The retina is the extension of the central nervous system that senses light. Cones and rods, situated in the outer retina, convert light into electrical signals that travel through intermediate neurons where these are further processed until they finally reach retinal ganglion cells (RGCs). The afferent neurons of the retina, the RGCs, send the visual information through their axons in the optic nerve to the retinorecipient nuclei in the brain, where is further analysed.
Until recently, it was thought that rods and cones (known as classical photoreceptors) were the only cells sensing light in the retina. However, we now know that light might be converted into electrical signals also in a specific subset of RGCs, the melanopsin-expressing RGCs (m+ RGCs). These cells account in the rat for 2% to 3% of the entire RGC population (García-Ayuso et al., 2015) and are involved in non-image forming visual functions such as the circadian photoentrainment or the pupillary reflex. RGCs not expressing melanopsin constitute approximately 98% of the RGC population and serve image-forming visual functions (García-Ayuso et al., 2015). RGCs not expressing melanopsin may be identified by their expression of Brn3a, a transcription factor which is, in turn, not expressed by m+ RGCs.
Inherited or acquired photoreceptor degenerations are a group of pathologies that involve the outer retina but that, with time, reach the inner retina affecting RGCs. Here we will review the current knowledge of retinal remodeling and loss of RGCs in photoreceptor degenerations caused by different aetiologies.
Age-related macular degeneration (AMD) and inherited retinal degenerations (RD) represent a major clinical problem (Benfenati and Lanzani, 2018; LaVail et al., 2018). AMD is at present the most frequent cause of irreversible blindness in developed countries. Inherited RD are less frequent but cause blindness at early ages, thus being an important cause of blindness at working ages. The most frequent inherited RD in humans is retinitis pigmentosa (RP). Both AMD and RP cause vision loss and irreversible blindness due to photoreceptor degeneration, but progress differently. While AMD triggers the loss of cones and retinal pigment epithelium at the macula, RP causes first rod degeneration and, secondarily, cone degeneration. Both diseases are due to intrinsic (genetic) and extrinsic (environmental) factors, and nutrition and light exposure have been proposed as predisposing risk factors. Indeed, light has been shown to cause photoreceptor death and to accelerate photoreceptor degenerations. Also, light-induced RD models have been documented to mimic some features of human AMD (Marco-Gomariz et al., 2006; Marc et al., 2008; García-Ayuso et al., 2011).
The main aim of RD research is to develop therapies to slow or prevent photoreceptor loss and to replace lost photoreceptors, since a relative survival of the inner retinal cells is assumed after photoreceptor loss. However, there is increasing evidence that the inner retina becomes progressively disorganized and remodeled as the outer retinal degeneration progresses (Villegas-Pérez et al., 1998; Marco-Gomariz et al., 2006; Marc et al., 2007, 2008; García-Ayuso et al., 2010, 2011, 2014, 2015; Kalloniatis et al., 2016). Concretely, when photoreceptors are lost, a sequence of progressive events is initiated in the outer retina that culminate with cell death and remodelling of the inner neural retina (Marc et al., 2007, 2008; Kalloniatis et al., 2016; LaVail et al., 2018). The degenerating retina is dynamic and some reprogramming of the neural retina occurs during retinal degeneration (Marc et al., 2007; Kalloniatis et al., 2016). Indeed, anatomical and neurochemichal changes have been proposed to be responsible for the observed activation of amacrine and RGCs in the absence of bipolar cell activation (Marc et al., 2007) and these changes may be responsible for long-term RGC viability.
The question is whether the RGCs are affected and die during the course of these diseases, and if so, when. This is important because therapies aimed to replace photoreceptors (i.e., photoreceptor transplantation or retinal prosthesis implantation) depend on the assumption that RGCs are alive and properly functioning (Benfenati and Lanzani, 2018). If they are not, these therapies will not restore vision because the retinorecipient areas of the brain will not be reached.
RD has been widely studied using laboratory animals. The rodent models have been the most extensively used because of their many experimental advantages. In our lab, we have studied the effect of retinal remodeling following photoreceptor degeneration on RGC survival. For this purpose, we have investigated RGC survival in three different rodent models with different etiologies: two models of inherited photoreceptor degeneration, the Royal College of Surgeons (RCS; Villegas-Pérez et al., 1998; García-Ayuso et al., 2014) and the P23H-1 (García-Ayuso et al., 2010) rat strains, and one model of light-induced photoreceptor degeneration in albino (García-Ayuso et al., 2011) and pigmented (Marco-Gomariz et al., 2006) rats.
RCS rats suffer a recessive mutation of the MERKT gene, an orthologous human gene defect that impairs the phagocytosis of the outer segments of photoreceptors by the retinal pigment epithelium cells (LaVail et al., 2018). P23H rats bare a proline to histidine substitution at codon 23 in the rhodopsin gene, one of the commonest mutations associated with human autosomal dominant RP (LaVail et al., 2018). Both mutations have been shown to occur in certain human diseases and thus, both models have been used to study the consequences of these mutations in the retina (Villegas-Pérez et al., 1998; García-Ayuso et al., 2010, 2014). One of the main differences between these two models is that RD in the P23H-1 rat is due to a rod genetic defect while in the RCS rat the mutant gene impedes retinal pigment epithelium phagocytosis.
Light-induced RD or phototoxicity has also been employed as a model to study RD (Marco-Gomariz et al., 2006; Marc et al., 2008; García-Ayuso et al., 2011, 2015). Different models were proposed, in albino and pigmented rats and mice, using various light intensities, duration and emission spectrum. In our experiments we have exposed the animals for 48 hours (albino rats; García-Ayuso et al., 2011) or 72 hours (pigmented rats; Marco-Gomariz et al., 2006) to 3000 luxes of white cold fluorescent light (containing all wavelengths), which presumably mimics the effect of excess exposure to daylight. To achieve light-induced RD in pigmented rats, the pupils need to be dilated (Marco-Gomariz et al., 2006), while this is not necessary in albino rats (García-Ayuso et al., 2011).
To investigate RGC survival, we have labeled the RGCs. First, we used fluorogold (FG), a tracer that after being applied onto the superior colliculi (the largest retinorecipient area in rodents) is retrogradely transported from the axonal terminals to the RGC somas in the retina and results in the labeling over 98% of the total rat RGC population (Villegas-Pérez et al., 1998; Nadal-Nicolás et al., 2009). A software previously developed by our group allowed us to quantify the whole population of FG-labeled RGCs (FG+ RGCs) in each retina (García-Ayuso et al., 2010, 2011).
When we studied the population of traced-RGCs in these models, we observed that the RGC remained unaffected during a period after photoreceptor degeneration. Thus, until postnatal day (P) 270 in the P23H-1 rat, until P450 in the RCS rat or until 90 days after light exposure, the mean number of traced-RGCs was similar to that observed in younger animals and control animals, respectively (Figure 1A). In the P23H-1 rat, however, shortly after birth, at P30, the mean number of RGCs was significantly smaller (7%; García-Ayuso et al., 2010) than the observed in age-matched control rats (Figure 1A). Because at P30 there is no photoreceptor loss in this strain nor retinal remodelling yet, we concluded that the P23H strain have fewer RGCs than wild type rats.
Figure 1.
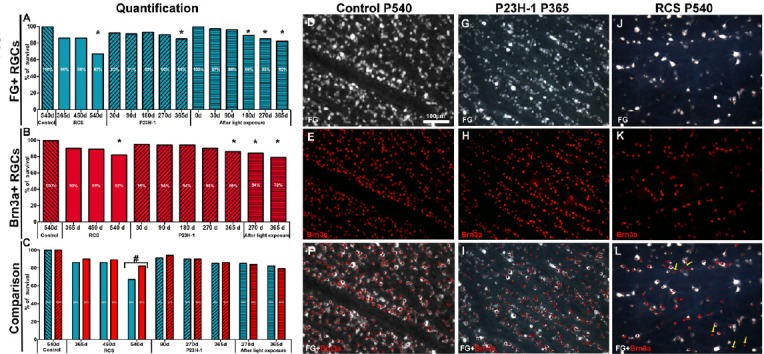
Retinal ganglion cell (RGC) loss in three models of photoreceptor degeneration.
Percentages of fluorogold (FG)+ RGCs (A), Brn3a+ RGCs (B) and comparison of both (C) at different postnatal days (P) for Royal College of Surgeons (RCS) and P23H rats or days after light exposure. These percentages were calculated considering 100% the number of RGCs in control retinas. *P < 0.05, vs. the earlier time points; #P < 0.05. (D–F) Microphotographs from a representative P540 control rat retina showing FG+ RGCs (D) and Brn3a+ RGCs (E); (F) is a merged image of D and E. (G–I) Microphotographs from a representative P365 P23H-1 rat retina showing FG+ RGCs (G) and Brn3a+ RGCs (H); (I) is a merged image of G and H. (J–L) Microphotographs from a representative P540 RCS rat retina showing FG+ RGCs (J) and Brn3a+ RGCs (K); (L) is a merged image of J and K and shows Brn3a+ RGCs that were not labelled with FG (arrows). FG was applied into both superior colliculi 7 days before animal processing. Scale bar: 100 μm. Data in Figure 1 are from García-Ayuso et al. (2010, 2011, 2014).
Next we analysed the numbers of RGCs in aged P23H and RCS animals and long term after light exposure, since retinal reorganization is a late event of photoreceptor loss. The number of traced-RGCs remained unaltered up to P365 and P540 in P23H-1 and RCS rats, respectively, when 14% and 36% of RGCs were lost (Figure 1A). In light-exposed animals, the loss of traced-RGCs occurred earlier, at 180 days after light exposure in albino animals. Interestingly, an almost complete loss of photoreceptors occurs at P365 and P90 in the P23H-1 rats and RCS rats, respectively, and 180 days after light exposure. Thus, the decrease of traced-RGCs is observed when there are almost no photoreceptors left and at the same time that the RGC axons become strangulated by the vessels of the inner retinal blood plexus (Villegas-Pérez et al., 1998; Marco-Gomariz et al., 2006; García-Ayuso et al., 2011).
Because FG is retrogradely transported (Villegas-Pérez et al., 1998; Marco-Gomariz et al., 2006; García-Ayuso et al., 2010, 2011), one could argue that the mean number of traced RGCs decrease after photoreceptor loss because at the strangulation points the retrograde axonal transport is impaired, but there is not really RGC death. To explore this possibility, RGCs were immunoidentified by their expression of Brn3a, a transcription factor that is a marker of RGC viability (Nadal-Nicolás et al., 2009) and is expressed by all image-forming RGCs (García-Ayuso et al., 2015). The whole population of Brn3a-labelled RGCs (Brn3a+ RGCs) in each retina was quantified using the software developed by our group (García-Ayuso et al., 2010, 2011). The mean numbers of Brn3a+ RGCs (Figure 1B) matched those observed for FG-traced RGCs in all models, ages and times ALE (Figure 1C; García-Ayuso et al., 2010, 2011, 2014, 2015). However, in RCS rats at P540, we observed that the mean numbers of FG-traced RGCs (71% out of total in control rats) were significantly lower than the mean number of immunodetected Brn3a+ RGCs (87% out of total in control rats; Figure 1C, J–L; García-Ayuso et al., 2014). Therefore, we concluded that there is RGC death in all models, but that the RCS rats show in addition an impairment of their axonal transport that precedes their death (García-Ayuso et al., 2014).
Overall, our results confirm that retinal remodelling after advanced photoreceptor loss causes RGC death, and that this may be caused and preceded by axonal transport interruption, as observed in the RCS strain. We cannot rule out the possibility that the same sequence of events may also occur in the other 2 models of photoreceptor degeneration. We speculate that we may have not found the exact, and probably narrow, time window where the axonal transport is impaired but the RGC is still alive in these latter models.
We thus conclude that RGC death after photoreceptor loss is a delayed event that occurs when there is an almost complete loss of photoreceptors. Therefore, the therapies aimed to replace the degenerated photoreceptors should be attempted promptly, before complete loss of photoreceptor occurs, to ensure its effectiveness.
This work was supported by grants from Fundación Séneca, Agencia de Ciencia y Tecnología Región de Murcia (19881/GERM/15), Spanish Ministry of Economy and Competitiveness, Instituto de Salud Carlos III, Fondo Europeo de Desarrollo Regional ‘‘Una Manera de Hacer Europa’’ (SAF2015-67643-P, PI16/00380, RD16/0008/0026, PI16/00031).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
References
- 1.Benfenati F, Lanzani G. New technologies for developing second generation retinal prostheses. Lab Anim (NY) 2018;47:71–75. doi: 10.1038/s41684-018-0003-1. [DOI] [PubMed] [Google Scholar]
- 2.García-Ayuso D, Salinas-Navarro M, Agudo-Barriuso M, Alarcón-Martínez L, Vidal-Sanz M, Villegas-Pérez MP. Retinal ganglion cell axonal compression by retinal vessels in light-induced retinal degeneration. Mol Vis. 2011;17:1716–1733. [PMC free article] [PubMed] [Google Scholar]
- 3.García-Ayuso D, Salinas-Navarro M, Agudo M, Cuenca N, Pinilla I, Vidal-Sanz M, Villegas-Pérez MP. Retinal ganglion cell numbers and delayed retinal ganglion cell death in the P23H rat retina. Exp Eye Res. 2010;91:800–810. doi: 10.1016/j.exer.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 4.García-Ayuso D, Salinas-Navarro M, Nadal-Nicolás FM, Ortín-Martínez A, Agudo-Barriuso M, Vidal-Sanz M, Villegas-Pérez MP. Sectorial loss of retinal ganglion cells in inherited photoreceptor degeneration is due to RGC death. Br J Ophthalmol. 2014;98:396–401. doi: 10.1136/bjophthalmol-2013-303958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Ayuso D, Di Pierdomenico J, Esquiva G, Nadal-Nicolás FM, Pinilla I, Cuenca N, Vidal-Sanz M, Agudo-Barriuso M, Villegas-Pérez MP. Inherited photoreceptor degeneration causes the death of melanopsin-positive retinal ganglion cells and increases their coexpression of Brn3a. Invest Ophthalmol Vis Sci. 2015;56:4592–4604. doi: 10.1167/iovs.15-16808. [DOI] [PubMed] [Google Scholar]
- 6.Kalloniatis M, Nivison-Smith L, Chua J, Acosta ML, Fletcher EL. Using the rd1 mouse to understand functional and anatomical retinal remodelling and treatment implications in retinitis pigmentosa: A review. Exp Eye Res. 2016;150:106–121. doi: 10.1016/j.exer.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 7.LaVail MM, Nishikawa S, Steinberg RH, Naash MI, Duncan JL, Trautmann N, Matthes MT, Yasumura D, Lau-Villacorta C, Chen J, Peterson WM, Yang H, Flannery JG. Phenotypic characterization of P23H and S334ter rhodopsin transgenic rat models of inherited retinal degeneration. Exp Eye Res. 2018;167:56–90. doi: 10.1016/j.exer.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marc RE, Jones BW, Watt CB, Vazquez-Chona F, Vaughan DK, Organisciak DT. Extreme retinal remodeling triggered by light damage: implications for age related macular degeneration. Mol Vis. 2008;14:782–806. [PMC free article] [PubMed] [Google Scholar]
- 9.Marc RE, Jones BW, Anderson JR, Kinard K, Marshak DW, Wilson JH, Wensel T, Lucas RJ. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:3364–3371. doi: 10.1167/iovs.07-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marco-Gomariz MA, Hurtado-Montalbán N, Vidal-Sanz M, Lund RD, Villegas-Pérez MP. Phototoxic-induced photoreceptor degeneration causes retinal ganglion cell degeneration in pigmented rats. J Comp Neurol. 2006;498:163–179. doi: 10.1002/cne.21028. [DOI] [PubMed] [Google Scholar]
- 11.Nadal-Nicolás FM, Jiménez-López M, Sobrado-Calvo P, Nieto-López L, Cánovas-Martínez I, Salinas-Navarro M, Vidal-Sanz M, Agudo M. Brn3a as a marker of retinal ganglion cells: qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Invest Ophthalmol Vis Sci. 2009;50:3860–3868. doi: 10.1167/iovs.08-3267. [DOI] [PubMed] [Google Scholar]
- 12.Villegas-Pérez MP, Lawrence JM, Vidal-Sanz M, Lavail MM, Lund RD. Ganglion cell loss in RCS rat retina: a result of compression of axons by contracting intraretinal vessels linked to the pigment epithelium. J Comp Neurol. 1998;392:58–77. [PubMed] [Google Scholar]


