH. Sleep attacks (sleep episodes) with pergolide. Lancet 2000;355:1332–1333. [DOI] [PubMed] [Google Scholar]
- 40. Ferreira JJ, Galitzky M, Montastruc JL, Rascol O. Sleep attacks and Parkinson's disease treatment. Lancet 2000;355:1333–1334. [DOI] [PubMed] [Google Scholar]
- 41. Ryan M, Slevin JT, Wells A. Non‐ergot dopamine agonist‐induced sleep attacks. Pharmacotherapy 2000;20:724–726. [DOI] [PubMed] [Google Scholar]
- 42. Hauser RA, Gauger L, Anderson WM, Zesiewicz TA. Pramipexole‐induced somnolence and episodes of daytime sleep. Mov Disord 2000;15:658–663. [DOI] [PubMed] [Google Scholar]
- 43. Hirayama M, Nakamura T, Hori N, Koike Y, Sobue G. The video images of sleep attacks in Parkinson's disease. Mov Disord 2008;23:288–290. [DOI] [PubMed] [Google Scholar]
- 44. Garcia Ruiz PJ. Sleep attack associated to rotigotine. Clin Neuropharmacol 2009;32:365. [DOI] [PubMed] [Google Scholar]
- 45. Pal S, Bhattacharya KF, Agapito C, Chaudhuri KR. A study of excessive daytime sleepiness and its clinical significance in three groups of Parkinson's disease patients taking pramipexole, cabergoline and levodopa mono and combination therapy. J Neural Transm 2001;108:71–77. [DOI] [PubMed] [Google Scholar]


