Abstract
Endothelin-1 (ET-1), a potent vasoconstrictor, is involved in retinal vascular dysregulation and oxidative stress in glaucomatous eyes. Taurine (TAU), a naturally occurring free amino acid, is known for its neuroprotective and antioxidant properties. Hence, we evaluated its neuroprotective properties against ET-1 induced retinal and optic nerve damage. ET-1 was administered intravitreally to Sprague-Dawley rats and TAU was injected as pre-, co- or post-treatment. Animals were euthanized seven days post TAU injection. Retinae and optic nerve were examined for morphology, and were also processed for caspase-3 immunostaining. Retinal redox status was estimated by measuring retinal superoxide dismutase, catalase, glutathione, and malondialdehyde levels using enzyme-linked immuosorbent assay. Histopathological examination showed significantly improved retinal and optic nerve morphology in TAU-treated groups. Morphometric examination showed that TAU pre-treatment provided marked protection against ET-1 induced damage to retina and optic nerve. In accordance with the morphological observations, immunostaining for caspase showed a significantly lesser number of apoptotic retinal cells in the TAU pre-treatment group. The retinal oxidative stress was reduced in all TAU-treated groups, and particularly in the pre-treatment group. The findings suggest that treatment with TAU, particularly pre-treatment, prevents apoptosis of retinal cells induced by ET-1 and hence prevents the changes in the morphology of retina and optic nerve. The protective effect of TAU against ET-1 induced retinal and optic nerve damage is associated with reduced retinal oxidative stress.
Keywords: endothelin-1, retina, optic nerve, taurine, oxidative stress
Introduction
Retinal ganglion cells (RGCs) apoptosis, a characteristic feature of glaucomatous neuropathy, is often associated with elevated intraocular pressure (IOP). However, the IOP reduction does not arrest the disease progression in several patients (Agarwal et al., 2009). Hence, direct neuroprotective strategies are of significant importance.
Retinal ischemia has been widely implicated in glaucomatous RGC loss. According to the vascular theory, retinal and optic nerve damage in glaucoma occurs due to insufficient ocular blood flow (Flammer, 1994; Gramer et al., 2015). In fact, vasospasm was considered a specific risk factor for glaucoma independent of IOP levels (Broadway and Drance, 1998). Importantly, increased levels of endothelin-1 (ET-1), a potent vasoconstrictor, were detected in the plasma and ocular tissue of glaucoma patients (Emre et al., 2005; Iwabe et al., 2010). For this reason, intravitreal injection of ET-1 has been used to model glaucomatous changes in retina and optic nerve of animals (Agarwal and Agarwal, 2017). In the present study, we also used rat model of ET-1 induced retinal and optic nerve damage to investigate the neuroprotective effects of the amino acid taurine (TAU).
TAU is one of the amino acids not used for protein synthesis. As such, it is often labelled as a “non-essential” amino acid. TAU, however, has key functional significance in processes like cell development, nutrition and survival and hence undoubtedly is one of the most essential substances (Sturman and Gaull, 1975; Sturman and Hayes, 1980). Furthermore, it is the most abundant amino acid found in the ocular tissue and its highest levels were detected in vertebrate retina (Cohen et al., 1973; Heinämäki et al., 1986). High concentration of TAU throughout retinal tissue was suggested to provide neuroprotection (Ripps and Shen, 2012). TAU has been shown to prevent ethanol-induced Purkinje cell apoptosis in mice cerebellum (Taranukhin et al., 2010), and protect against ischemic brain injury in mice after middle cerebral artery occlusion (Zhang et al., 2017). TAU enhanced RGC survival in serum deprived conditions and in NMDA exposed rat retinal explants (Froger et al., 2012). The protective effects of TAU were also reported against secondary RGC degeneration in rat with retinitis pigmentosa and vein occlusion and in DBA/2J mice (Froger et al., 2012). Several potential targets have been proposed for the neuroprotective effects of TAU, such as the inhibition of calcium influx through voltage gated calcium channels; inhibition of glutamate induced excitotoxicity; restoration of the expression of anti- and pro-apoptotic proteins and its potent antioxidant properties (Sun and Xu, 2008). It is noteworthy that tissue hypoxia-induced reactive oxygen species accumulation is associated with optic nerve damage (Ko et al., 2005). Indeed, increased oxidative stress may also result from ischemia induced excitotoxicity (Lee et al., 2012). In our previous studies, a combined salt of magnesium and TAU, magnesium acetyltaurate (MgAT), provided neuroprotection against ET-1 and NMDA induced retinal and optic nerve damage (Arfuzir et al., 2016; Jafri et al., 2017; Lambuk et al., 2017). In this study, we investigated whether TAU alone reduces the ET-1 induced retinal oxidative stress and protects against retinal and optic nerve injury in rats.
Materials and Methods
Animals
All experimental procedures in this study followed the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The study was approved by The Committee on Animal Research and Ethics (UiTM Care: 216/6/2017 (6/10/2017)) and was done in compliance with the ethical guidelines of Universiti Teknology MARA. Ninety Sprague-Dawley (SD) rats of either sex, aged 6–8 weeks and weighing 200–250 g, were procured from the Laboratory Animal Care Unit, Faculty of Medicine, Universiti Teknologi MARA, Malaysia. The animals were kept under standard laboratory conditions with a 12-hour light/dark cycle, and free access to food and water. Healthy rats were included in this study after ophthalmic and general examination. ET-1 was purchased from Sigma Aldrich and TAU was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Study design
The rats were randomly divided into five groups with 18 animals each (n = 36 eyes). Groups 1 and 2 were intravitreally injected with phosphate buffer saline (0.1 M PBS, pH 7.4) and ET-1 2.5 nM, respectively. Group 3 received an intravitreal injection of 320 nM of TAU, 24 hour before injection of 2.5 nM ET-1 (TAU pre-treatment group). Rats in group 4 were injected with 2.5 nM ET-1 and 320 nM TAU simultaneously (TAU co-treatment group). Group 5 received an intravitreal injection of 320 nM of TAU, 24 hour after 2.5 nM ET-1 injection (TAU post-treatment group). The dose of ET-1 was selected based on the results of our previous studies and the dose of TAU used in this study was equimolar to the dose of MgAT used in the previous study (Arfuzir et al., 2016). Seven days after the intravitreal injection, animals were sacrificed with an intraperitoneal injection of pentobarbital (100 mg/kg). Bilateral enucleation was done and eyes were processed for morphological examination of retinal and optic nerve. Retinal sections were also subjected to immunohistochemical staining for caspase-3 expression. In addition, we processed the retinae for detection of the reduced glutathione (GSH) and melondialdehyde (MDA) contents, superoxide dismutase (SOD) and catalase (CAT) activity to estimate the retinal oxidative stress. For each retinal parameter, six eyes from six different animals were used. Six optic nerve sections were obtained from the six eyes that were subjected to histopathological examination. Before enucleation of eyes, a suture was inserted at the 12 O’clock position for proper orientation.
Intravitreal injections were given as described previously (Arfuzir et al., 2016; Jafri et al., 2017; Lambuk et al., 2017). Accordingly, intraperitoneal injection of sodium pentobarbital (65 mg/kg) was used to anaesthetize the rats. One drop of alcaine 0.5% was instilled on the ocular surface for local anaesthesia. A 30-gauge needle was used to puncture the sclera 1 mm behind the limbus, superionasally. Subsequently, a 10 µL Hamilton syringe was inserted through the puncture site and the injections were made using a Hamilton syringe. A total of 2 µL volume was used for all injections that were made slowly to avoid pressure induced retinal damage.
Histopathological examination of retina
Retinal sections were stained with hematoxylin and eosin (H&E) and morphology was studied under the light microscope. To obtain the retinal sections, firstly, the fixation of enucleated eyes was done in 10% formaldehyde for 24 hours. Then whole eyeballs were paraffin embedded and sectioning was done at 3 µm thickness. The sections were taken at 1 mm from the temporal edge of the optic disc and were H&E stained. A digital camera attached with the light microscope (NIS-Elements Basic Research, Nikon Instrument Inc.) was used to capture the images. Retinal morphological changes were quantified as described previously (Arfuzir et al., 2016; Mohd Lazaldin et al., 2018). Accordingly, in each section, three fields of view were randomly selected and were calibrated at 20× magnification. Images were saved in jpg format and quantification was done using image analysis software (ImageJ 1.31, National Institutes of Health, Bethesda, MD, USA). We estimated the fractional (%) thickness of the ganglion cell layer (GCL) within the inner retina (IR), linear cell density within the GCL, which refers to the number of retinal cells/100 μm length of GCL and retinal cell density within GCL and IR (number of nuclei per 100 µm2 of GCL and IR). The count of the cell nuclei included all types of neuronal cells. We did not differentiate between various types of neuronal and displaced amacrine cells. Cell nuclei with a diameter less than 7 µm were excluded. Furthermore, only cells that were identified to have a definite neuronal morphology (with light nuclei containing decondensed chromatin, prominent nucleoli) were included, whereas morphologically distinguishable vascular endothelial cells and glial cells were not included in the cell count, as described previously (Lagreze et al., 1998; Takahata et al., 2003; Razali et al., 2015, 2016; Arfuzir et al., 2016; Lambuk et al., 2017). All estimations were done by two masked observers. The average of estimations from three random areas by each observer was calculated and subsequently, average of the values obtained by both observers was taken as final estimate.
Histopathological examination of optic nerve
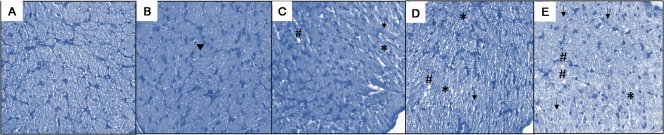
Toluidine blue was used to stain the optic nerve sections as described previously (Arfuzir et al., 2016). The observations were made by two independent observers under light microscope. Entire cross section of each optic nerve was examined, and a histopathological grade was assigned as described previously (Jia et al., 2000). Accordingly, grade 1: normal optic nerve with intact axons; grade 2: early mild lesions with axonal swelling and appearance of glial cells; grade 3: vacuolation, axonal swelling and focal presence of degenerating axons; grade 4: nuclear clearing, axonal swelling and numerous degenerating axons spread all over the optic nerve section; grade 5: nearly all axons degenerating across the entire section (Figure 1).
Figure 1.
Toluidine blue stained retinal sections representing grading of degenerative changes in the optic nerve.
(A) Grade 1: normal optic nerve with intact axons. (B) Grade 2: early mild lesions with axonal swelling and appearance of glial cells (arrowhead). (C) Grade 3: *indicates nuclear clearing, # indicates vacuolation, arrow indicates axonal swellings and focal presence of degenerating axons. (D) Grade 4: nuclear clearing (*), vacuolation (#), axonal swellings and numerous degenerating axons spread all over the optic nerve section as indicated by arrow. (E) Grade 5: shows numerous nuclear clearing (*), vacuolation (#), axonal swelling and nearly all axons degenerating (arrows) across the entire section.
Immunostaining for caspase-3
Retinal sections were deparaffinized with xylene and dehydrated in graded series of alcohols. Sodium citrate (10 mM, 0.05% Tween-20, pH 6.0) was used for the antigen retrieval for 20 minutes at boiling point. Sections were treated with 100 μL of rabbit anti active and procaspase 3 polyclonal primary antibody (ABCAM, Cambridge, MA, USA) diluted at 2:98 with 10 mM Tris 1% BSA and the incubation was performed overnight at 4°C. Subsequently, incubation with secondary antibody, which was conjugated with Texas red fluorochrome (goat anti rabbit IgG antibody - H&L Alexa Fluor® 594, ABCAM, Cambridge, MA), was done for 1 hour. Next, Tris-buffered saline (TBS) with 0.025% Triton X-100 was used to wash the sections for 5 minutes. The counterstaining was done for 10 minutes with 4,6-diamidino-2-phenylindole (DAPI) diluted in PBS 1 mM at a ratio of 1:999, and then the slides were mounted. The observations were made in a dark room using a fluorescence microscope (BX61TRF-FL-CCD; Olympus, Florida, USA). For Texas red 596 nm and for DAPI 358 nm filter was used.
Measurement of retinal oxidative stress
To estimate retinal oxidative stress, we measured the retinal contents of MDA and GSH, and the activities of the CAT and SOD using ELISA kit (Cayman Chemical, Ann Arbor, MI, USA). Retinae were homogenized for retinal MDA estimation in 10 μL of radio-immunoprecipitation assay lysis (RIPA) buffer (Cayman Chemical, Ann Arbor, MI, USA) with protease inhibitor per 1 mg retina. For GSH estimation, homogenization of retinal samples was performed in 5–10 mL of 2-morpholinoethanesulfonic acid (MES) buffer (0.4 M 2-(N-morpholino) ethane-sulphonic acid, 0.1 M phosphate, and 1 mM EDTA). For the catalase and SOD activity, homogenization of the retinae was performed in cold 50 mM PBS with 1 mM ethylenediaminetetraacetic acid (EDTA) at pH 7.0 and in cold 20 mM 4-(2-hydroxyethyl)-1-piperazinee thanesulfonic acid (HEPES) buffer at pH 7.2, respectively. The homogenates were centrifuged at 15,000 r/min at 4°C for 5 minutes and then supernatants were used for assay as per manufacturer's instructions. We used the Bradford's method to estimate the total protein content in the samples.
Statistical analysis
All data is expressed as the mean ±SD. Statistical analysis was done using SPSS version 20 (IBM, Armonk, NY, USA). Significant differences among groups were calculated using one-way analysis of variance followed by Tukey's post-hoc analysis. P-value of < 0.05 was considered statistically significant.
Results
Effect of TAU on retinal morphology
In the ET-1 treated group, the H&E stained sections of retinae showed significantly thinner GCL and loss of retinal cells in IR. Among the treated groups, the TAU pre-treatment group in particular showed a relatively preserved thickness of retina and number of retinal cells. Morphometric measurements showed 2.28-fold thinner GCL in the ET-1 group compared with the PBS group (P < 0.001). The number of nuclei per 100 μm length of GCL, per 100 μm2 of GCL and per 100 μm2 of IR in ET-1 group was also 3.62-, 2.82-, and 2.36-folds lower, respectively, compared to the PBS group (P < 0.001, P < 0.001 and P < 0.01 respectively). When comparing the ET-1 group to the TAU pre-treatment group, we observed that there was 1.89-fold greater thickness of GCL (P < 0.01), 3.18-fold greater linear density of nuclei in the GCL (P < 0.001), 2.41-fold greater cell density in the GCL (P < 0.01) and 2.02-fold greater cell density in the IR (P < 0.05) in the latter group. In TAU co- and post-treatment groups, all the four parameters remained significantly lower than PBS group, except for the cell density in the IR in the TAU co-treatment group, which showed no significant difference from both the PBS and ET-1 groups (Figures 2 and 3).
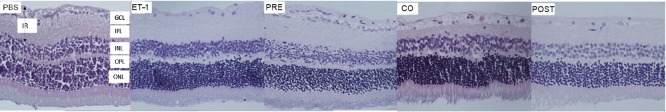
Figure 2.
Representative haematoxylin and eosin stained retinal section as viewed under light microscope.
PBS group was intravitreally injected with PBS and 7 days post-injection normal retinal morphology was observed. In the ET-1 group, 7 days after similar injection of ET-1 there was significant reduction in the number of cells in the IR and thickness of the GCL. In the PRE group, rats received TAU 24 hours before ET-1 and subsequently 7 days post-injection, there was an increase in the GCL thickness and number of nuclei in the IR compared to the ET-1 group. The retinal morphology in this group was comparable to the PBS group. In the CO group, ET-1 and TAU were co-administered. GCL thickness and the number of nuclei in the IR in this group remained significantly lower than the PBS group. Animals in the POST group received TAU 24 hours after ET-1 and subsequently 7 days post-injection the thickness of GCL as well as number of nuclei in the IR were significantly lower than in the PBS group. PBS: Phosphate buffer saline; ET-1: endothelin-1; TAU: amino acid taurine; GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; OPL: outer plexiform layer; ONL: outer nuclear layer; IR: inner retina.
Figure 3.
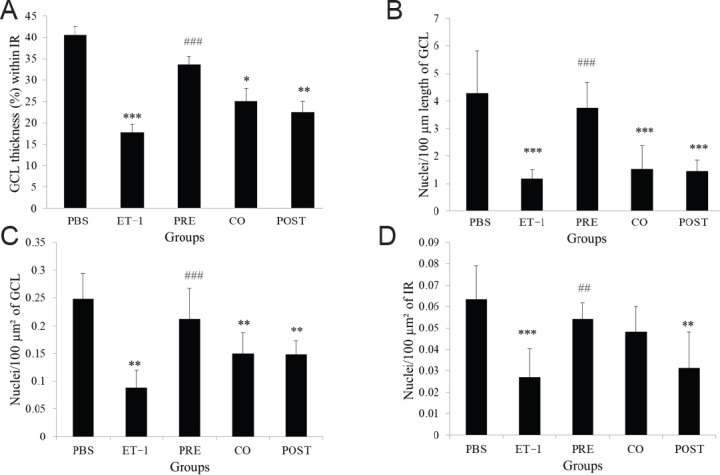
Quantification of retinal morphological changes after intravitreal administration of ET-1 and TAU.
(A) Fractional thickness of GCL within the IR. (B) Number of retinal cell nuclei per 100 µm length of GCL. (C) Number of retinal cell nuclei per 100 µm² of GCL. (D) Number of retinal cell nuclei per 100 µm² of IR. Groups - PBS: Phosphate-buffered saline; ET-1: endothelin-1; PRE: pre-treatment with TAU 24 hours before ET-1; CO: co-administration of TAU and ET-1; POST: post-treatment with TAU 24 hours after ET-1. All values are expressed as the mean ± SD (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001, vs. PBS group; ##P < 0.01, ###P < 0.001, vs. ET-1 group (one-way analysis of variance followed by Tukey's post-hoc analysis). TAU: amino acid taurine; GCL: ganglion cell layer; IR: inner retina.
Effect of TAU on optic nerve morphology
Intravitreal injection of ET-1 caused widespread degenerative changes in the optic nerve. The degeneration was relative less prominent in three TAU treatment groups, particularly in the TAU pre-treatment group. Quantitative estimation of the extent of optic nerve damage showed significantly greater damage in ET-1 group compared with the PBS group as the mean grade was 2.62 times higher (P < 0.001). Among the TAU treatment groups, pre-treatment group showed significantly lesser optic nerve damage (2.44-fold decrease) compared with the ET-1 group (P < 0.001). In the co-treatment group, the optic nerve damage was significantly lesser than the ET-1 group with 1.63-fold lower mean grade (P < 0.05). Extent of optic nerve damage in the post-treatment group was similar to that in the ET-1 group (Figure 4A and B).
Figure 4.
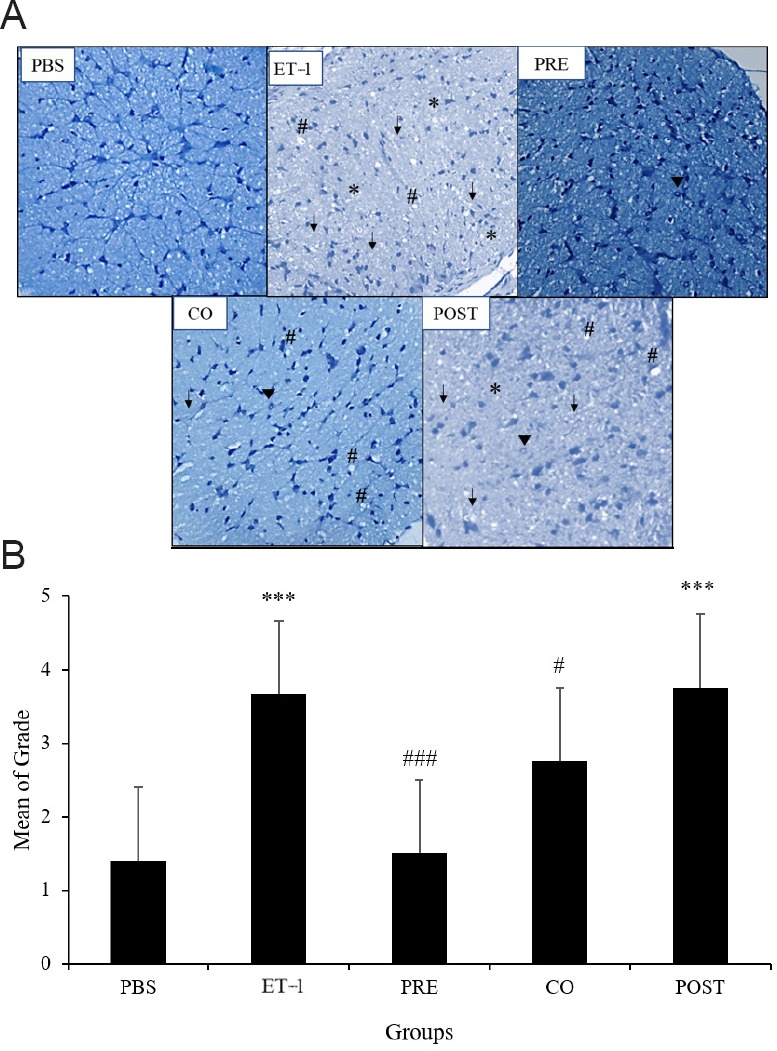
Effect of TAU on ET-1-induced changes in optic nerve morphology.
(A) Representative optic nerve sections stained with toluidine blue. PBS: A normal intact optic nerve was observed in PBS injected rats; ET-1: degenerative changes were seen across the entire optic nerve section; PRE: amino acid taurine (TAU) pretreatment group showed localized degenerative changes; CO: TAU and ET-1 co-administered group showed dense vacuolation and numerous degenerating fibers; POST: TAU posttreatment group showed severe axonal degeneration. *shows nuclear clearing; arrow head indicates increase in the number of glial cells; #shows dense vacuolation with degenerating fibers, arrow indicates extensive nuclear clearing and degenerating fibers (original magnification, 20×). (B) Quantitative estimation of changes in optic nerve morphology. Groups - PBS: Phosphate-buffered saline; ET-1: endothelin-1; PRE: pre-treatment with TAU 24 hours before ET-1; CO: co-administration of TAU and ET-1; POST: post-treatment with TAU 24 hours after ET-1. ***P < 0.001, vs. PBS group; #P < 0.05, ###P < 0.001, vs. ET-1 group (one-way analysis of variance followed by Tukey's post-hoc analysis). n = 6 for all groups.
Effect of TAU on ET-1-induced caspase-3 activation
Retinal sections from the PBS and TAU pre-treatment groups did not show any caspase-3-immunoreactive cells in the GCL. However, in the ET-1, TAU co-treatment, and TAU post-treatment groups, several caspase-3-immunoreactive cells were observed. Quantitatively, the number of apoptotic cells in the ET-1 group was 2.6-fold greater compared with the PBS group (P < 0.05). The TAU co- and post-treatment groups showed no significant difference from the PBS and ET-1 groups (P > 0.05). The TAU-pre-treatment group, however, showed a 1.99-fold lower number of caspase-3-immunoreactive cells compared with the ET-1 group (P < 0.05) (Figure 5A and B).
Figure 5.
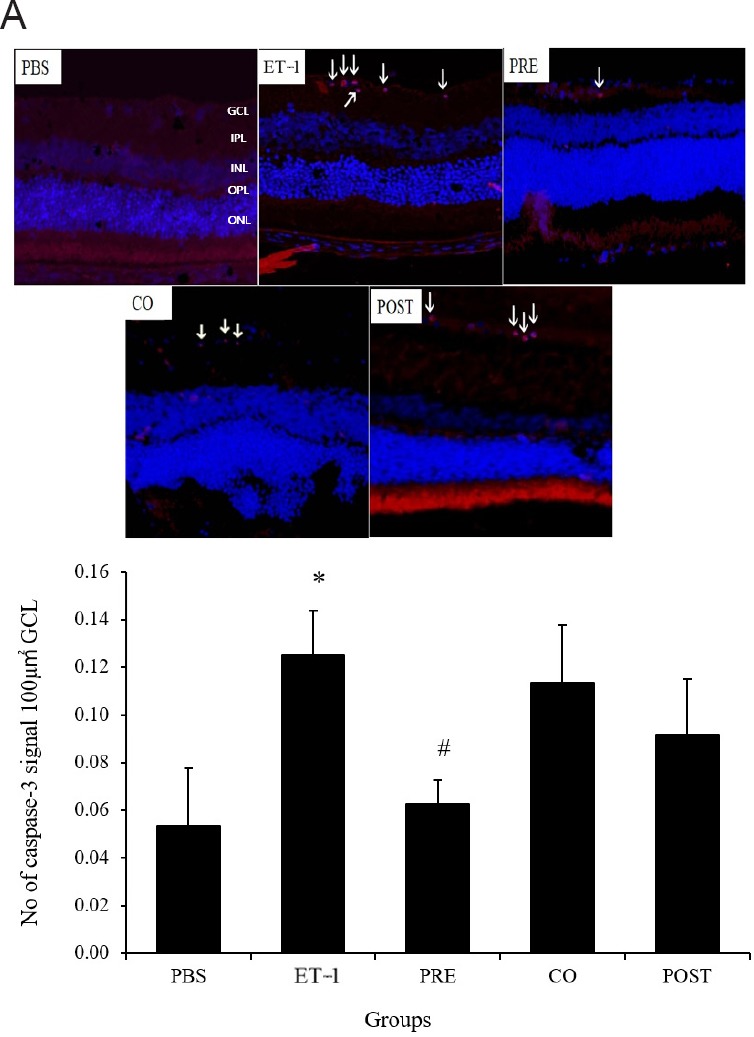
Effect of TAU on ET-1-induced retinal cell apoptosis as estimated by caspase-3 immunostaining.
(A) Representative retinal sections with caspase 3 staining showing apoptotic retinal cells as indicated by red fluorescent spots (arrows) in retinal sections; counterstaining with DAPI is seen in blue (original magnification, 60×). The PBS group showed no caspase positive cells, while in ET-1 group numerous caspase positive cells were observed. Among TAU treated groups, the PRE group showed minimal number of caspase positive cells, while in the CO and POST groups relatively greater number of caspase positive cells were observed (B). Quantitative estimation of caspase 3 positive retinal cells showed significantly greater number of caspase positive cells in the ET-1 group compared to the PBS group. In the PRE group number of caspase positive cells was significantly lower than the ET-1 group whereas the same in the CO and POST groups did not show significant difference either from the ET-1 or PBS group. Groups - PBS: Phosphate-buffered saline; ET-1: endothelin-1; PRE: pre-treatment with TAU 24 hours before ET-1; CO: co-administration of TAU and ET-1; POST: post-treatment with TAU 24 hours after ET-1. All values are the mean ± SD (n = 6), *P < 0.05, vs. PBS group; #P < 0.05, vs. ET-1 group (one-way analysis of variance followed by Tukey's post-hoc analysis). TAU: Amino acid taurine; GCL: ganglion cell layer.
Effect of TAU on ET-1 induced retinal oxidative stress
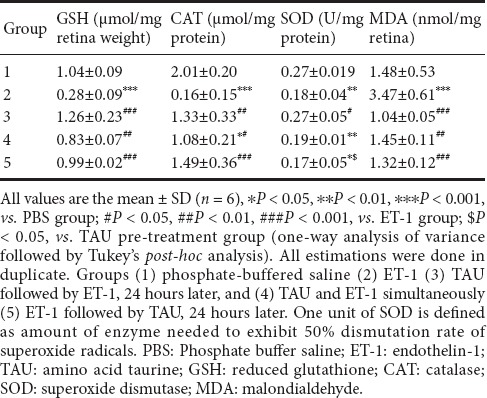
Intravitreal ET-1 caused a 3.71-fold reduction in the retinal GSH contents compared with the PBS group (P < 0.001). CAT and SOD activities were also lowered by 7.44 and 1.50 folds in the ET-1 group compared with the PBS group (P < 0.001 and P < 0.01, respectively). There was a 2.34-fold increase in retinal MDA contents in the ET-1 group compared with the PBS group (P < 0.001). In the TAU-pre-treatment group, GSH, CAT, SOD and MDA did not differ from the PBS group, however, there were 4.5-, 8.3-, 1.5- and 3.37-fold differences, respectively, from the ET-1 group (P < 0.001, P < 0.01, P < 0.05 and P < 0.001, respectively). Although the TAU co- and post-treatment groups showed significant improvement in retinal GSH, CAT and MDA compared with the ET-1 group, no difference was observed for SOD activity. Furthermore, in TAU co-treatment group, both CAT and SOD levels remained significantly lower than the PBS group (P < 0.05 and P < 0.01, respectively) and in the TAU post-treatment group, the SOD level was significantly lower than the PBS (P < 0.05) and TAU-pre-treatment groups (P < 0.05) (Table 1).
Table 1.
Effect of TAU on ET1 induced retinal oxidative stress
Discussion
Glaucomatous optic neuropathy is a heterogeneous disease and it is widely accepted that several factors other than elevated IOP are involved in its pathogenesis. Among these factors, vasospasm, resulting in decreased blood flow to the optic nerve head or reduced capacity to autoregulate blood flow to optic nerve head, has been suggested to play a significant role (Gasser and Flammer, 1987; Gasser et al., 1990). Several studies have provided indirect evidence to support the association of vasospasm and glaucoma. For example, an association has been shown with systemic hypertension and migraine (Drance et al., 2001; Bae et al., 2014). Vasospastic stimuli have been shown to cause an abnormal increase in plasma ET-1 level in glaucoma patients (Nicolela et al., 2003). Endothelial dysfunction may also have a role in primary open angle glaucoma (Bukhari et al., 2016). In view of this evidence, several experimental studies have used the intravitreal injection of ET-1 to induce retinal and optic nerve injury as was the case in the present study. The changes observed in the rat retina and optic nerve 7 days post-ET-1 injection in this study were in accordance with the findings of previous studies (Nagata et al., 2014).
Notably, this study has demonstrated for the first time that TAU provides protective effects against retinal and optic nerve damage induced by ET-1. We evaluated these effects of TAU by studying retinal and optic nerve morphology. While qualitative assessment of retinal sections showed prominent protective effects of TAU, further confirmation was done using quantitative morphometric estimations. To make an overall conclusive assessment, four morphometric parameters were compared among the groups. GCL thickness (%) within IR was expected to reduce with neuronal damage. However, it may not show significant changes despite obvious atrophy, as the thickness of the IR may also reduce with the neuronal damage. For the same reason, retinal cell density per unit volume of GCL and IR may not show the difference from control, if the volume of these layers reduces with neuronal loss. In this case, linear cell density may provide a better assessment of retinal damage as it does not take into account the volume but rather the length of GCL/IR. Nonetheless, all four parameters showed significant retinal damage in ET-1 injected animals and a restoration of retinal morphology, particularly in the pre-treatment group and, to some extent, in other TAU treated groups. The morphological observations made on the optic nerve were in accordance with those on retinas with greater effectiveness of TAU pre-treatment.
The protective effect of TAU may be attributed to the direct counteraction of ET-1-induced vasoconstriction. Studies have shown that a high dose of taurocholate causes dilation of superior mesenteric and hepatic artery, but a similar effect was not observed in renal vessels (Lautt and Daniels, 1983; Brezis et al., 1984). In vitro studies indicate that, depending on the vessel studied, TAU may affect both vasodilation and vasoconstriction, and these effects of the TAU appear to be Ca2+ dependent (Nishida and Satoh, 2009). Furthermore, ischemia induced changes in the structure and functions of neuronal cells involve a multitude of pathophysiological events. Neuronal death induced by ischemia is primarily attributed to dysfunction of the homeostasis of glutamate, the major excitatory neurotransmitter in the nervous system (Arundine and Tymianski, 2004). The interaction of glutamate with its NMDA subtype of receptors causes an influx of Ca2+ and Na+, resulting in depolarization of post-synaptic neuron. Under physiological conditions, this depolarization is short-lasting, as glutamate is quickly cleared from synapses by glutamate transporters. Under ischemic conditions, however, cellular respiration is compromised resulting in depletion of ATP. This impairs the functions of glutamate transporters causing synaptic accumulation of glutamate and excitotoxic neuronal damage (Taoufik and Probert, 2008). TAU has been shown to protect against glutamate induced neuronal apoptosis by inhibiting the intracellular increase in Ca2+ (Leon et al., 2009). Bulley and Shen (2010) showed that glutamate induced Ca2+ influx could be prevented by TAU via voltage-dependent Ca2+ channels and ionotropic glutamate receptors in the retinal neurons, hence preventing excitotoxic damage. In accordance with these observations, we observed reduced caspase staining in TAU treated groups and therefore, the protective effects of TAU, may be attributed, in part, to the inhibition of ischemia induced excitotoxicity.
Ischemia, besides initiating excitotoxicity, also results in oxidative stress as significantly high level of reactive oxygen species have been detected in glaucomatous eyes (Flammer et al., 2002). Oxidative stress may result from excessive production of ROS, impaired antioxidant defence system or a dysfunction of mitochondria. It may also result from a combined effect of these factors. Increased generation of free radicals in retina and optic nerve as a consequence of ischemia may involve an overstimulation of glutamate ionotropic receptors (Bonne et al., 1998). Additionally, during ischemic states, the production of free radicals by neuronal tissue can also cause an increased output of glutamate, perpetuating excitotoxic neuronal damage (Pellegrini-Giampietro et al., 1990). Hence, a vicious cycle of events involving oxidative stress and excitotoxicity subsequent to ischemia results in neuronal apoptosis. In this study, we measured the retinal levels of GSH, catalase, SOD and MDA to assess the retinal redox status. Glutathione is a part of the retinal defence system against oxidative stress and is distributed in different types of retinal cells. Its reduced form, GSH, helps in maintaining the thiol groups in reduced form (Ganea and Harding, 2006). SOD and catalase are also antioxidant defences against ROS induced tissue damage. SOD causes dismutation of superoxide radicals and the hydrogen peroxide produced as a consequence is removed by CAT (Ernster, 1988). MDA is a major byproduct of lipid peroxidation and its levels indirectly represent the redox status (Sevanian and Hochstein, 1985). The present study has shown that ET-1 induced retinal oxidative stress was reduced in the TAU-treated groups. Based on the overall assessment, and taking into account all four parameters of oxidative stress, it could be concluded that pre-treatment with TAU has a greater antioxidant effect compared with co- or post-treatment. Since this is in accordance with the observations made on the morphological examination of retina and optic nerve, it appears likely that the protective effect of TAU against ET-1 induced retinal and optic nerve morphology may, at least in part, be attributed to reduction of retinal oxidative stress.
It is worth noting that one of our earlier studies had demonstrated that TAU, as well as its Mg salt, MgAT, reduced ET-1 induced retinal nitrosative stress. It was also observed that both MgAT and TAU restored the retinal expression of nitric oxide synthase (NOS) isoforms and reduced the formation of peroxynitrite, a powerful oxidant, produced by a reaction of NO with superoxide radicals. This effect of MgAT and TAU on retinal nitrosative stress correlated with reduced retinal cell apoptosis, as detected by TUNEL staining (Arfuzir et al., 2018). It is likely that this reduction in peroxynitrite production was a consequence of not only a normalized NOS expression but also due to a reduced availability of superoxide radicals, resulting from improved retinal anti-oxidant defence as observed in the current study. Our previous study also showed that the effects of MgAT exceed those of TAU alone. MgAT is a chemical synthesized from Mg and TAU, and it hydrolyses to release Mg and TAU. Hence the superior effects of MgAT compared to TAU alone could be attributed to the combined actions of Mg and TAU (Arfuzir et al., 2016).
The present study has also shown that the benefits of treatment with TAU were particularly evident when it was given as pre-treatment rather than as co- or post-treatment. This was also in accordance with our previous study, which showed the restoration of ET-1 induced changes in retinal NOS expression, particularly after pre-treatment with both MgAT and TAU. This could be attributed to the possible protective effect of TAU against the onset of pathophysiological events by subsequent ET-1 administration. The effects of co- or post-treatment with TAU seem to be overwhelmed by the simultaneously initiated or already established ET-1 induced deleterious consequences. It was also noted that the higher effectiveness of TAU pre-treatment compared to co- or post-treatment as observed by morphology and caspase staining appeared to be proportionately greater than similar differences in retinal redox status in three TAU treated groups. Hence, it is likely that the additional molecular mechanisms contribute to the greater protective effect of TAU when given as pre-treatment. Effectiveness of pre-treatment rather than co- and post-treatment indicates the potential application of TAU as a prophylactic agent. However, it is worth noting that the effects of TAU in this study were observed after intravitreal administration, which may not be a suitable route of administration with regards to therapeutic application. Nevertheless, it is the first study to date that revealed the potential benefits of TAU against ischemia induced retinal and optic nerve damage, and these benefits may further be investigated using formulations that can be more suitable for therapeutic application.
In conclusion, treatment with TAU, particularly the pre-treatment, prevents retinal cell apoptosis induced by ET-1 by reducing retinal oxidative stress, hence preventing changes in the morphology of retina and optic nerve. Additional cellular mechanisms are likely to be involved in the protective effect of TAU. Further studies to explore the molecular mechanisms of action using suitable formulations of TAU will reveal its true therapeutic potential in retinal and optic nerve degeneration.
Acknowledgments
We would like to acknowledge the administrative and facility support by Research Management Institute, Laboratory Animal Care Unit and Institute of Medical Molecular Biotechnology (IMMB), Universiti Teknologi MARA, Malaysia.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Financial support: This study obtained the financial support by Universiti Teknologi MARA under grant No. 600-IRMI/DANA5/3/BESTARI (006/2017). The funding bodies played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: All experimental procedures in this study followed the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. The study was approved by The Committee on Animal Research and Ethics (UiTM Care: 216/6/2017 (6/10/2017)) and was done in compliance with the ethical guidelines of Universiti Teknology MARA.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study obtained the financial support by Universiti Teknologi MARA under grant No. 600-IRMI/DANA5/3/BESTARI (006/2017).
References
- 1.Agarwal R, Agarwal P. Rodent models of glaucoma and their applicability for drug discovery. Expert Opin Drug Discov. 2017;12:261–270. doi: 10.1080/17460441.2017.1281244. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R, Gupta SK, Agarwal P, Saxena R, Agrawal SS. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol. 2009;57:257–266. doi: 10.4103/0301-4738.53049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arfuzir NNN, Lambuk L, Jafri AJ, Agarwal R, Iezhitsa I, Sidek S, Agarwal P, Bakar NS, Kutty MK, Yusof AP, Krasilnikova A, Spasov A, Ozerov A, Mohd Ismail N. Protective effect of magnesium acetyltaurate against endothelin-induced retinal and optic nerve injury. Neuroscience. 2016;325:153–164. doi: 10.1016/j.neuroscience.2016.03.041. [DOI] [PubMed] [Google Scholar]
- 4.Arundine M, Tymianski M. Molecular mechanisms of glutamate-dependent neurodegeneration in ischemia and traumatic brain injury. Cell Mol Life Sci. 2004;61:657–668. doi: 10.1007/s00018-003-3319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae HW, Lee N, Lee HS, Hong S, Seong GJ, Kim CY. Systemic hypertension as a risk factor for open-angle glaucoma: a meta-analysis of population-based studies. PLoS One. 2014;9:e108226. doi: 10.1371/journal.pone.0108226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonne C, Muller A, Villain M. Free radicals in retinal ischemia. Gen Pharmacol. 1998;30:275–280. doi: 10.1016/s0306-3623(97)00357-1. [DOI] [PubMed] [Google Scholar]
- 7.Brezis M, Silva P, Epstein FH. Amino acids induce renal vasodilatation in isolated perfused kidney: coupling to oxidative metabolism. Am J Physiol. 1984;247:H999–1004. doi: 10.1152/ajpheart.1984.247.6.H999. [DOI] [PubMed] [Google Scholar]
- 8.Broadway DC, Drance SM. Glaucoma and vasospasm. Br J Ophthalmol. 82:862–870. doi: 10.1136/bjo.82.8.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bukhari SMI, Kiu KY, Thambiraja R, Sulong S, Rasool AHG, Liza-Sharmini AT. Microvascular endothelial function and severity of primary open angle glaucoma. Eye (Lond) 2016;30:1579–1587. doi: 10.1038/eye.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulley S, Shen W. Reciprocal regulation between taurine and glutamate response via Ca 2+-dependent pathways in retinal third-order neurons. J Biomed Sci. 2010;17:S5. doi: 10.1186/1423-0127-17-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen AI, McDaniel M, Orr H. Absolute levels of some free ammo acids in normal and biologically fractionated retinas. Invest Ophthalmol Vis Sci. 1973;12:686–693. [PubMed] [Google Scholar]
- 12.Drance S, Anderson DR, Schulzer M. Collaborative Normal-Tension Glaucoma Study Group. Risk factors for progression of visual field abnormalities in normal-tension glaucoma. Am J Ophthalmol. 2001;131:699–708. doi: 10.1016/s0002-9394(01)00964-3. [DOI] [PubMed] [Google Scholar]
- 13.Emre M, Orgül S, Haufschild T, Shaw SG, Flammer J. Increased plasma endothelin-1 levels in patients with progressive open angle glaucoma. Br J Ophthalmol. 2005;89:60–63. doi: 10.1136/bjo.2004.046755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernster L. Biochemistry of reoxygenation injury. Crit Care Med. 1988;16:947–953. doi: 10.1097/00003246-198810000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Flammer J. The vascular concept of glaucoma. Surv Ophthalmol. 1994;38:S3–6. doi: 10.1016/0039-6257(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 16.Flammer J, Orgül S, Costa VP, Orzalesi N, Krieglstein GK, Serra LM, Renard JP, Stefánsson E. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 17.Froger N, Cadetti L, Lorach H, Martins J, Bemelmans AP, Dubus E, Degardin J, Pain D, Forster V, Chicaud L, Ivkovic I, Simonutti M, Fouquet S, Jammoul F, Léveillard T, Benosman R, Sahel JA, Picaud S. Taurine provides neuroprotection against retinal ganglion cell degeneration. PLoS One. 2012;7:e42017. doi: 10.1371/journal.pone.0042017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganea E, Harding JJ. Glutathione-related enzymes and the eye. Curr Eye Res. 2006;31:1–11. doi: 10.1080/02713680500477347. [DOI] [PubMed] [Google Scholar]
- 19.Gasser P, Flammer J. Influence of vasospasm on visual function. Doc Ophthalmol. 1987;66:3–18. doi: 10.1007/BF00144735. [DOI] [PubMed] [Google Scholar]
- 20.Gasser P, Flammer J, Guthauser U, Mahler F. Do vasospasms provoke ocular diseases? Angiology. 1990;41:213–220. doi: 10.1177/000331979004100306. [DOI] [PubMed] [Google Scholar]
- 21.Gramer G, Weber BH, Gramer E. Migraine and Vasospasm in Glaucoma: Age-Related Evaluation of 2027 Patients With Glaucoma or Ocular Hypertension. Invest Ophthalmol Vis Sci. 2015;56:7999–8007. doi: 10.1167/iovs.15-17274. [DOI] [PubMed] [Google Scholar]
- 22.Heinämäki AA, Muhonen ASH, Piha RS. Taurine and other free amino acids in the retina, vitreous, lens, irisciliary body, and cornea of the rat eye. Neurochem Res. 1986;11:535–542. doi: 10.1007/BF00965323. [DOI] [PubMed] [Google Scholar]
- 23.Iwabe S, Lamas M, Vásquez Pélaez CG, Carrasco FG. Aqueous humor endothelin-1 (ET-1), vascular endothelial growth factor (VEGF) and cyclooxygenase-2 (COX-2) levels in Mexican glaucomatous patients. Curr Eye Res. 2010;35:287–294. doi: 10.3109/02713680903545315. [DOI] [PubMed] [Google Scholar]
- 24.Jafri AJA, Arfuzir NNN, Lambuk L, Iezhitsa I, Agarwal R, Agarwal P, Razali N, Krasilnikova A, Kharitonova M, Demidov V, Serebryansky E, Skalny A, Spasov A, Yusof APM, Ismail NM. Protective effect of magnesium acetyltaurate against NMDA-induced retinal damage involves restoration of minerals and trace elements homeostasis. J Trace Elem Med Biol. 2017;39:147–154. doi: 10.1016/j.jtemb.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Jia L, Cepurna WO, Johnson EC, Morrison JC. Patterns of intraocular pressure elevation after aqueous humor outflow obstruction in rats. Invest Ophthalmol Vis Sci. 2000;41:1380–1385. [PubMed] [Google Scholar]
- 26.Ko ML, Peng PH, Ma MC, Ritch R, Chen CF. Dynamic changes in reactive oxygen species and antioxidant levels in retinas in experimental glaucoma. Free Radic Biol Med. 2005;39:365–373. doi: 10.1016/j.freeradbiomed.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 27.Lagreze WA, Knörle R, Bach M, Feuerstein TJ. Memantine is neuroprotective in a rat model of pressure-induced retinal ischemia. Invest Ophthalmol Vis Sci. 1998;39:1063–1066. [PubMed] [Google Scholar]
- 28.Lambuk L, Jafri AJ, Arfuzir NNN, Iezhitsa I, Agarwal R, Rozali KN, Agarwal P, Bakar NS, Kutty MK, Yusof AP, Krasilnikova A, Spasov A, Ozerov A, Ismail NM. Neuroprotective effect of magnesium acetyltaurate against NMDA-induced excitotoxicity in rat retina. Neurotox Res. 2017;31:31–45. doi: 10.1007/s12640-016-9658-9. [DOI] [PubMed] [Google Scholar]
- 29.Lautt WW, Daniels TR. Differential effect of taurocholic acid on hepatic arterial resistance vessels and bile flow. Am J Physiol. 1983;244:G366–369. doi: 10.1152/ajpgi.1983.244.4.G366. [DOI] [PubMed] [Google Scholar]
- 30.Lee D, Kim KY, Noh YH, Chai S, Lindsey JD, Ellisman MH, Weinreb RN, Ju WK. Brimonidine blocks glutamate excitotoxicity-induced oxidative stress and preserves mitochondrial transcription factor a in ischemic retinal injury. PLoS One. 2012;7:10. doi: 10.1371/journal.pone.0047098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leon R, Wu H, Jin Y, Wei J, Buddhala C, Prentice H, Wu JY. Protective function of taurine in glutamate‐induced apoptosis in cultured neurons. J Neurosci Res. 2009;87:1185–1194. doi: 10.1002/jnr.21926. [DOI] [PubMed] [Google Scholar]
- 32.Mohd Lazaldin MA, Iezhitsa I, Agarwal R, Bakar NS, Agarwal P, Mohd Ismail N. Time- and dose-related effects of amyloid beta1-40 on retina and optic nerve morphology in rats. Int J Neurosci. 2018;20:1–14. doi: 10.1080/00207454.2018.1446953. [DOI] [PubMed] [Google Scholar]
- 33.Nagata A, Omachi K, Higashide T, Shirae S, Shimazaki A, Nakamura M, Ishida N, Sugiyama K. OCT evaluation of neuroprotective effects of tafluprost on retinal injury after intravitreal injection of endothelin-1 in the rat eye. Invest Ophthalmol Vis Sci. 2014;55:1040–1047. doi: 10.1167/iovs.13-13056. [DOI] [PubMed] [Google Scholar]
- 34.Nicolela MT, Ferrier SN, Morrison CA, Archibald ML, LeVatte TL, Wallace K, Chauhan BC, LeBlanc RP. Effects of cold-induced vasospasm in glaucoma: the role of endothelin-1. Invest Ophthalmol Vis Sci. 2003;44:2565–2572. doi: 10.1167/iovs.02-0913. [DOI] [PubMed] [Google Scholar]
- 35.Nishida S, Satoh H. Vascular modulation of rat aorta by taurine. Adv Exp Med Biol. 2009;643:37–46. doi: 10.1007/978-0-387-75681-3_4. [DOI] [PubMed] [Google Scholar]
- 36.Nor Arfuzir NN, Agarwal R, Iezhitsa I, Agarwal P, Sidek S, Spasov A, Ozerov A, Mohd Ismail N. Effect of magnesium acetyltaurate and taurine on endothelin1-induced retinal nitrosative stress in rats. Curr Eye Res. 2018;20:1–9. doi: 10.1080/02713683.2018.1467933. [DOI] [PubMed] [Google Scholar]
- 37.Pellegrini-Giampietro DE, Cherici G, Alesiani M, Carla V, Moroni F. Excitatory amino acid release and free radical formation may cooperate in the genesis of ischemia-induced neuronal damage. J Neurosci. 1990;10:1035–1041. doi: 10.1523/JNEUROSCI.10-03-01035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Razali N, Agarwal R, Agarwal P, Kapitonova MY, Kutty MK, Smirnov A, Bakar NS, Ismail NM. Anterior and posterior segment changes in rat eyes with chronic steroid administration and their responsiveness to antiglaucoma drugs. Eur J Pharmacol. 2015;749:73–80. doi: 10.1016/j.ejphar.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 39.Razali N, Agarwal R, Agarwal P, Tripathy M, Kapitonova MY, Kutty MK, Smirnov A, Khalid Z, Ismail NM. Topical trans-resveratrol ameliorates steroid-induced anterior and posterior segment changes in rats. Exp Eye Res. 2016;143:9–16. doi: 10.1016/j.exer.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 40.Ripps H, Shen W. Taurine: a “very essential” amino acid. Mol Vis. 2012;18:2673. [PMC free article] [PubMed] [Google Scholar]
- 41.Sevanian A, Hochstein P. Mechanisms and consequences of lipid peroxidation in biological systems. Annu Rev Nutr. 1985;5:365–390. doi: 10.1146/annurev.nu.05.070185.002053. [DOI] [PubMed] [Google Scholar]
- 42.Sturman JA, Gaull GE. Taurine in the brain and liver of the developing human and monkey. J Neurochem. 1975;25:831–835. doi: 10.1111/j.1471-4159.1975.tb04414.x. [DOI] [PubMed] [Google Scholar]
- 43.Sturman JA, Hayes KC. The biology of taurine in nutrition and development. In: Draper HH, editor; Draper HH, editor. Advances in Nutritional Research. Boston, MA: Springer; 1980. pp. 231–299. [Google Scholar]
- 44.Sun M, Xu C. Neuroprotective mechanism of taurine due to up-regulating calpastatin and down-regulating calpain and caspase-3 during focal cerebral ischemia. Cell Mol Neurobiol. 2008;28:593–611. doi: 10.1007/s10571-007-9183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahata K, Katsuki H, Kume T, Nakata D, Ito K, Muraoka S, Yoneda F, Kashii S, Honda Y, Akaike A. Retinal neuronal death induced by intraocular administration of a nitric oxide donor and its rescue by neurotrophic factors in rats. Invest Ophthalmol Vis Sci. 2003;44:1760–1766. doi: 10.1167/iovs.02-0471. [DOI] [PubMed] [Google Scholar]
- 46.Taoufik E, Probert L. Ischemic neuronal damage. Curr Pharm Des. 2008;14:3565–3573. doi: 10.2174/138161208786848748. [DOI] [PubMed] [Google Scholar]
- 47.Taranukhin AG, Taranukhina EY, Saransaari P, Podkletnova IM, Pelto-Huikko M, Oja SS. Neuroprotection by taurine in ethanol-induced apoptosis in the developing cerebellum. J Biomed Sci. 2010;17(Suppl 1):S12. doi: 10.1186/1423-0127-17-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Yu R, Cao L. Neuroprotection of taurine through inhibition of 12/15 lipoxygenase pathway in cerebral ischemia of rats. Neurol Res. 2017;39:453–458. doi: 10.1080/01616412.2017.1297906. [DOI] [PubMed] [Google Scholar]