Abstract
Museum fluid collections preserve important biological specimens for study. Tissues are often fixed in 10% buffered formalin to halt metabolic activities and transferred to a solution of ethanol for long‐term storage. This process, however, forces water from the tissues and has been shown to alter the morphology of preserved specimens in ways that may influence the biological interpretation of results. The degree to which fluid preservation alters morphology is linked to multiple biological factors, such as tissue size and composition, and should therefore be examined prior to functional analysis. This study is undertaken as part of a more inclusive examination of mammalian volar morphology. A sample of five adult male and five adult female rats (Rattus norvegicus) was utilized to evaluate longitudinal changes in the dimensions of the volar pads across fixation in 10% buffered formalin and preservation in 70% ethanol for 1 year. No significant changes to the measured dimensions of the rat volar pads were present across stages of fixation and preservation, and no significant interactions of specimen size or sex were noted. These findings indicate that small mammalian volar pads that have been fixed in 10% buffered formalin and stored in 70% ethanol are appropriate for morphological study using the measurements described here without corrective algorithms. This finding is rare among preservation studies but highlights the variability of tissue behavior during chemical preservation and the necessity of preliminary investigations of preservation artifacts. Concurrence here between the preserved and unpreserved samples is likely related to the anhydrous nature of the volar pads and the supporting skeletal structure, and their confined position between major joints of the hands and feet.
Keywords: dental molding gel, fluid preservation, microCT, museum specimens, preservation artifacts, soft‐tissue preservation, walking pads
Introduction
Interest in the use of museum‐curated collections in morphological study is currently growing, as they provide access to rare, endangered or protected species, and reduce the need to sacrifice or injure additional subjects to examine their morphology (Suarez & Tsutsui, 2004; Winker, 2004; Rainbow, 2009; Casas‐Marce et al. 2012; Monfils et al. 2017). Successful preservation of soft tissues requires both halting metabolic and autolytic processes, and eliminating microbes that aide decomposition, ideally through means that do not alter the structure or composition of the tissue itself. To date, however, no known preservative or storage medium successfully accomplishes all three objectives (Fox et al. 1985; Kiernan, 2000; Vickerton et al. 2013). The current preferred practice of museums for curating soft tissues specimens consists of fixation in formalin (an aldehyde) and storage in a solution of 70% ethanol (Hopwood, 1969; Fox et al. 1985; Simmons & Voss, 2009; Suvarna et al. 2013; Hughes et al. 2016). This combination of chemical fixation and subsequent transfer to an antimicrobial media has proven highly effective at halting metabolic and autolytic processes and providing a sterile environment for long‐term storage (Hopwood, 1969; Fox et al. 1985; Suvarna et al. 2013). However, the process of chemical infiltration often produces changes in the size and shape of the preserved tissues, and a wealth of preservation artifacts have been documented in the morphological and histological literature (Parker, 1963; Hopwood, 1969; Kirkeby & Moe, 1986; Steedman, 1976; Fowler & Smith, 1983; Fox et al. 1985; Leslie & Moore, 1986; Kruse & Dalley, 1990; Bininda‐Emonds & Russell, 1993, 1994; Lasenby et al. 1994; Takizawa et al. 1994; Fox, 1996; Shields & Carlson, 1996; Fisher et al. 1998; Fey, 1999, 2012; Moku et al. 2004; Buchheister & Wilson, 2005; Fey & Hare, 2005; Gagliano et al. 2006; Thorstad et al. 2007; Edwards et al. 2009; Santos et al. 2009; Vervust et al. 2009; Yokogawa, 2009; Beamish et al. 2011; Lee et al. 2012; Berbel‐Filho et al. 2013; Gaston et al. 2013; Vickerton et al. 2013; Gómez et al. 2014; Kansu et al. 2017; Hedrick et al. 2018). Thus, morphologists interested in the study of soft tissues are left with a quandary: how accurately do the dimensions of preserved tissues reflect those found in live subjects and, further, do the benefits of utilizing curated collections outweigh the complications introduced by preservation artifacts?
Preservation artifacts, ubiquitous though they are, are generally reported to be relatively small. For two‐dimensional measures, shrinkage due to preservation in formalin or ethanol is predominantly reported to be within 0–5% of the original dimensions (Parker, 1963; Leslie & Moore, 1986; Bininda‐Emonds & Russell, 1993, 1994; Fox, 1996; Shields & Carlson, 1996; Fey & Hare, 2005; Gagliano et al. 2006; Thorstad et al. 2007; Vervust et al. 2009; Yokogawa, 2009; Lee et al. 2012; Gaston et al. 2013). Further, this shrinkage and associated shape changes have been demonstrated by many authors to be predictable and resolvable with appropriate transformation algorithms (Parker, 1963; Lasenby et al. 1994; Takizawa et al. 1994; Fox, 1996; Fey, 1999, 2001, 2012; Moku et al. 2004; Buchheister & Wilson, 2005; Fey & Hare, 2005; Santos et al. 2009; Vervust et al. 2009; Lee et al. 2012). The benefits of utilizing museum collections, on the other hand, are quite great. The collections are often extensive in size – the Natural History Museum of London, for example, houses over 70 million specimens alone (Rainbow, 2009) – and include type series that are used to demonstrate intraspecies variation and provide a standard reference for taxonomic classification. The collections further provide access to rare, endangered or otherwise difficult to obtain species (Suarez & Tsutsui, 2004; Winker, 2004; Rainbow, 2009; Monfils et al. 2017; Casas‐Marce et al. 2012). Centralization of a large number of species reduces the research costs associated with travel, collection, and private curation (Suarez & Tsutsui, 2004; Rainbow, 2009), so it is perhaps unsurprising to find that, in addition to the taxonomists and biodiversity specialists that traditionally call upon these collections, morphologists are increasingly interested in their sampling potential (Bininda‐Emonds & Russell, 1993; Vervust et al. 2009; Hughes et al. 2016; Gaston et al. 2013; Gignac & Kley, 2014; Gignac et al. 2016; Hedrick et al. 2018; Vickerton et al. 2013).
This simplistic equation, however, is complicated by the variable interactions of chemical preservatives with biological tissues. Formalin fixation halts metabolic and autolytic processes by binding to the tissue molecules, altering their shape and in turn arresting enzyme activity (Kiernan, 2000). During this process, methylene bridges form between tissue molecules, cross‐linking them and rendering the tissue stiff (Hopwood, 1969; Kiernan, 2000). Formalin binds to proteins within 24 h of exposure, making it useful for fixing large blocks of tissue (Helander, 1994). Its full range of reactions, however, takes a longer period to achieve; cross‐linking of molecules requires additional hours of exposure, and chemical bonds with lipids and carbohydrates only form after several weeks of exposure (Kiernan, 2000). During this period, the formalin solution can dissolve glycogen, glucose, phospholipids, and inorganic salts – including those that comprise the bone matrix (Steedman, 1976; Tucker & Chester, 1984; Kiernan, 2000; Suvarna et al. 2013). Despite these deleterious effects, formalin remains a popular fixative due to its relative speed and effectiveness, and because its effects on morphology are comparatively limited (Hopwood, 1969; Steedman, 1976; Helander, 1994; Fox et al. 1985; Kiernan, 2000; Suvarna et al. 2013; Simmons & Voss, 2009; Hughes et al. 2016).
Long‐term storage in ethanol solutions produces its own set of artifacts. Ethanol is less acidic than formalin and does not dissolve inorganic salts. However, it has the potential to dissolve fats and may dissolve free lipids in stored tissues, though the magnitude of this effect is likely small, as ethanol is a short‐chain, polar molecule and less prone to lipid dissolution than are long‐chain alcohols (Gagliano et al. 2006; Suvarna et al. 2013). Additionally, due to the high concentration necessary to prevent microbe proliferation, ethanol solutions have been demonstrated to be the one of the greater perpetrators of tissue shrinkage (Parker, 1963; Hay, 1982; Fowler & Smith, 1983; Tucker & Chester, 1984; Kruse & Dalley, 1990; Fox, 1996; Fisher et al. 1998; Moku et al. 2004; Buchheister & Wilson, 2005; Neave et al. 2006; Vervust et al. 2009; Vickerton et al. 2013).
Although these chemical changes certainly contribute to preservation artifacts, the primary driver of morphological changes is not the chemical used but rather the process of chemical infiltration itself (Tucker & Chester, 1984; Margo & Lee, 1995; Vickerton et al. 2013; Hedrick et al. 2018). To be effective, the chemical preservative must diffuse across the semi‐permeable membranes of the component cells of the tissue. This diffusion requires the presence of an osmotic gradient to occur; that is, the concentrations of solute (in this case, the preservative) on each side of the membrane must be unequal. The solute will then distribute across the membrane, displacing solvent (here, water) as needed, until both concentrations are equal (Hopwood, 1969; Fox et al. 1985; Kiernan, 2000). The concentration of the preservative solution and the living tissue composition (especially its water content) therefore both play an important role in determining the magnitude of morphological changes.
The relationship between preservative solution concentration and shrinkage is well documented. The fixatives formalin and glutaraldehyde have been demonstrated to produce greater degrees of shrinkage with increasing concentrations (Parker, 1963; Hay, 1982; Tucker & Chester, 1984; Meyer & Melzer, 2004; Moku et al. 2004; Buchheister & Wilson, 2005). Increasing concentrations of ethanol have likewise been shown to produce correspondingly greater tissue shrinkage when used for storage (Fisher et al. 1998; Moku et al. 2004; Santos et al. 2009). This phenomenon is not limited to preservatives: the concentration of histological stains has also been reported to correlate positively with tissue shrinkage (Vickerton et al. 2013; Hedrick et al. 2018).
In all cases, the changes to tissue morphology result from water exiting the tissue during infiltration, thus it follows that the initial composition of the tissue also plays a role in determining the magnitude of change. Specifically, tissues with a high initial water content, such as muscle (65–70% per unit volume, Schmidt, 1989), experience a greater exchange of fluids during preservation than those with low initial water content, such as adipose (10% per unit volume; Schmidt, 1989) or bone (22% per unit volume, Schmidt, 1989) (Fox et al. 1985; Kiernan, 2000). This, too, is well illustrated through comparisons of preserved specimens of different seasonal body compositions (Butler, 1992; Takizawa et al. 1994), as well as documentation of differential changes in gross shape and proportion within individual specimens comprising multiple tissue types (Vervust et al. 2009; Weisbecker, 2012; Berbel‐Filho et al. 2013; Gaston et al., 2013). This unequal warping affects soft tissues more drastically than hard tissues, which comprise lower percentages of water and receive additional support from rigid minerals in their matrices (Schmidt, 1989; Butler, 1992; Takizawa et al. 1994; Margo & Lee, 1995; Vervust et al. 2009; Korwin‐Kossakowski, 2014; Kansu et al. 2017; Hedrick et al. 2018). These disparities also highlight the potential for inconsistent artifact formation when full and partial specimens are preserved, as well as the potential for allometric size effects in the formation of preservation artifacts (Fox et al. 1985; Fey, 1999, 2012; Edwards et al. 2009; Vickerton et al. 2013; Korwin‐Kossakowski, 2014; Jeyakumar et al. 2015).
Because many factors may potentially influence the post‐preservation morphology of specimens, many authors caution – and rightly so – that investigation of preservation artifacts is integral to a rigorous and reproducible study (Parker, 1963; Lasenby et al. 1994; Takizawa et al. 1994; Fox, 1996; Fey, 1999, 2001, 2012; Moku et al. 2004; Buchheister & Wilson, 2005; Fey & Hare, 2005; Santos et al. 2009; Vervust et al. 2009; Beamish et al. 2011; Lee et al. 2012; Berbel‐Filho et al. 2013; Gaston et al. 2013). Moreover, species classified by traits or measurements that experience preservation changes may be erroneously placed when these changes are poorly understood (Worsaae, 2001; Oliveira et al. 2010). At present, the preponderance of studies in the literature on preservation artifacts focus on small fishes, reptiles, and soft‐bodied marine life. Studies of mammal preservation are sparse but suggest similar results: isolated soft tissues are prone to extreme shrinkage (Margo & Lee, 1995; Weisbecker, 2012; Vickerton et al. 2013; Jeyakumar et al. 2015; Hedrick, 2018) , but composite tissues experience small, predictable changes in dimensions that may be mitigated through extension of joints during preservation (Bininda‐Emonds & Russell, 1993, 1994; Kansu et al. 2017). With so few mammalian studies, it remains difficult for morphologists to use fluid‐preserved specimens with confidence. This study is carried out as part of a broader investigation of variation in mammalian volar fat pad morphology wherein utilization of preserved specimens provides a significant benefit in terms of cost and access to samples. Use of these specimens, however, is complicated by lack of studies targeting similar tissues for study; it remains unclear how chemical infiltration may affect the relatively anhydrous fat pads (10% water per unit volume; Schmidt, 1989) or what potential interactions or limitations the closely associated metapodial bones (22% water per unit volume; Schmidt, 1989) might impose on tissue warping. This study presents an opportunity both to address how specific variables of interest are affected by a common fluid preservation technique and to document the behavior of previously unexamined tissues. To this end, the dimensions of a sample of laboratory rat (Rattus norvegicus) cheiridia are tracked from perimortem through fixation in formalin and 1 year of preservation in 70% ethanol.
Methods
Sample
The volar pads are composed of adipose tissue surrounded by a fibrous sheath of collagen and elastin. In general, mammals possess six volar pads on the palms of the hands and soles of the feet – though these may fuse in different configurations – which are separated by prominent flexion creases. Additional volar pads are found on the ventral surfaces of the free digits; however, these are not discussed here. Here, the term ‘volar pad’ is used exclusively to refer to one of the six pads overlying the metapodial and carpal/tarsal bones. The thenar pad sits proximal to the hallux or pollex, the hypothenar pad sits proximal to the fifth digit, and the four interdigital pads sit between the digits near the bases of the proximal phalanges. Positions of these pads are illustrated in Fig. 1.
Figure 1.
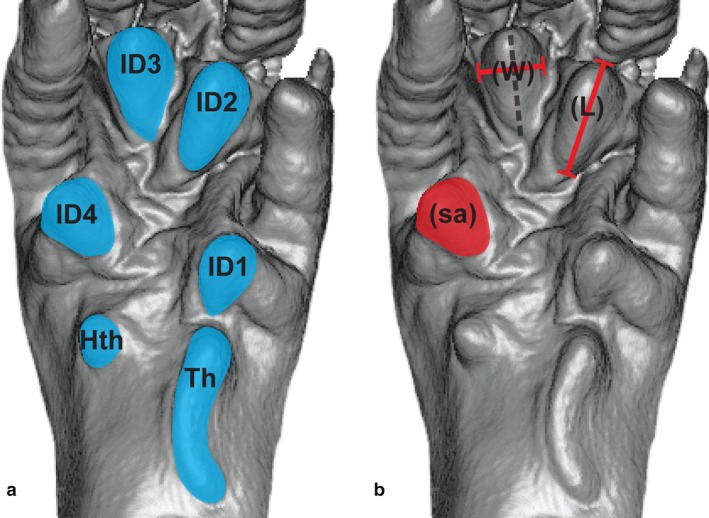
Illustration of volar anatomy and dimensions. (a) Location of the six volar pads on the pes of Rattus norvegicus. Pad abbreviations: Th, Thenar; Hth, Hypothenar; ID1, first interdigital; ID2, second interdigital; ID3, third interdigital; ID4, fourth interdigital. (b) An approximation of measured dimensions. Length (L) is defined as the greatest proximo‐distal distance; width (W) is defined as the greatest mediolateral distance perpendicular to the length of the pad (represented by the gray dashed line). Pad surface area (sa) is defined as the total area of the volar pad including all surface features.
This study examined longitudinal changes in manual and pedal volar morphology pre‐ and post‐preservation in 10 laboratory rats (Rattus norvegicus). Laboratory rats were selected as a model mammal due to their ubiquitous presence in university animal resource laboratories and regular scheduled culling. The rats were previously housed in Stony Brook University's Division of Laboratory Animal Resources (DLAR), which operates under Assurance #A3011‐01, approved by the NIH Office of Laboratory Animal Welfare (OLAW), and were received by the author after a routine colony cull. Five adult male and five adult female rats were obtained, and the right hand and foot of each were removed for study.
Molding procedure
To facilitate study across multiple locations, high resolution molds were created of volar surfaces by applying Coltene President light body dental molding compound (low‐viscosity polyvinylsiloxane) to the entire surface and allowing it to harden. Once solidified, the molds peeled away easily from volar surfaces and left no residue behind. This molding process followed Boyer's (2008) protocol for obtaining high‐resolution molds of primate teeth.
Two sets of molds were produced for each sampled individual. The first set was created from unpreserved right manual and pedal surfaces within the first hour postmortem. One hand and foot from each individual were removed and fixed in 10% buffered formalin for 24 h, then stored with the joints extended in 70% ethanol for 1 year (365 days). The second set of molds were created from the preserved cheiridia after 1 year.
Digitization and reconstruction
Molds were digitized following Boyer (2008). Digital scans were conducted via microcomputed tomography (μCT). All scans were conducted using the VivaCT75 μCT scanner located in the main DLAR facility in Stony Brook University's Health Sciences Center. Specimens were scanned at a resolution of 30 μm (integration time of 100 ms, 70 kVp, 114 μA) and exported as dicom images. Image files were stacked and converted to three‐dimensional digital objects in amira (Thermo Scientific) and imported into geomagic studio (Raindrop). Digital measurements were taken in geomagic studio for the thenar, hypothenar, and each of the four interdigital pads. Definitions of measurements are as follows (see also Fig. 1): Pad length: the longest dimension of the pad in a plane radiating from the wrist joint toward the associated digits. Pad width: the longest dimension of the pad in a plane perpendicular to the length. Pad surface area: the entire area of the skin covering the pad, including all surface features. Measurements were collected to the nearest 0.01 mm as bounded by the scanning resolution (30 μm = 0.003 mm).
Accuracy and precision of methodology
Each step of processing before analysis presented an opportunity to introduce error to the measured dimensions of the examined samples. In this study, samples were extensively processed – molded, scanned, and digitally reconstructed – and measured digitally via manually selected landmarks. Additionally, the molding and scanning procedure employed here has previously only been validated for reconstruction of hard tissues (Boyer, 2008; Blatch et al. 2011; Ledogar et al. 2013; St. Clair & Boyer, 2016). The accuracy and precision of data collected here were therefore examined in two ways. First, to ensure the dental molding compound produced molds that accurately represented the physical specimens, the measurements collected directly from scans of the physical specimens were included in a repeated measures analysis of covariance (ancova) with matched measurements collected from both the molds created at the time of death and the molds collected after fixation and preservation. This analysis is described in greater detail in the following section. Secondly, the precision of the manually selected landmarks was assessed by calculating the mean (x̄) and relative standard deviations (srel = (s/x̄)*100%) of repeated measurements. The repeated measurements (length, width, surface area) were carried out by a single observer on five independent occasions from reconstructed scans of the unpreserved rat cheiridia. Statistical computations were performed in spss software. Power analyses were performed in g*power 3.1.9.2 Faul et al., 2007.
Longitudinal analysis
The longitudinal analysis aimed to identify changes in volar pad dimension after fluid fixation and storage. A repeated measures ancova was utilized to compare the dimensions of measurements collected directly from scans of the physical specimens with matched measurements collected from both the molds created at the time of death and the molds collected after fixation and preservation. Sex of the specimens was included in the analysis as a fixed factor. The geometric mean of manual and pedal volar pad surface areas was calculated as a proxy for relative specimen size and included in the analysis as a covariate. This measure was employed as a proxy for specimen size in place of body mass, as the hands and feet were removed from the body to facilitate fixation and storage. All measurements were transformed by the natural logarithm to increase distribution normality, and alpha was set at 0.05. Mauchly's W test was used examine data sphericity; when sphericity was violated, Greenhouse–Geisser's correction was performed in computing the F statistic. Further contrasts were not indicated by the results. Statistical computations were performed in spss software.
Results
Accuracy and precision of methodology
The high‐definition volar molds produce measurements that are statistically indistinguishable from those collected from the physical specimens. Descriptive statistics and results of the repeated measures ancova are provided in Table 1; means and quartiles for these groups are illustrated in Fig. 2. This result indicates that high‐definition molds represent the original surfaces accurately and are appropriate for use in data collection involving soft tissues.
Table 1.
Results of statistical analyses
| Repeated measures anova | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Preservation | Size interaction | Sex interaction | |||||||
| Extremity | Pad | Dimension | SDRel | F | P | F | P | F | P |
| Manual | Th | Length | 0.65% | 1.11 | 0.36 | 1.24 | 0.32 | 0.35 | 0.71 |
| Width | 1.56% | 3.85 | 0.06 | 3.85 | 0.06 | 0.41 | 0.67 | ||
| Surface area | 2.82% | 0.54 | 0.60 | 0.43 | 0.66 | 0.78 | 0.47 | ||
| ID2 | Length | 1.24% | 0.41 | 0.68 | 0.36 | 0.70 | 1.64 | 0.23 | |
| Width | 1.15% | 0.25 | 0.78 | 0.17 | 0.85 | 0.20 | 0.82 | ||
| Surface area* | 0.99% | 0.07 | 0.82 | 0.09 | 0.79 | 0.02 | 0.90 | ||
| ID3 | Length* | 1.23% | 1.31 | 0.29 | 1.45 | 0.69 | 0.23 | 0.67 | |
| Width | 1.34% | 2.45 | 0.12 | 3.26 | 0.07 | 0.40 | 0.68 | ||
| Surface area | 1.20% | 2.95 | 0.09 | 3.15 | 0.08 | 2.48 | 0.12 | ||
| ID4 | Length | 0.96% | 0.90 | 0.43 | 0.89 | 0.43 | 2.75 | 0.10 | |
| Width | 1.64% | 1.07 | 0.37 | 0.98 | 0.40 | 1.12 | 0.35 | ||
| Surface area | 1.71% | 1.75 | 0.21 | 1.78 | 0.20 | 0.18 | 0.84 | ||
| Hth | Length | 1.12% | 0.62 | 0.65 | 0.74 | 0.49 | 2.98 | 0.09 | |
| Width | 1.18% | 0.30 | 0.74 | 0.48 | 0.63 | 2.50 | 0.12 | ||
| Surface area | 0.85% | 0.04 | 0.96 | 0.05 | 0.95 | 0.40 | 0.68 | ||
| Pedal | Th | Length | 2.75% | 0.13 | 0.88 | 0.20 | 0.82 | 0.07 | 0.93 |
| Width | 3.83% | 0.96 | 0.41 | 0.99 | 0.39 | 0.11 | 0.90 | ||
| Surface area | 1.87% | 0.68 | 0.52 | 0.55 | 0.59 | 0.01 | 0.99 | ||
| ID1 | Length | 1.28% | 0.06 | 0.94 | 0.18 | 0.84 | 1.36 | 0.29 | |
| Width | 1.92% | 0.02 | 0.98 | 0.05 | 0.96 | 0.88 | 0.44 | ||
| Surface area* | 1.98% | 0.56 | 0.51 | 0.49 | 0.54 | 0.02 | 0.92 | ||
| ID2 | Length | 2.15% | 0.23 | 0.79 | 0.15 | 0.86 | 0.79 | 0.47 | |
| Width | 2.84% | 0.48 | 0.63 | 0.32 | 0.73 | 0.57 | 0.58 | ||
| Surface area | 1.90% | 1.62 | 0.21 | 1.76 | 0.21 | 0.59 | 0.57 | ||
| ID3 | Length | 2.53% | 0.49 | 0.62 | 0.45 | 0.64 | 0.45 | 0.65 | |
| Width | 2.96% | 0.14 | 0.87 | 0.14 | 0.87 | 0.35 | 0.71 | ||
| Surface area | 1.42% | 0.17 | 0.85 | 0.09 | 0.91 | 0.44 | 0.65 | ||
| ID4 | Length | 3.08% | 1.18 | 0.34 | 0.93 | 0.42 | 2.64 | 0.11 | |
| Width | 2.94% | 0.41 | 0.67 | 0.46 | 0.64 | 1.17 | 0.34 | ||
| Surface Area* | 2.65% | 0.73 | 0.44 | 0.62 | 0.48 | 0.09 | 0.82 | ||
| Hth | Length | 1.75% | 1.15 | 0.35 | 1.13 | 0.35 | 0.79 | 0.47 | |
| Width | 2.10% | 0.00 | 0.99 | 0.00 | 0.99 | 1.31 | 0.30 | ||
| Surface area | 1.60% | 0.81 | 0.47 | 0.72 | 0.51 | 2.35 | 0.13 | ||
The relative standard deviations of repeated measurements of digital models are provided in the column headed SDRel. Results of repeated measures anova comparing dimensions collected from (1) the physical volar surfaces, (2) molds created at the time of death and (3) molds created after fixation and 1‐year storage in ethanol are presented in the columns on the right.
Greenhouse–Geiser correction for non‐spherical data employed.
Figure 2.
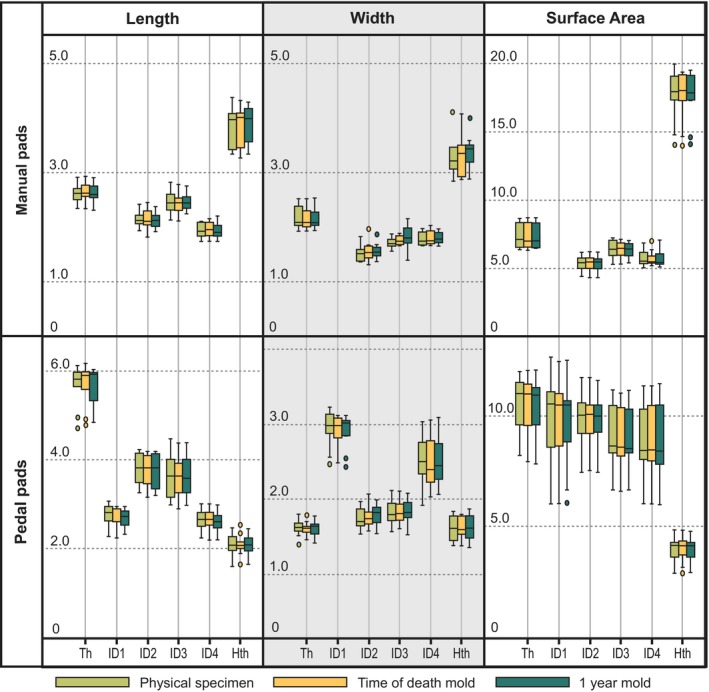
Box‐and‐whisker plots of measurements collected from Rattus norvegicus specimens and molds at different stages of preservation. Black lines indicate sample means, colored boxes indicate quartiles. Boxes cluster on the X‐axis by individual manual and pedal volar pads within columnar groupings of measured dimensions.
Manual selection of digital landmarks also allowed for precise measurements, with all relative standard deviations for all measurements falling between a minimum of 0.65% and maximum of 3.83% of the dimension mean, with an average of 1.70% for measurements of length, 1.73% for width, and 2.13% for surface area. The relative standard deviations for each precision measurement are also provided in Table 1.
Longitudinal analysis
No significant differences between measurements collected from the physical volar pad specimens, the molds created at the time of death or the molds created after fixation and 1‐year storage in ethanol were indicated by the repeated measures ancova. No significant interaction effects of sex or body size were found. F statistics and probabilities are provided in Table 1 and means and quartiles are illustrated in Fig. 2 for each dimension.
Discussion
Effects of preservation
This study did not indicate significant differences in the measured dimensions of rat volar pads before and after fixation in 10% buffered formalin and long‐term preservation in 70% ethanol. In light of the wealth of previous studies detailing preservation artifacts under similar conditions, these findings may seem at first to be an aberration. However, although this study may be somewhat unique in its null findings for all examined measurements, it is not the first study to report negligible changes to individual measurements (Billy, 1982; Leslie & Moore, 1986; Jawad, 2003; Vervust et al. 2009). Further, it is important to note that the preserved rat hands and feet do indeed change in appearance. Specifically, the positions of the fingers and toes are changed; the digits have flexed as muscle tissue has shrunk, and the volar surface has been pulled into a concave posture. The volar pads, however, remain relatively unaffected by this positional shift. The morphometric resilience of the pads here may be explained their tissue composition, anatomic location and, potentially, the size of the specimen they come from.
There is a stark contrast in the existing literature detailing the shrinkage of isolated soft tissues and that of composite tissue supported by bone or cartilage. Invariably, the most extreme shrinkage is reported for isolated soft tissues, with up to 43% loss of volume reported for eyes (Margo & Lee, 1995; Hedrick et al. 2018), 38% of volume for brains (Hedrick et al. 2018), and 35% for linear measurements for skin samples (Jeyakumar et al. 2015). Findings are more varied for studies where full or composite specimens are examined, but morphometric studies of fish larvae and associated preservation artifacts prove particularly instructive. Several authors have reported a negative correlation between larval shrinkage and ossification of the skeleton; very young larvae without fully ossified skeletons shrink significantly more than their older, ossified counterparts (Butler, 1992; Takizawa et al. 1994; Gómez et al. 2014; Korwin‐Kossakowski, 2014). This has been attributed both to absence of a mineralized skeleton or shell (Butler, 1992; Edwards et al. 2009) and to the differing chemical – especially water – content of the larvae (Leslie & Moore, 1986; Gómez et al. 2014). These explanations are not necessarily mutually exclusive – bone contains a relatively small amount of water at 20% per unit volume (Schmidt, 1989), and recent work reveals that it is not immune to artifacts brought on by preservation and staining (Buytaert et al. 2014). Whichever the primary driver, a pattern emerges of relatively rigid and/or anhydrous tissues experiencing a lesser degree of morphometric change due to infiltration and preservation. These same tissues also appear to support surrounding tissues such that they also experience a lesser degree of change. In their study of iguanas, Vervust et al. (2009) note specifically that the bony hands and feet were the only structures that did not shrink significantly, and that structures of the head directly supported by the skeleton were far less affected by preservation than those supported by soft tissue or cartilage. Cartilage as a structural tissue itself provides an interesting insight here as well: 77% of nasal cartilage is composed of water (Homicz et al. 2003), yet Kansu et al. (2017) report that leaving it attached to excised and preserved nasal mucosa (90% water, Schmidt, 1989) significantly reduces shrinkage of the mucosa. This was not the case when muscle (65–70% water per unit volume, Schmidt, 1989) was left attached to excised skin (65% water, Schmidt, 1989); no reduction in shrinkage for either tissue was noted (Jeyakumar et al. 2015). Taken together, these pieces of evidence indicate that the most rigid and/or anhydrous tissue is a limiting factor to the amount of shrinkage experienced by composite tissues.
Like the iguana hands and feet described by Vervust et al. (2009), the hands and feet of the rats examined here are supported by, and their volume dominated by, metapodial bones and phalanges. These provide a rigid base for the surrounding tissues that remains relatively stable during fixation and storage, and likely contributes to the negligible changes recorded for the volar pads. The composition of the fat pads themselves also likely contributes to their stability. It remained unclear at the outset exactly how the fatty tissue would behave – its anhydrous constitution (10% per unit volume, Schmidt, 1989) suggested that the effects of infiltration might be minimal, but previous studies indicated that fixation might be incomplete (Kiernan, 2000) or that volume might be lost through partial dissolution in ethanol (Gagliano et al. 2006; Suvarna et al. 2013). The former appears to be the case, as neither the individual dimensions nor the surface areas of the pads show any significant changes to their size throughout fixation and storage.
The confined anatomic position of the volar fat pads on the ventral surfaces of the metapodial bones appears to protect them further from morphometric change during preservation. Movement at the intermetapodial joints in the palm and sole is limited; flexion and extension occur proximal to the pads at the wrist or talar joint and distal to the pads at the metapodiophalangeal joints. The volar pads do not cross the wrist or talar joints, thus movements at these joints does not affect their shape. The interdigital pads, positioned ventral to the metapodial heads, may potentially be constricted by digital flexion; however, the dearth of differences found here indicates that extending the metapodiophalangeal joints during preservation and creation of molds adequately rectifies this problem.
Additionally, the limited movements of the volar surface occur at flexion creases, which run between the volar pads rather than crossing them, and the discrete nature of each pad allows for a small range of movement without a corresponding change in shape (for example, from colliding with a neighbor). In contrast, many of the cases previously studied involved measurements taken across multiple joints, where muscles could shrink and alter the shape and size of the specimens in numerous ways (Bininda‐Emonds & Russell, 1993, 1994; Fey, 1999; Neave et al. 2006; Edwards et al. 2009; Vervust et al. 2009; Gaston et al. 2013). Here, the relative regional isolation of each pad likely spares them these changes; it is additionally likely that measurements of the volar surface as a whole (for example, the median length of the surface from the carpometacarpal joint to the 3rd metacarpophalageal joint) would indeed exhibit differences before and after preservation. It is also important to appreciate that only gross linear and area measurements have been examined here – a more finely tuned analysis of shape may reveal other differences.
Finally, it is possible that the pads do shrink or expand during preservation, but that the error introduced during the collection procedure here is large enough to mask them. Previous work by Muñoz‐Muñoz & Perpiñán (2010) demonstrates that as body size in subjects increases, observed variation among individuals increases as well, which reduces the proportion of the group variance accounted for by measurement error. This study sought to control for this by utilizing repeated measures ancova in its analysis; however, if preservation artifacts are quite small, their effects may still be masked by the scale used to measure them. Measurements here were constrained by scanning resolution to the nearest 0.01 mm, a fraction that represents 0.67% of the shortest linear measurement (pedal hypothenar width: 1.46 mm), 0.1% of the largest linear measurement (pedal thenar length: 6.14 mm), and 0.40% of the average linear measurement (2.45 mm). Although greater scanning resolution may reveal statistically significant changes to the volar dimensions, it is important to recognize that this potential effect size represents less than or equal to a 1% change in dimensional measurements. For rat volar pads, this effect would be so miniscule that it could not be detected without advanced technology.
Similarly, it is possible that any effect size associated with preservation changes is small (i.e. Cohen's d < 0.2) and the statistical analysis is not powerful enough to detect it. This study measured 10 specimens within two sex groups across three preservation stages (real, time of death mold, year of preservation mold). The collected measurements are significantly correlated across groups; the average correlation (Pearson's r) between measurements of the physical specimens and time of death molds is 0.94 (SD = 0.10): r = 0.96 (SD = 0.04) for measurements of the time of death molds and molds created after a year of preservation, and r = 0.93 (SD = 0.13) for measurements of the physical specimens and the molds collected after 1 year. Following Cohen (1988), the power for a 10‐specimen, two‐group, three repeated measures study, in which the correlation between measures is equal to r = 0.9, is 0.99 in detecting a large effect size (d = 0.8; effect explains 15% or more of the variance between groups) when alpha is 0.05. For a small size effect (d = 0.2; effect explains 1% of the variance between groups) the power is 0.81. The smallest effect size detectable within this analysis at a power greater than 0.5 is d = 0.15, which accounts for less than 1% of the variance between groups.
A compounding effect of specimen size has been variably implicated in previous reports of preservation artifacts. Several studies of fish larvae have shown that larger individuals shrank significantly less than their small counterparts (Leslie & Moore, 1986; Fey, 1999, 2012; Edwards et al. 2009; Korwin‐Kossakowski, 2014). Kansu et al. (2017) found a similar size effect for preserved 10‐ and 20‐mm diameter nasal mucosa and cartilage samples. Others specifically report no that discrepancies between differently sized specimens exist and suggest the phenomenon may be taxon‐specific or the result of incomplete fixation (Lee et al. 2012). This study found no significant interaction of body size with preservation changes; however, the portions of the fore‐ and hind‐limbs collected for preservation here measured between 2 and 5 cm, which makes them larger than many of the larval fishes previously studied (Leslie & Moore, 1986; Fey, 1999, 2012; Edwards et al. 2009; Korwin‐Kossakowski, 2014) It is possible this larger size protects them from extreme changes during preservation.
Implications for future studies
This study employs a combination of low‐viscosity polyvinylsiloxane dental molding gel and μCT scanning to create high‐resolution digital surface maps for data collection. This non‐invasive, non‐destructive combination of techniques has been previously described (Boyer, 2008) and employed in morphometric studies of hard tissues (Blatch et al. 2011; Ledogar et al. 2013; St. Clair & Boyer, 2016). Here, this combination of techniques is validated for use on preserved soft tissues as well. No changes to the soft tissue dimensions caused by the weight of the molding gel or its chemical properties were evident, and no residue was left behind on the museum specimens. This method may prove useful for use of museum collections or at field sites, where it is not possible to directly scan individual specimens.
Although this study indicates that the gross dimensions of rat volar pad morphology may be accurately studied from alcohol‐preserved museum specimens without need of correction, it also highlights the necessity of preservation studies to investigate the interactions of tissue types and preservatives. The volar pads examined in this study differ from previously examined tissues in four important ways: they are supported by a bony foundation, they are limited in composition (adipose), comprise a low percentage of water, and are limited to an isolated region of the body that experiences little movement. These factors likely interact to produce few or relatively small changes during the preservation process. Further study of tissue behavior will elucidate the relative contributions of different characteristics.
Conclusions
Impressions of volar surfaces created using high performance dental molding gel provide a good proxy for μCT scanning and digital measurement when the actual specimens are unavailable for direct use. Digital measurements taken from these molds are accurate when compared with corresponding measurements taken from the same subject, with a relative standard deviation that falls below 4% for all measurements, and below 2% for the majority.
Alcohol‐preserved museum specimens provide a rich source of research material, but investigators must be mindful of the ways preservation may alter the specimens. In this case, the volar fat pads of rats were not found to differ significantly before and after alcohol preservation. Their adipose composition and location on stable portions of the volar surfaces likely protect them from significant shrinkage or warping during preservation, and thus leave their preserved form appropriate for morphologic study. Despite this, caution should always be exercised when utilizing alcohol‐preserved specimens and the effects of preservation on specific traits investigated beforehand.
Author contributions
The research described in this article was designed, performed, analyzed, and drafted by Amanda K. Kingston.
Acknowledgements
Funding for this project was provided by Doctoral Dissertation Improvement Grant 1097438‐1‐58637 from the National Science Foundation. Many thanks belong to the helpful employees of Stony Brook University's Department of Lab Animal Resources, Duke Lemur Center, and the American and National Museums of Natural History, and most especially to Susan Larson, who allowed me to store preserved specimens in her fume hood for a year, even though looking at them put her off her lunch. Thanks as well to two anonymous reviewers who provided important insights and helped shape the manuscript.
References
- Beamish FWH, Plongsesthee R, Chanintarapoomi P, et al. (2011) Total length‐weight relationships among Thai freshwater fishes and the influence of capture location and preservation. J Appl Ichthyol 27, 955–958. [Google Scholar]
- Berbel‐Filho WM, Jacobina UP, Martinez PA (2013) Preservation effects in geometric morphometric approaches: freezing and alcohol in a freshwater fish. Ichthyol Res 60, 268–271. [Google Scholar]
- Billy AJ (1982) The effects of formalin and isopropyl alcohol on length and weight measurements of Sarotherodon mossambicus Trewavas. J Fish Biol 21, 107–112. [Google Scholar]
- Bininda‐Emonds ORP, Russell AP (1993) Effects of preservation on wing morphometry of the little brown bat (Myotis lucifugus). J Zool 230, 141–158. [Google Scholar]
- Bininda‐Emonds ORP, Russell AP (1994) Flight style in bats as predicted from wing morphometry: the effects of specimen preservation. J Zool 234, 275–287. [Google Scholar]
- Blatch S, Boyer DM, King SJ, et al. (2011) Changes in orientation of attritional wear facets with implications for jaw motion in a mixed longitudinal sample of Propithecus edwardsi from Ranomafana National Park, Madagascar. Am J Phys Anthropol 146, 130–147. [DOI] [PubMed] [Google Scholar]
- Boyer DM (2008) Relief index of second mandibular molars in a correlate of diet among prosimian primates and other euarchontan mammals. J Hum Evol 55, 1118–1137. [DOI] [PubMed] [Google Scholar]
- Buchheister A, Wilson MT (2005) Shrinkage correction and length conversion equations for Theragra chalcogramma, Mallotus villosus and Thaleichthys pacificus . J Fish Biol 67, 541–548. [Google Scholar]
- Butler JL (1992) Otolith microstructure examination and analysis long‐term otolith growth chronologies in relation to cod stock dynamics and climate in the Northeast Atlantic View project In Otolith Microstructure Examination and Analysis. (eds Stevenson DK, Campana SE), pp. 13–17. Ottawa: Canadian Special Publication of Fisheries and Aquatic Sciences, 117. [Google Scholar]
- Buytaert J, Goyens J, De Greef D, et al. (2014) Volume shrinkage of bone, brain and muscle tissue in sample preparation for micro‐CT and light sheet fluorescence microscopy (LSFM). Microsc Microanal 20, 1208–1217. [DOI] [PubMed] [Google Scholar]
- Casas‐Marce M, Revilla E, Fernandes M, et al. (2012) The value of hidden scientific resources: preserved animal specimens from private collections and small museums. Bioscience 62, 1077–1082. [Google Scholar]
- Edwards FK, Lauridsen RB, Armand L, et al. (2009) The relationship between length, mass and preservation time for three species of freshwater leeches (Hirudinea). Fundam Appl Limnol/Arch für Hydrobiol 173, 321–327. [Google Scholar]
- Faul F, Erdfelder E, Lang A‐G, et al. (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 39, 175–191. [DOI] [PubMed] [Google Scholar]
- Fey DP (1999) Effects of preservation technique on the length of larval fish: methods of correcting estimates and their implication for studying growth rates. Arch Fish Mar Res 47, 17–29. [Google Scholar]
- Fey DP (2001) Differences in temperature conditions and somatic growth rate of larval and early juvenile spring‐spawned herring from the Vistula Lagoon, Baltic Sea manifested in the otolith to fish size relationship. J Fish Biol 58, 1257–1273. [Google Scholar]
- Fey DP (2012) Length adjustment of larval and early‐juvenile cod (Gadus morhua) after up to 3 years of preservation in alcohol. J Appl Ichthyol 28, 665–666. [Google Scholar]
- Fey DP, Hare JA (2005) Length correction for larval and early‐juvenile Atlantic menhaden (Brevoortia tyrannus) after preservation in alcohol. Fish Bull 103, 725–727. [Google Scholar]
- Fisher SJ, Anderson MR, Willis DW (1998) Total length reduction in preserved yellow perch larvae. North Am J Fish Manag 18, 739–742. [Google Scholar]
- Fowler GM, Smith SJ (1983) Length changes in silver hake (Merluccius bilinearis) larvae: effects of formalin, ethanol, and freezing. Can J Fish Aquat Sci 40, 866–870. [Google Scholar]
- Fox CJ (1996) Length changes in herring (Clupea harengus) larvae: effects of capture and storage in formaldehyde and alcohol. J Plankton Res 18, 483–493. [Google Scholar]
- Fox CH, Johnson FB, Whiting J, et al. (1985) Formaldehyde fixation. J Histochem Cytochem 33, 845–853. [DOI] [PubMed] [Google Scholar]
- Gagliano M, Kowalewsky S, McCormick MI (2006) An alternative method for the preservation of tropical fish larvae. J Fish Biol 68, 634–639. [Google Scholar]
- Gaston KA, Jacquemin SJ, Lauer TE (2013) The influence of preservation on fish morphology in museum collections based on two species of the genus lepomis (Actinopterygii: Perciformes: Centrarchidae). Acta Ichthyol Piscat 43, 219–227. [Google Scholar]
- Gignac PM, Kley NJ (2014) Iodine‐enhanced micro‐CT imaging: methodological refinements for the study of the soft‐tissue anatomy of post‐embryonic vertebrates. J Exp Zool Part B Mol Dev Evol 322, 166–176. [DOI] [PubMed] [Google Scholar]
- Gignac PM, Kley NJ, Clarke JA, et al. (2016) Diffusible iodine‐based contrast‐enhanced computed tomography (diceCT): an emerging tool for rapid, high‐resolution, 3‐D imaging of metazoan soft tissues. J Anat 228, 889–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez MI, Sánchez S, Fuentes CM (2014) Shrinkage of Prochilodus lineatus (Valenciennes, 1847) larvae preserved in either ethyl‐alcohol or formalin in relation to their developmental stage and feeding condition. J Appl Ichthyol 30, 140–144. [Google Scholar]
- Hay DE (1982) Fixation shrinkage of herring larvae: effects of salinity, formalin concentration, and other factors. Can J Fish Aquat Sci 39, 1138–1143. [Google Scholar]
- Hedrick BP, Yohe L, Vander Linden A, et al. (2018) Assessing soft‐tissue shrinkage estimates in museum specimens imaged with diffusible iodine‐based contrast‐enhanced computed tomography (diceCT). Microsc Microanal 1, 1352–1353. [DOI] [PubMed] [Google Scholar]
- Helander KG (1994) Kinetic studies of formaldehyde binding in tissue. Biotech Histochem 69, 177–179. [DOI] [PubMed] [Google Scholar]
- Homicz MR, McGowan KB, Lottman LM, et al. (2003) A compositional analysis of human nasal septal cartilage. Arch Facial Plast Surg 5, 53–58. [DOI] [PubMed] [Google Scholar]
- Hopwood D (1969) Fixatives and fixation: a review. Histochem J 1, 323–360. [DOI] [PubMed] [Google Scholar]
- Hughes DF, Walker EM, Gignac PM, et al. (2016) Rescuing perishable neuroanatomical information from a threatened biodiversity hotspot: remote field methods for brain tissue preservation validated by cytoarchitectonic analysis, immunohistochemistry, and x‐ray microcomputed tomography. PLoS ONE 11, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jawad LA (2003) The effect of formalin, alcohol and freezing on some body proportions of Alepes djeddaba (Pisces: Carangidae) collected from the Red Sea coast of Yemen. Rev Biol Mar Oceanogr 38, 77–80. [Google Scholar]
- Jeyakumar S, Smith AN, Schleis SE, et al. (2015) Effect of histologic processing on dimensions of skin samples obtained from cat cadavers. Am J Vet Res 76, 939–945. [DOI] [PubMed] [Google Scholar]
- Kansu L, Aydın E, Akkaya H, et al. (2017) Shrinkage of nasal mucosa and cartilage during formalin fixation. Balkan Med J 3, 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan JA (2000) Formaldehyde, formalin, paraformaledhyde and glutaraldehyde: what they are and what they do. Microsc Today 00–1, 8–12. [Google Scholar]
- Kirkeby S, Moe D (1986) Studies on the actions of glutaraldehyde, formaldehyde, and mixtures of glutaraldehyde and formaldehyde on tissue proteins. Acta Histochem 79, 115–121. [DOI] [PubMed] [Google Scholar]
- Korwin‐Kossakowski M (2014) Physical changes in specimens of five species of Cyprinidae preserved in ethanol and frozen. J Fish Biol 85, 736–751. [DOI] [PubMed] [Google Scholar]
- Kruse GH, Dalley EL (1990) Length changes in capelin, Mallotus villosus (Müller), larvae due to preservation in formalin and anhydrous alcohol. J Fish Biol 36, 619–621. [Google Scholar]
- Lasenby DC, Yan ND, Futter MN (1994) Changes in body dimensions of larval Chaoborus in ethanol and formalin. J Plankton Res 16, 1601–1608. [Google Scholar]
- Ledogar JA, Winchester JM, St. Clair EM, et al. (2013) Diet and dental topography in pitheciine seed predators. Am J Phys Anthropol 150, 107–121. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kodama K, Horiguchi T (2012) Change in body size of juvenile marbled sole Pseudopleuronectes yokohamae after preservation in ethanol. Ichthyol Res 59, 49–52. [Google Scholar]
- Leslie JK, Moore JE (1986) Changes in lengths of fixed and preserved young freshwater fish. Can J Fish Aquat Sci 43, 1079–1081. [Google Scholar]
- Margo CE, Lee A (1995) Fixation of whole eyes: the role of fixative osmolarity in the production of tissue artifact. Graefe's Arch Clin Exp Ophthalmol 233, 366–370. [DOI] [PubMed] [Google Scholar]
- Meyer R, Melzer R (2004) Scanning EM diagnosis of marine decapoda larvae: a comparison of preparation techniques. Crustaceana 77, 883–886. [Google Scholar]
- Moku M, Mori K, Watanabe Y (2004) Shrinkage in the body length of myctophid fish (Diaphus Slender‐Type spp.) larvae with various preservatives. Copeia 2004, 647–651. [Google Scholar]
- Monfils AK, Powers KE, Marshall CJ, et al. (2017) Natural history collections: teaching about biodiversity across time, Space, and digital platforms. BioOne 16, 47–57. [Google Scholar]
- Muñoz‐Muñoz F, Perpiñán D (2010) Measurement error in morphometric studies: comparison between manual and computerized methods. Ann Zool Fenn 47, 46–56. [Google Scholar]
- Neave FB, Mandrak NE, Docker MF, et al. (2006) Effects of preservation on pigmentation and length measurements in larval lampreys. J Fish Biol 68, 991–1001. [Google Scholar]
- Oliveira VM, Santos CSG, Lana PC, et al. (2010) Morphological variations caused by fixation techniques may lead to taxonomic confusion in Laeonereis (Polychaeta: Nereididae). Zoologia 27, 146–150. [Google Scholar]
- Parker RR (1963) Effects of formalin on length and weight of fishes. J Fish Res Bd Can 20, 1441–1455. [Google Scholar]
- Rainbow PS (2009) Marine biological collections in the 21st century. Zool Scr 38, 33–40. [Google Scholar]
- Santos JNS, Araújo FG, Silva DS (2009) Length correction for early‐juvenile Brazilian herring Sardinella janeiro (Eigenmann, 1894) after preservation in formalin, ethanol and freezing. Neotrop Ichthyol 7, 87–92. [Google Scholar]
- Schmidt RF (1989) Human Physiology. Berlin: Springer‐Verlag. [Google Scholar]
- Shields PA, Carlson SR (1996) Effects of formalin and alcohol preservation on lengths and weights of juvenile Sockeye Salmon. Alaska Fish Res Bull 3, 81–93. [Google Scholar]
- Simmons N, Voss R (2009) Collection, preparation, and fixation of specimens and tissues In: Ecological and Behavioral Methods for the Study of Bats (eds Kunz TH, Parsons S.), pp. 849–867. Baltimore: The Johns Hopkins University Press. [Google Scholar]
- St. Clair EM, Boyer DM (2016) Lower molar shape and size in prosimian and platyrrhine primates. Am J Phys Anthropol 161, 237–258. [DOI] [PubMed] [Google Scholar]
- Steedman HF (1976) Zooplankton Fixation and Preservation. Monographs on Oceanographic Methodology 4. Paris: Unesco Press. [Google Scholar]
- Suarez AV, Tsutsui ND (2004) The value of museum collections for research and society. Bioscience 54, 6–74. [Google Scholar]
- Suvarna SK, Layton C, Bancroft JD (2013) Bancroft's Theory and Practice of Histological Techniques. 8th edn Amsterdam: Elsevier Ltd. [Google Scholar]
- Takizawa K, Fujita Y, Ogushi Y, et al. (1994) Relative change in body length and weight in several fish larvae due to formalin fixation and preservation. Fish Sci 60, 355–359. [Google Scholar]
- Thorstad EB, Finstad AG, Jensen AJ, et al. (2007) To what extent does ethanol and freezing preservation cause shrinkage of juvenile Atlantic salmon and European minnow? Fish Manag Ecol 14, 295–298. [Google Scholar]
- Tucker JW, Chester AJ (1984) Effects of salinity, formalin concentration and buffer on quality of preservation of southern flounder (Paralichthys lethostigma) larvae. Copeia 1984, 981–988. [Google Scholar]
- Vervust B, Van Dongen S, Van Damme R (2009) The effect of preservation on lizard morphometrics – An experimental study. Amphib Reptil 30, 321–329. [Google Scholar]
- Vickerton P, Jarvis J, Jeffery N (2013) Concentration‐dependent specimen shrinkage in iodine‐enhanced microCT. J Anat 223, 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbecker V (2012) Distortion in formalin‐fixed brains: using geometric morphometrics to quantify the worst‐case scenario in mice. Brain Struct Funct 217, 677–685. [DOI] [PubMed] [Google Scholar]
- Winker K (2004) Natural history museums in a postbiodiversity era. Bioscience 54, 455–459. [Google Scholar]
- Worsaae K (2001) The systematic significance of palp morphology in the Polydora complex (Polychaeta: Spionidae). Zool Anz 240, 47–59. [Google Scholar]
- Yokogawa K (2009) Changes of lengths of body proportions and body weight by fixation with 10% formalin in largemouth bass, Micropterus salmoides, and bluegill, Lepomis macrochirus . Biogeography 11, 151–161. [Google Scholar]


