Signals of touch, pressure, pain, temperature, position, and vibration are transmitted from the peripheral nervous system (PNS) to the dorsal horn of the segmental spinal cord via pseudounipolar dorsal root ganglia (DRG) neurons. Sensory information gathering relies on functional integrity of DRG neurons and can be interrupted by PNS injury due to trauma, disease or exposure to drugs, toxins or viral pathogens. Despite the high regenerative capacity of DRG neurons, sensory recovery after PNS injury is often incomplete and severe neuropathic pain may last for years. Although numerous mechanisms of PNS injury have been established, neuropathic pain is refractory to therapy, contributing to the physical and emotional suffering of patients and the enormous economic burden to society.
Among thousands of genes with known pro- and anti-inflammatory, -nociceptive, -survival and -regenerative functions are regulated in response to PNS injury, tissue inhibitor of metalloproteases (TIMP)-1 is one of the top-induced genes in the damaged nerve (Kim et al., 2012), yet its functions in PNS injury, repair or pain remain largely obscure. Herein, we discuss the known and probable roles of TIMPs in the damaged PNS.
TIMPs: The TIMP family of four multifunctional proteins (TIMP-1, -2, -3 and -4) are potent inhibitors of the extracellular matrix (ECM) proteases of the matrix metalloproteinase (MMP), a disintegrin and metalloproteinase (ADAM) and ADAM with thrombospondin motifs (ADAMTS) families (Brew and Nagase, 2010). TIMPs also regulate signal transduction through direct binding to cell-surface receptors (Stetler-Stevenson, 2008; Brew and Nagase, 2010). As a result, TIMPs play a critical role in regulating ECM remodeling and cellular activity in normal and pathological conditions. All four TIMPs are expressed in the damaged PNS (Chernov et al., 2015). According to our genome-wide transcriptional profiling, TIMP-1 is the top 6th gene induced in the murine model of sciatic nerve axotomy (Kim et al., 2012). In both cultured and in vivo DRG, TIMP-1 is expressed among regeneration-associated genes as a component of the cyclic adenosine monophosphate (cAMP) second messenger regenerative program (Liu et al., 2015). However, it is presently unknown whether or not TIMP-1 directly exhibits pro-regenerative and pro-survival functions in sensory neurons.
The MMP:TIMP ratio determines the net proteolytic activity of active MMPs and, consequently, the level of cleavage of the respective MMP target proteins. The N-terminal domain of TIMPs binds to the MMP active site with a sub-nanomolar affinity and in 1:1 ratio, inactivating the protease (Brew and Nagase, 2010). As a result, TIMPs control pericellular proteolysis and cell signaling, adhesion, migration, survival and proliferation, as well as the release of cytokines, chemokines, trophic factors and biologically active fragments. In addition, TIMP-1 through its C-terminal domain forms a 1:1 stoichiometric complex with the MMP-9 latent zymogen, making the enzyme resistant to activation, while TIMP-2 forms a similar complex with the MMP-2 proenzyme. TIMPs also exhibit non-proteolytic functions and bind to integrins and cell receptors [reviewed in Stetler-Stevenson (2008)]. For example, TIMP-1 binds to CD63 to regulate the Wnt and PI3K/Akt signaling pathways.
Neurovascular permeability and immune cell function: Neurovascular barriers protect peripheral nerve, albeit not DRG. An array of immune cells, including neutrophils, mast cells, monocytes and lymphocytes, infiltrate the injured PNS. Since first proposed by Zena Werb and colleagues, TIMPs are expected to control immune cell infiltration into the nerve across neurovascular barriers by inhibition of MMP proteolysis of the basement membrane proteins, such as collagen IV. In a series of subsequent studies (Kim et al., 2012; Nishihara et al., 2015; Hong et al., 2017; Remacle et al., 2018), we have shown that TIMP-2, MMP-2 and MMP-14 are constitutively expressed in nerve and induced post-injury at the blood-nerve barrier and perineurial barrier (deposited by fibroblasts). MMP-9, expressed in the PNS only post-injury, promotes endoneurial recruitment of immune cells, including macrophages and lymphocytes. Both MMP-9 and TIMP-1 are produced by immune, endothelial and Schwann cells in the PNS. On the nerve-infiltrating macrophages, MMP-14 regulates proteolysis of the cell surface chondroitin sulfate proteoglycan 4/nerve/glial-antigen 2, although the functional role of this event remains elusive. In addition to the general control of the catalytic MMP activity, TIMPs are expected to control neurovascular permeability, immune and endothelial cell function via the MMP-independent, e.g., vascular endothelial growth factor (Stetler-Stevenson, 2008), signaling.
Neuronal survival: MMPs and TIMPs exhibit, respectively, pro-apoptotic and pro-survival activities in neurons. It is therefore reasonable to propose that the TIMP:MMP imbalance contributes to DRG neuronal loss after PNS injury. TIMP-1 has been repeatedly shown to protect neurons against oxygen-glucose deprivation injury and staurosporine, human immunodeficiency virus-1) and glutamate-induced apoptosis. TIMP-1 has been suggested to inhibit neuron death by inhibiting pro-apoptotic MMP-9 proteolysis of laminin and attenuating the glutamate-evoked intracellular calcium flux. In the DRG, TIMPs are produced by cAMP-dependent transcription factor-positive neurons and their expression is under the pro-regenerative cAMP control (Liu et al., 2015). The regenerative and neuroprotective activity, however, may be specific for some rather than all TIMPs, as TIMP-3 is known to stimulate Fas-mediated cell death by preventing FasL shedding and stabilization of the Fas receptor.
Axonal growth: The high regenerative capacity of the PNS is linked to the function of its unique structure, Schwann cell basal lamina tube, a natural conduit rich in growth-permissive ECM. The individual roles of MMPs and TIMPs in axonal growth are complex, diverse and incompletely understood. This area of research remains contentious in part due to the diverse MMP substrates ECM, the incompletely understood biological activities of the fragmented vs. the full-length individual proteins, and catalytic vs. non-catalytic activities of MMPs. The works by David Muir and colleagues have shown that by degradation and inactivation of growth-inhibitory CSPG, MMP-2, albeit not MMP-9, promotes DRG axonal growth in cell culture or nerve cryoculture. The team of Jerry Silver implicated MMP-9 in inhibition of DRG axonal growth by forging macrophage attack of the dystrophic axons, an action reversed by MMP inhibition. MMP inhibition also stimulates DRG neurite outgrowth by preventing the release of growth-inhibitory myelin associated glycoprotein (MAG) fragments. Indirectly, MMP inhibition stimulates DRG axonal growth in vivo by stimulating Schwann cell mitosis (discussed below). But the effects of TIMPs on axonal growth is likely not solely MMP-dependent. In cortical neurons expressing low MMP-9, TIMP-1 increases the growth cone size, yet reduces the neurite length (Ould-yahoui et al., 2009). MMP inhibition, however, may inhibit the release of the mature nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) from their respective precursors. But since cAMP activity is critical to regeneration of DRG neurons, it is reasonable to suggest that the cAMP-induced TIMP-1 expression in DRG neurons (Liu et al., 2015) is a part of the pro-regenerative program.
Schwann cell signaling and myelination: Schwann cells provide the trophic, metabolic and physical support for neurons. Non-myelinating Schwann cells organize pain C-fibers into Remak bundles while myelinating Schwann cells insulate mechanosensory (A-afferent) and motor (efferent) axons by a multilamellar myelin sheath. PNS injury induces extensive phenotypic changes in Schwann cells; they de-differentiate, divide, migrate and align in Bungner's bands, re-differentiate, and regulate both axon regeneration and remyelination. Schwann cells also regulate myelin phagocytosis, antigen presentation and metabolic activity of axons. By activation of the stop-mitosis neuregulin 1 (NRG1)/ErbB signaling, MMP-9 inhibits Schwann cell mitosis and MMP-28 promotes myelination. MMP-2/MMP-9 inhibition and MMP-9 gene deletion increase the rate of Schwann cell mitosis and alter myelin domain organization (the length of internodes and Cajal bands and apposition size). MMP inhibition also activates JNK and p38 signaling in Schwann cells and inactivates insulin growth factor receptor-mediated ERK signal transduction. Although the role of TIMPs in PNS myelination remains elusive, myelinating Schwann cells overexpressing Raf/MEK stimulate TIMP-1 expression. In the CNS, the team of Stephen Crocker has shown that TIMP-1 promotes myelination by facilitating maturation of oligodendrocyte progenitor cells. The inability of the central TIMP-1-null axons to myelinate is not rescued by broad-range MMP inhibition, suggesting that the pro-myelinating TIMP-1 function is MMP-independent. Yet the N-terminal domain of TIMP-3 is responsible for its pro-myelinating function in DRG neurons (Kim et al., 2017). These observations imply that MMP-dependent and MMP-independent roles of TIMPs may exist in Schwann cell signaling and functions.
Central plasticity: PNS injury may produce adaptive or maladaptive CNS plasticity that determines the outcome of sensorimotor recovery. Spinal astrocytes and microglia have been implicated in analgesic activity of TIMPs (Kawasaki et al., 2008). The increase in spinal TIMP-1 after PNS injury may also relate to function of oligodendrocyte progenitor cells (Liu et al., 2015). While multiple known MMP substrates (ephrins, cadherins, tenascin, laminin, brain-derived neurotrophic factor, insulin growth factor and N-methyl-D-aspartate receptors) play an important role in synaptogenesis, synaptic plasticity and long-term potentiation in the CNS, the role of the MMP/TIMP axis in the molecular reorganization of the spinal cord after PNS injury remains largely unexplored.
Neuropathic pain: Intractable neuropathic pain can be provoked by an innocuous stimulus or occur spontaneously, without a definite stimulus. The intrathecal TIMP-1 and TIMP-2 therapy prevents the development and attenuates the established neuropathic pain, respectively, by blocking spinal glial activation (Kawasaki et al., 2008). This team has since shown that intrathecal injection of TIMP-1 potentiated and prolonged morphine analgesia. TIMPs are expected to inhibit the established pro-algesic roles of multiple MMPs (MMP-2, MMP-9, MMP-3, MMP-14/membrane-type 1-matrix metalloproteinase (MT1-MMP), MMP-25/membrane-type 5-matrix metalloproteinase (MT5-MMP)) and ADAMs by the proteolytic release of the soluble pro-inflammatory cytokines, such as tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β), and the pro-algesic fragments of myelin basic protein, fibronectin and tenascin, as well as by regulation of the less appreciated MMP substrates involved in nociception, including voltage-gated sodium channels (Remacle et al., 2015), N-methyl-D-aspartate receptors, NGF and BDNF. Whether TIMPs exhibit MMP-independent action in pain is yet to be elucidated.
Balancing act: A number of factors are expected to influence TIMP function in the PNS. At least 17 individual MMPs and 4 TIMPs are expressed in the damaged PNS in rats (Chernov et al., 2015), with distinct temporal and spatial distribution patterns. Thus, the individual TIMP:MMP and TIMP:proMMP ratios and the levels of target and binding proteins will determine whether the catalytic and non-catalytic, hemopexin domain-dependent, MMP activity, or MMP-independent, trophic TIMP activity take place in the course of PNS injury. For example, MMP-2 is localized in the blood-nerve and perineurial barriers of the normal and injured PNS, whereas MMP-9 is produced only post-injury, mainly by immune, endothelial and Schwann cells. The proMMP-9 and active MMP-9 levels dominate, respectively, the early (1 day) and the late (1 month) phases of PNS injury, whereas the level of the latent to active MMP-2 remains roughly constant (Remacle et al., 2018). Biological variables, including sex, age, species, strains, and other variables, such as extent of physical activity/exercise, may influence MMP or TIMP activity in the PNS. While the MMP patterns are comparable in mouse and rat (Kim et al., 2012; Chernov et al., 2015) female and male (Remacle et al., 2018) nerves, TIMP expression is species-specific. In rat nerves, TIMPs are constitutively high and are moderately induced post-injury (Chernov et al., 2015), and in mouse nerves, TIMP levels are constitutively low and robustly induced post-injury (Kim et al., 2012). Our ongoing findings suggest that TIMPs are regulated differentially in male and female PNS.
Significance: TIMPs are among the top-regulated genes after PNS injury, yet only several reports relate to their function in the PNS. As pan-inhibition of the catalytic MMP activity (by broad-spectrum hydroxamate inhibitors) at the early stage of PNS injury promotes regeneration, inhibits neuroinflammation, limits demyelination and delays significantly the development of neuropathic pain, it is tempting to speculate of TIMPs’ beneficial effects. However, negative impact of inhibiting beneficial MMP activities in nerve regrowth and remyelination as well as the trophic, MMP-independent TIMP functions in the PNS are yet to be elucidated. Stimulated as part of the cAMP-induced pro-regenerative program in DRG neurons (Liu et al., 2015), endogenous TIMP-1 likely promotes PNS regeneration. The exogenous TIMP therapy has a strong, albeit short-acting, analgesic effect after PNS injury, estimated as 1000 times more potent than that of opioids (Kawasaki et al., 2008). Given the opioid crisis and the enormous economic burden (~$600 billion annually in the US alone) of chronic pain conditions, investigation of the analgesic properties of TIMPs is a high-priority area of research (Figure 1).
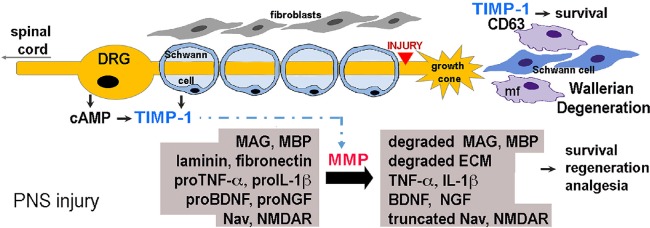
Figure 1.
Tissue inhibitors of metalloproteases (TIMPs) control peripheral nervous system (PNS) injury (a model diagram).
During PNS injury-induced Wallerian degeneration, fibroblasts, Schwann cells (SC) and immune cells (e.g., macrophages, mf) express up to 17 individual MMPs and 4 TIMPs (Chernov et al, 2015). In DRG neurons, TIMP-1 is induced by cAMP-regenerative program (Liu et al., 2015). TIMPs inhibit MMPs (e.g., TIMP-1 inhibits all MMPs, except MMP-14; TIMP-2 inhibits MMP-14 (Brew and Nagase, 2010)), promoting growth-permissive ECM (e.g., laminin, fibronectin) and preventing the formation of growth-inhibitory (e.g., myelin associated glycoprotein, MAG) and pro-algesic (e.g., myelin basic protein, MBP) fragments. TIMPs regulate cleavage (i.e., activation or inactivation) of other MMP substrates involved in cell survival, axonal regeneration and nociception, including cytokines [e.g., tumor necrosis factor (TNF)-alpha and interleukin (IL)-1-beta]; trophic factors [e.g., nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF)]; voltage-gated sodium (Nav) channels and N-methyl-D-aspartate receptor (NMDAR). In addition, TIMPs facilitate PNS repair via MMP-independent ability to regulate cell signaling; e.g., TIMP-1 stimulates cell survival by binding to the cell surface tetraspanin, CD63 [reviewed in Stetler-Stevenson (2008)].
Department of Veteran's Affairs Merit Review Award 5I01BX000638 (to VIS) and National Institutes of Health R01DE022757 (to VIS and AYS) supported this study.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chernov AV, Dolkas J, Hoang K, Angert M, Srikrishna G, Vogl T, Baranovskaya S, Strongin AY, Shubayev VI. The calcium-binding proteins S100A8 and S100A9 initiate the early inflammatory program in injured peripheral nerves. J Biol Chem. 2015;290:11771–11784. doi: 10.1074/jbc.M114.622316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong S, Remacle AG, Shiryaev SA, Choi W, Hullugundi SK, Dolkas J, Angert M, Nishihara T, Yaksh TL, Strongin AY, Shubayev VI. Reciprocal relationship between membrane type 1 matrix metalloproteinase and the algesic peptides of myelin basic protein contributes to chronic neuropathic pain. Brain Behav Immun. 2017;60:282–292. doi: 10.1016/j.bbi.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–336. doi: 10.1038/nm1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim J, Elias A, Lee T, Maurel P, Kim HA. Tissue inhibitor of metalloproteinase-3 promotes schwann cell myelination. ASN Neuro. 2017;9:1759091417745425. doi: 10.1177/1759091417745425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim Y, Remacle AG, Chernov AV, Liu H, Shubayev I, Lai C, Dolkas J, Shiryaev SA, Golubkov VS, Mizisin AP, Strongin AY, Shubayev VI. The MMP-9/TIMP-1 axis controls the status of differentiation and function of myelin-forming Schwann cells in nerve regeneration. PLoS One. 2012;7:e33664. doi: 10.1371/journal.pone.0033664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Angert M, Nishihara T, Shubayev I, Dolkas J, Shubayev VI. Spinal glia division contributes to conditioning lesion-induced axon regeneration into the injured spinal cord: potential role of cyclic amp-induced tissue inhibitor of metalloproteinase-1. J Neuropathol Exp Neurol. 2015;74:500–511. doi: 10.1097/NEN.0000000000000192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishihara T, Remacle AG, Angert M, Shubayev I, Shiryaev SA, Liu H, Dolkas J, Chernov AV, Strongin AY, Shubayev VI. Matrix metalloproteinase-14 both sheds cell surface neuronal glial antigen 2 (NG2) proteoglycan on macrophages and governs the response to peripheral nerve injury. J Biol Chem. 2015;290:3693–3707. doi: 10.1074/jbc.M114.603431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ould-yahoui A, Tremblay E, Sbai O, Ferhat L, Bernard A, Charrat E, Gueye Y, Lim NH, Brew K, Risso JJ, Dive V, Khrestchatisky M, Rivera S. A new role for TIMP-1 in modulating neurite outgrowth and morphology of cortical neurons. PLoS One. 2009;4:e8289. doi: 10.1371/journal.pone.0008289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remacle AG, Hullugundi SK, Dolkas J, Angert M, Chernov AV, Strongin AY, Shubayev VI. Acute- and late-phase matrix metalloproteinase (MMP)-9 activity is comparable in female and male rats after peripheral nerve injury. J Neuroinflammation. 2018;15:89. doi: 10.1186/s12974-018-1123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remacle AG, Kumar S, Motamedchaboki K, Cieplak P, Hullugundi S, Dolkas J, Shubayev VI, Strongin AY. Matrix metalloproteinase (MMP) proteolysis of the extracellular loop of voltage-gated sodium channels and potential alterations in pain signaling. J Biol Chem. 2015;290:22939–22944. doi: 10.1074/jbc.C115.671107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stetler-Stevenson WG. Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities. Sci Signal. 2008;1:re6. doi: 10.1126/scisignal.127re6. [DOI] [PMC free article] [PubMed] [Google Scholar]