Abstract
Contralateral C7 (cC7) root transfer to the healthy side is the main method for the treatment of brachial plexus root injury. A relatively new modification of this method involves cC7 root transfer to the lower trunk via the prespinal route. In the current study, we examined the effectiveness of this method using electrophysiological and histological analyses. To this end, we used a rat model of total brachial plexus injury, and cC7 root transfer was performed to either the lower trunk via the prespinal route or the median nerve via a subcutaneous tunnel to repair the injury. At 4, 8 and 12 weeks, the grasping test was used to measure the changes in grasp strength of the injured forepaw. Electrophysiological changes were examined in the flexor digitorum superficialis muscle. The change in the wet weight of the forearm flexor was also measured. Atrophy of the flexor digitorum superficialis muscle was assessed by hematoxylin-eosin staining. Toluidine blue staining was used to count the number of myelinated nerve fibers in the injured nerves. Compared with the traditional method, cC7 root transfer to the lower trunk via the prespinal route increased grasp strength of the injured forepaw, increased the compound muscle action potential maximum amplitude, shortened latency, substantially restored tetanic contraction of the forearm flexor muscles, increased the wet weight of the muscle, reduced atrophy of the flexor digitorum superficialis muscle, and increased the number of myelinated nerve fibers. These findings demonstrate that for finger flexion functional recovery in rats with total brachial plexus injury, transfer of the cC7 root to the lower trunk via the prespinal route is more effective than transfer to the median nerve via subcutaneous tunnel.
Keywords: nerve regeneration, total brachial plexus injury, contralateral C7 root, nerve transfer, lower trunk, median nerve, neural regeneration
Introduction
For patients with total brachial plexus injuries, the restoration of hand function remains the main weakness of surgical reconstruction. Contralateral C7 (cC7) root transfer to the median nerve, bridged by a vascularized or non-vascularized ulnar nerve graft, is one of the few effective methods for restoration of wrist and finger flexion and hand sensation (Gu et al., 1992, 1998, 2002; Giuffre et al., 2010; Chuang and Hernon, 2012; Sammer et al., 2012). However, function cannot be fully restored after this type of operation. The unsatisfactory outcome might be mainly attributed to the long distance the regenerated axons must extend along the ulnar nerve graft and the long denervation period at the target muscles because of the slow reinnervation speed (Waikakul et al., 1999). In addition, the two neurorrhaphy sites that must be passed by the regenerating axons also hamper the effectiveness of the procedure (Wang et al., 2013).
Wang et al. (2007) invented a surgical procedure involving transfer of the cC7 root to the lower trunk via the prespinal route by direct neurorrhaphy to reconstruct total brachial plexus injuries, and they reported good clinical outcome (i.e. finger and wrist flexion). In a long-term retrospective study of this procedure, Wang et al. (2013) reported satisfactory functional recovery of finger and wrist flexion. In 2014, our team succeeded in establishing a rat model of this procedure by Wang and colleagues, which allowed us to demonstrate efficacy by electrophysiological, histological and functional analyses (Wang et al., 2014). In the present study, we performed cC7 root transfer to the lower trunk via the prespinal route and compared functional outcome with that of cC7 transfer to the median nerve, bridged by vascularized ulnar nerve graft via subcutaneous tunnel, for the treatment of total brachial plexus injury in rats.
Materials and Methods
Animals
A total of 90 adult male specific-pathogen-free Sprague- Dawley rats, aged 7 weeks and weighing approximately 300 g, were obtained from the Experiment Animal Center of Fudan University of China (license number: SYXK 2014-0029). The experiments were performed in accordance with the principles of the Laboratory Animal Care and Use Guidelines issued by the Animal Research Committee of Shanghai Medical College, Fudan University. The rats were fed in a light- and temperature-controlled room. The experimental protocol was approved by the Animal Ethics Committee of Fudan University (20120521A091).
The rats were equally and randomly assigned to three groups according to surgical method. In group A, the cC7 root was transferred to the lower trunk via the prespinal route. In group B, the cC7 root was transferred to the median nerve via subcutaneous tunnel. The rats in group C received a sham operation and served as controls.
Surgical procedures
The rats were intraperitoneally anesthetized with 10% chloral hydrate (3 mL/100 g body weight). Under sterile conditions, the left side was subjected to total brachial plexus injury, and the right side was kept intact. The left brachial plexus roots were completely dissected through a supraclavicular incision and severed at the intervertebral foramen to create the total brachial plexus injury.
cC7 root transfer to the lower trunk
In group A, a supraclavicular transverse incision approximately 1 cm long was made to expose the contralateral C5 to T1. The C7 nerve root was then separated and cut off at the division-to-cord level following 2% lidocaine blocking. The prespinal route was made along the anterior vertebral body by bluntly dissecting the sternocleidomastoid muscle and carotid sheath along their medial margin and severing the right anterior scalene muscle. The cC7 root was then gently passed through the tunnel to the injured side. The coaptation was made utilizing end-to-end neurorrhaphy with 11/0 prolene under 10× magnification (Wang et al., 2014) (Figure 1A).
Figure 1.
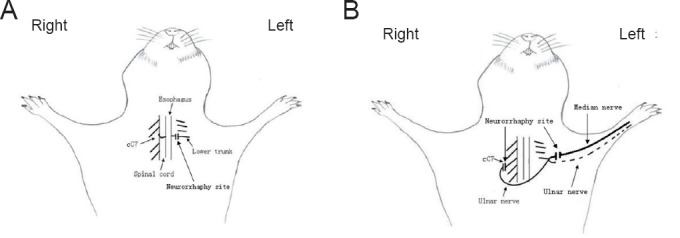
Schematic diagrams of contralateral C7 (cC7) root transfer to the lower trunk vs. the median nerve for the repair of total brachial plexus injury.
(A) Modified cC7 root transfer to the lower trunk: the C7 nerve root was separated and cut off at the division-to-cord level. The prespinal route was made along the anterior vertebral body by bluntly dissecting the sternocleidomastoid muscle and carotid sheath along their medial margin and severing the right anterior scalene muscle. The cC7 root was passed through the tunnel to the injured side. The coaptation was made utilizing end-to-end neurorrhaphy. (B) Traditional cC7 root transfer to the median nerve: the left pedicular vascularized ulnar nerve grafts were dissected, and the distal end was severed at the wrist level, moved to the contralateral side through the ventral thoracic subcutaneous tunnel and connected to the proximal cC7 root. Four weeks later, the ulnar nerve graft and median nerve were dissected and cut off from an incision at the middle of the injured arm. The distal stump of the ulnar nerve graft was coapted to the distal end of the median nerve with end-to-end neurorrhaphy.
cC7 root transfer to the median nerve
In group B, the cC7 roots were harvested through the same surgical procedure as described above. The left pedicular vascularized ulnar nerve grafts were dissected, and the distal end was severed at the wrist level, moved to the contralateral side through the ventral thoracic subcutaneous tunnel and connected to the proximal cC7 root. Four weeks later, the ulnar nerve graft and median nerve were dissected and cut off from an incision at the middle of the injured arm. At 10× magnification, the distal stump of the ulnar nerve graft was coapted to the distal end of the median nerve with end-to-end neurorrhaphy. Two stitches were made for each tension-free neurorrhaphy site (Figure 1B).
After surgery, the rats were evaluated every 4 weeks using 10 rats from each group per time point. The ambient temperature during the evaluations was maintained at 24°C. The exposed nerve and muscle were kept moist with warm saline during the relevant tests.
Grasping test
An iron frame was placed on the electronic balance (KERN Company, Balingen, Germany), and its weight was recorded. The rat's tail was held, and the injured forepaw was guided to grasp the iron frame with the healthy forepaw fixed with a rope. When the injured forepaw was loosened from the iron frame, the reading on the electronic balance was recorded. The grip strength was calculated as the change before and after the loosening of the injured forepaw.
Electrophysiologic testing
The rats were anesthetized following the grasping test. A Neuromatic 2000M electrophysiological apparatus (Dantec, Les Ulis, France) was used to test the compound muscle action potential of the flexor digitorum superficialis muscle. An electric stimulus (2.0 mA supramaximal current, 0.2 ms pulse width and 1 Hz frequency) was applied to the cC7 root by a stimulating electrode, and then the maximum amplitude and latency of the compound muscle action potential were obtained from the flexor digitorum superficialis muscle by inserting a concentric needle recording electrode.
The RM6240BD multichannel physiology signal collection system and JZJ101 force displacement transducer (Chengdu Instruments, Chengdu, China) were used to measure the force of the forearm flexor muscle's tetanic contraction. The forearm flexor muscles were dissected, and the distal tendinous portions were cut at the level of the proximal transverse carpal ligament. The distal end was then fixed on the force displacement transducer by a silk suture. The peak amplitudes of tetanic contraction were recorded using continuous single stimulation (2.0 V amplitude, 1 ms duration and 20 Hz frequency) by placing two hooked AgCl stimulating electrodes on the muscle surface. The recovery rate of the peak amplitudes was calculated by comparing them with the control group.
Muscle wet weight
Subsequently, the forearm flexor muscles were cut off from the humeral medial epicondyle. The tendinous portions and connective tissue on the muscle surface were fully removed. Immediately, the muscle wet weight was recorded with an electronic scale (R200D, 0.0001 precision; KERN Company). The muscle wet weight in the experimental groups was expressed as a percentage of that in the control group.
Muscle cross-sectional area measurements
After the muscle weights were measured, 10% formalin was used to fix the muscles. The muscles were dehydrated in a graded alcohol series and embedded in olefin. The middle of each muscle was cut into 5-mm-thick sections and stained with hematoxylin and eosin. Five random images were captured from each section at 200× magnification. The average cross-sectional area of the flexor digitorum superficialis muscle was measured and calculated with digital image analysis software (Leica-Qwin, Heidelberg, Germany).
Myelinated nerve fiber counts
A 1-mm median nerve segment was harvested at the site 1 cm proximal to the elbow joint and then fixed in 2.5% glutaraldehyde for 72 hours. Subsequently, the specimen was dehydrated with a graded alcohol series and embedded in Epon. Cross sections (0.5 μm thickness) were cut and stained with 5% toluidine blue, and observed on a microscope (DWLB2; Leica, Heidelberg, Germany) at 400× magnification. Analysis software (FW2000; Leica) was used to measure the area of the field of view, to count myelinated nerve fibers, and calculate mean myelinated nerve fiber density.
Statistical analysis
Data are expressed as the mean ± SD, and were analyzed with SPSS 11.5 software (SPSS, Chicago, IL, USA). Student's t-test was used to compare the two experimental groups. P-values less than 0.05 were regarded statistically significant.
Results
All the rats survived and were infection-free until they were killed for the experiments.
Grasp strength of the injured forepaw in the rat model of total brachial plexus injury after cC7 root transfer to the lower trunk or the median nerve
The grasping test was performed to assess function of the injured forepaw at intervals of 8 weeks postoperatively in groups A and B. The grasp strength was significantly greater in group A than in group B (P < 0.05), although it was not restored to the normal level (Table 1).
Table 1.
Grasp strength (g) of the injured forepaw in the rat model of total brachial plexus injury with cC7 root transfer to the lower trunk or the median nerve
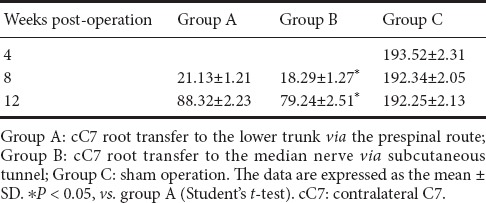
Electrophysiological characteristics of the flexor digitorum superficialis muscle in the rat model of total brachial plexus injury given cC7 root transfer to the lower trunk or median nerve
The compound muscle action potential maximum amplitude and latency for the flexor digitorum superficialis muscle at 4, 8 and 12 weeks postoperatively are shown in Table 2. Over time, the compound muscle action potential maximum amplitude increased, and the latency decreased, in both groups A and B, although at 12 weeks postoperatively, they had not reached the normal levels observed in group C (P < 0.05). No significant difference was observed between groups A and B (P > 0.05).
Table 2.
Compound muscle action potential maximum amplitude (mV) and latency (ms) of the flexor digitorum superficialis muscle in the rat model of total brachial plexus injury with cC7 root transfer to the lower trunk or median nerve
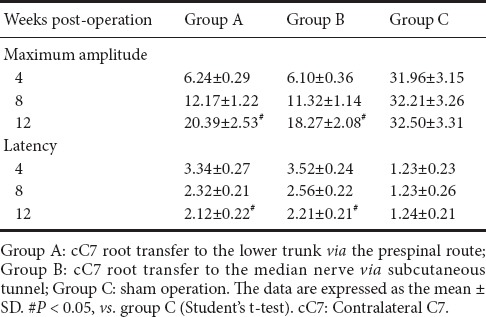
Tetanic contraction of the forearm flexor muscles in the rat model of total brachial plexus injury given cC7 root transfer to the lower trunk or the median nerve
The peak amplitudes of the tetanic contraction of the forearm flexor muscles at each time point postoperatively are shown in Table 3. The muscle tension in both groups A and B began to recover after 4 weeks postoperatively, and no significant difference was seen between groups A and B (P > 0.05). At 8 and 12 weeks postoperatively, the peak amplitudes of tetanic contraction were significantly greater in group A than in group B (P < 0.05). Although muscle function did not recover to the normal level (P < 0.05, vs. Group C), it tended to improve in both experimental groups.
Table 3.
The peak amplitudes (% of control) of tetanic contraction of the forearm flexor muscles in the rat model of total brachial plexus injury with cC7 root transfer to the lower trunk or median nerve
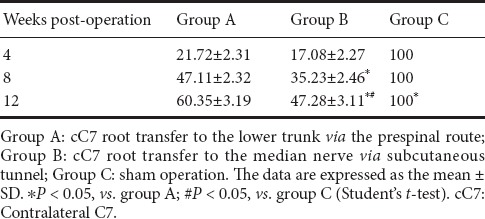
Forearm flexor muscle wet weight in the rat model of total brachial plexus injury following cC7 root transfer to the lower trunk or the median nerve
The forearm flexor muscle wet weights are shown in Table 4. The flexor muscle wet weights in groups A and B increased postoperatively (P < 0.05). At 4, 8 and 12 weeks following surgery, the weights were significantly greater in group A than in group B (P < 0.05).
Table 4.
The wet weight of the forearm flexor muscles (expressed as % of the control group) in the rat model of total brachial plexus injury with cC7 root transfer to the lower trunk or median nerve
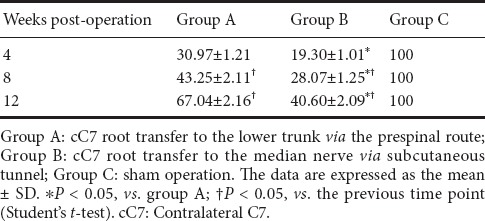
Atrophy of the flexor digitorum superficialis muscle in the rat model of total brachial plexus injury given cC7 root transfer to the lower trunk or the median nerve
Hematoxylin-eosin staining showed significant atrophy of the flexor digitorum superficialis muscle on the injured side in groups A and B, 4 weeks after surgery (Figure 2). The muscle cross-sectional area recovered with time, as shown in Table 5. The flexor digitorum superficialis muscle cross-sectional area was marginally greater in group B than in group A at each time point postoperatively, but the differences were not statistically significant (P > 0.05).
Figure 2.
Morphology of the flexor digitorum superficialis muscle in the rat model of total brachial plexus injury with cC7 root transfer to the lower trunk or the median nerve (hematoxylin-eosin staining, 200× magnification)
Group A: cC7 root transfer to the lower trunk via the prespinal route; Group B: cC7 root transfer to the median nerve via subcutaneous tunnel; Group C: sham operation. Significant atrophy of the flexor digitorum superficialis muscle was seen on the injured side in groups A and B at 4, 8 and 12 weeks after the surgery. cC7: Contralateral C7.
Table 5.
Cross-sectional area (μm2) of the flexor digitorum superficialis muscle in the rat model of total brachial plexus injury with cC7 root transfer to the lower trunk or median nerve
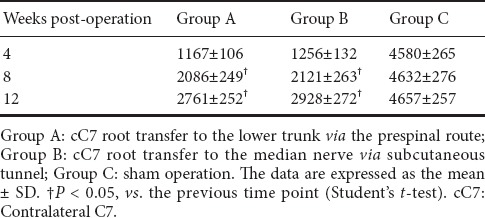
Myelinated nerve fiber counts in rats with total brachial plexus injury after cC7 root transfer to the lower trunk or the median nerve
The total number of myelinated fibers in the median nerve in groups A and B increased with time postoperatively, as detected by toluidine blue staining (Figure 3 and Table 6). No significant difference was seen between groups A and B at any of the three time points (P > 0.05), although axons were slightly more numerous in group B than in group A.
Figure 3.
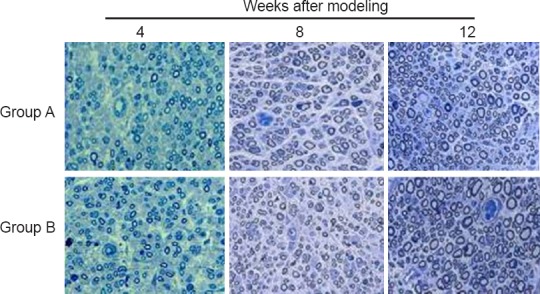
Neuromorphology of the median nerve in the rat model of total brachial plexus injury with cC7 root transfer to the lower trunk or the median nerve (toluidine blue staining, 400× magnification)
Group A: cC7 root transfer to the lower trunk via the prespinal route; Group B: cC7 root transfer to the median nerve via subcutaneous tunnel; Group C: sham operation. The total number of myelinated fibers in the median nerve in both groups A and B increased with time postoperatively. cC7: Contralateral C7.
Table 6.
Total number of myelinated nerve fibers in the median nerve in the rat model of total brachial plexus injury with cC7 root transfer to the lower trunk or median nerve
Discussion
Total brachial plexus injury is very difficult to treat, and the prognosis is generally unfavorable. Various neurotizations of the brachial plexus by extraplexal donor nerves, including the cervical plexus, spinal accessory nerve, phrenic nerve, intercostal nerves and the cC7 root, have been used to restore function of the injured upper extremity (Brunelli and Monini, 1984; Gu and Ma, 1996; Songcharoen et al., 1996; Nagano, 2001; Midha, 2004; Lu et al., 2012; Bertelli and Ghizoni, 2013). The clinical efficacy of extraplexal nerve transfer for restoring shoulder and elbow function has currently improved (Narakas, 1981; Chuang, 1995; Chuang et al., 1995; Tu et al., 2014). Before the development of cC7 transfer, hand function was very difficult to restore by other extraplexal neurotizations. The main reasons include the long distance between the neurotization site and the target muscle, and the inadequate number of myelinated donor nerve fibers. The cC7 root can serve as an ideal potential donor nerve for neurotization, because it contains abundant (~40,000) nerve fibers (Bentolila et al., 1999), and its function can be compensated by other nerves (Lin et al., 2013). However, the recovery of finger flexion strength has hitherto been unsatisfactory after cC7 transfer to the median nerve (bridged by vascularized ulnar nerve graft) for the treatment of total brachial plexus avulsion injury (Waikakul et al., 1999; Songcharoen et al., 2001; Gu et al., 2002; Wood and Murray, 2007; Giuffre et al., 2010). The shortcomings of the surgical technique might be caused by the long distance the regenerating axons must extend, the long reinnervation period at the target muscles, and the two neurorrhaphy sites. Accordingly, numerous research groups have focused on these hurdles to improve the procedure. Wang et al. (2007, 2013) reported a modified surgical procedure involving cC7 root transfer to the injured lower trunk via the prespinal route. This method shortens the distance of nerve regeneration and reduces the reinnervation period of the target muscles, with direct coaptation without requiring a nerve graft. The follow-up outcomes demonstrated that 73.4% (47/64) of patients regained M4 strength in the flexor carpi ulnaris, and 64.1% (41/64) of patients regained it in the flexor digitorum superficialis. This modified technique therefore appears to provide better outcome (restoration of finger flexion) compared with previous techniques. However, Wang's report remains the only one to describe the modified cC7 transfer technique, and there is no other study on efficacy. Therefore, our team established the rat model of cC7 root transfer to the lower trunk for the repair of total brachial plexus injuries, with the aim of facilitating further comparative study (Wang et al., 2014).
In the present study, we used our rat model to evaluate the treatment results and help validate the modified technique. Better functional recovery of forepaw function was observed in the group given cC7 root transfer to the lower trunk compared with the group given cC7 root transfer to the median nerve, at 8 and 12 weeks postoperatively, as evaluated by measuring grasp strength, tetanic contraction amplitude and wet muscle weight. The short regeneration distance and the presence of only one neurorrhaphy site (without the need for nerve graft) likely contribute to the better outcome of this procedure. However, no significant difference was found between the two groups by electrophysiological and histological study of the flexor digitorum superficialis muscle or by counting myelinated axons in the nerve. In group B, only the median nerve was repaired by cC7 transfer, and the ulnar nerve was used for grafting. In contrast, the median nerve and ulnar nerve were both repaired simultaneously by cC7 transfer to the lower trunk in group A. Therefore, all motor output from the cC7 was neurotized to the median nerve in group B, while only a portion of regenerated axons were neurotized to the median nerve in group A. This difference might account for why the quantity of myelinated axons in the median nerve was slightly greater in group B than in group A (although not statistically significant). Our findings suggest that the recovery of finger and wrist flexion by cC7 transfer to the lower trunk (in the present study and in our previous study (Wang et al., 2014)) is associated with reinnervation of the muscles dominated by the ulnar nerve.
The surgical procedure of cC7 transfer to the lower trunk provides the possibility of reinnervation of the intrinsic hand muscle, because of ulnar nerve neurotization, as long as muscle atrophy can be slowed down or prevented. Moreover, the prespinal route is theoretically the shortest one for functional restoration. It is worth noting that the transhemispheric functional reorganization of the cortex following cC7 transfer is another important factor that influences functional recovery (Jiang et al., 2010), although the underlying mechanisms are unclear. The main limitation is the complexity of both surgical techniques, although all the operations were skillfully performed by one researcher in the present study.
In summary, cC7 root transfer to the lower trunk via the prespinal route for finger flexion restoration in rats with total brachial plexus injury is more effective than the traditional method (cC7 transfer to the median nerve bridged by vascularized ulnar nerve graft via subcutaneous tunnel). Therefore, it can serve as an alternative method for the treatment of total brachial plexus injuries.
Additional file: Open peer review reports 1 (40.8KB, pdf) and 2 (40.8KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81572127. The funding body played no role in the study design, collection, analysis and interpretation of data, in the writing of the paper, or in the decision to submit the paper for publication.
Institutional review board statement: The experiments were approved by the Animal Ethics Committee of Fudan University of China (approval number: 20120521A091).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Ming-Chao Huang, Taipei Veterans General Hospital, China; Wengbo Yang, Nanjing First People's Hospital Affiliated to Nanjing Medical University, China.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81572127.
(Copyedited by Patel B, Hindle A, Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Bentolila V, Nizard R, Bizot P, Sedel L. Complete traumatic brachial plexus palsy. Treatment and outcome after repair. J Bone Joint Surg Am. 1999;81:20–28. doi: 10.2106/00004623-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Bertelli JA, Ghizoni MF. Transfer of a flexor digitorum superficialis motor branch for wrist extension reconstruction in C5-C8 root injuries of the brachial plexus: a case series. Microsurgery. 2013;33:39–42. doi: 10.1002/micr.22027. [DOI] [PubMed] [Google Scholar]
- 3.Brunelli G, Monini L. Neurotization of avulsed roots of brachial plexus by means of anterior nerves of cervical plexus. Clin Plast Surg. 1984;11:149–152. [PubMed] [Google Scholar]
- 4.Chuang DC. Neurotization procedures for brachial plexus injuries. Hand Clin. 1995;11:633–645. [PubMed] [Google Scholar]
- 5.Chuang DC, Hernon C. Minimum 4-year follow-up on contralateral C7 nerve transfers for brachial plexus injuries. J Hand Surg Am. 2012;37:270–276. doi: 10.1016/j.jhsa.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Chuang DC, Lee GW, Hashem F, Wei FC. Restoration of shoulder abduction by nerve transfer in avulsed brachial plexus injury: evaluation of 99 patients with various nerve transfers. Plast Reconstr Surg. 1995;96:122–128. doi: 10.1097/00006534-199507000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Giuffre JL, Kakar S, Bishop AT, Spinner RJ, Shin AY. Current concepts of the treatment of adult brachial plexus injuries. J Hand Surg Am. 2010;35:678–688. doi: 10.1016/j.jhsa.2010.01.021. quiz 688. [DOI] [PubMed] [Google Scholar]
- 8.Gu Y, Xu J, Chen L, Wang H, Hu S. Long term outcome of contralateral C7 transfer: a report of 32 cases. Chin Med J (Engl) 2002;115:866–868. [PubMed] [Google Scholar]
- 9.Gu YD, Ma MK. Use of the phrenic nerve for brachial plexus reconstruction. Clin Orthop Relat Res. 1996:119–121. doi: 10.1097/00003086-199602000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Gu YD, Zhang GM, Chen DS, Yan JG, Cheng XM, Chen L. Seventh cervical nerve root transfer from the contralateral healthy side for treatment of brachial plexus root avulsion. J Hand Surg Br. 1992;17:518–521. doi: 10.1016/s0266-7681(05)80235-9. [DOI] [PubMed] [Google Scholar]
- 11.Gu YD, Chen DS, Zhang GM, Cheng XM, Xu JG, Zhang LY, Cai PQ, Chen L. Long-term functional results of contralateral C7 transfer. J Reconstr Microsurg. 1998;14:57–59. doi: 10.1055/s-2007-1006902. [DOI] [PubMed] [Google Scholar]
- 12.Jiang S, Li ZY, Hua XY, Xu WD, Xu JG, Gu YD. Reorganization in motor cortex after brachial plexus avulsion injury and repair with the contralateral C7 root transfer in rats. Microsurgery. 2010;30:314–320. doi: 10.1002/micr.20747. [DOI] [PubMed] [Google Scholar]
- 13.Lin H, Sheng J, Hou C. The effectiveness of contralateral C7 nerve root transfer for the repair of avulsed C7 nerve root in total brachial plexus injury: an experimental study in rats. J Reconstr Microsurg. 2013;29:325–330. doi: 10.1055/s-0033-1343498. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Xu J, Xu W, Xu L, Fang Y, Chen L, Gu Y. Combined nerve transfers for repair of the upper brachial plexus injuries through a posterior approach. Microsurgery. 2012;32:111–117. doi: 10.1002/micr.20962. [DOI] [PubMed] [Google Scholar]
- 15.Midha R. Nerve transfers for severe brachial plexus injuries: a review. Neurosurg Focus. 2004;16:E5. doi: 10.3171/foc.2004.16.5.6. [DOI] [PubMed] [Google Scholar]
- 16.Nagano A. Intercostal nerve transfer for elbow flexion. Tech Hand Up Extrem Surg. 2001;5:136–140. doi: 10.1097/00130911-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Narakas A. Brachial plexus surgery. Orthop Clin North Am. 1981;12:303–323. [PubMed] [Google Scholar]
- 18.Sammer DM, Kircher MF, Bishop AT, Spinner RJ, Shin AY. Hemi-contralateral C7 transfer in traumatic brachial plexus injuries: outcomes and complications. J Bone Joint Surg Am. 2012;94:131–137. doi: 10.2106/JBJS.J.01075. [DOI] [PubMed] [Google Scholar]
- 19.Songcharoen P, Mahaisavariya B, Chotigavanich C. Spinal accessory neurotization for restoration of elbow flexion in avulsion injuries of the brachial plexus. J Hand Surg Am. 1996;21:387–390. doi: 10.1016/S0363-5023(96)80349-2. [DOI] [PubMed] [Google Scholar]
- 20.Songcharoen P, Wongtrakul S, Mahaisavariya B, Spinner RJ. Hemi-contralateral C7 transfer to median nerve in the treatment of root avulsion brachial plexus injury. J Hand Surg Am. 2001;26:1058–1064. doi: 10.1053/jhsu.2001.27764. [DOI] [PubMed] [Google Scholar]
- 21.Tu YK, Tsai YJ, Chang CH, Su FC, Hsiao CK, Tan JS. Surgical treatment for total root avulsion type brachial plexus injuries by neurotization: a prospective comparison study between total and hemicontralateral C7 nerve root transfer. Microsurgery. 2014;34:91–101. doi: 10.1002/micr.22148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waikakul S, Orapin S, Vanadurongwan V. Clinical results of contralateral C7 root neurotization to the median nerve in brachial plexus injuries with total root avulsions. J Hand Surg Br. 1999;24:556–560. doi: 10.1054/jhsb.1999.0264. [DOI] [PubMed] [Google Scholar]
- 23.Wang L, Jiang Y, Lao J, Zhao X. Contralateral C7 transfer to lower trunk via the prespinal route in the repair of brachial plexus injury: an experimental study in rats. J Plast Reconstr Aesthet Surg. 2014;67:1282–1287. doi: 10.1016/j.bjps.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 24.Wang SF, Li PC, Xue YH, Yiu HW, Li YC, Wang HH. Contralateral C7 nerve transfer with direct coaptation to restore lower trunk function after traumatic brachial plexus avulsion. J Bone Joint Surg Am. 2013;95:821–827. doi: 10.2106/JBJS.L.00039. S1-2. [DOI] [PubMed] [Google Scholar]
- 25.Wang SF, Li PC, Chu Y, Lu J, Li YC, Pan YW, Xue YH, Hu Q, Tian GL. The primary clinical study of direct anastomosis of contralateral C7 transfered through the prespinal route with lower trunk in patient with brachial plexus root avulsion. Zhonghua Guke Zazhi. 2007;27:438–441. [Google Scholar]
- 26.Wood MB, Murray PM. Heterotopic nerve transfers: recent trends with expanding indication. J Hand Surg Am. 2007;32:397–408. doi: 10.1016/j.jhsa.2006.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


