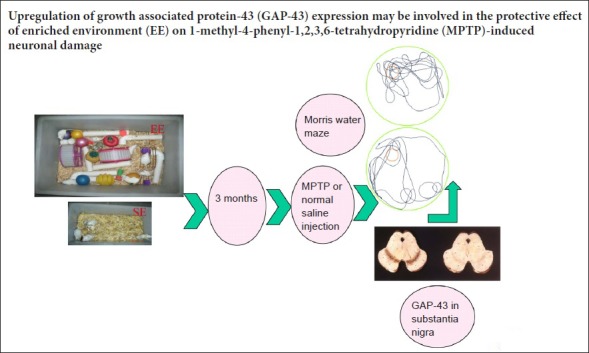
Keywords: nerve regeneration, Parkinson's disease, neural plasticity, senescence-accelerated mouse prone 8, growth associated protein-43, substantia nigra, learning and memory, neural regeneration
Abstract
An enriched environment protects dopaminergic neurons from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neuronal injury, but the underlying mechanism for this is not clear. Growth associated protein-43 (GAP-43) is closely associated with neurite outgrowth and axon regeneration during neural development. We speculate that an enriched environment can reduce damage to dopaminergic neurons by affecting the expression of GAP-43. This study is designed to test this hypothesis. Three-month-old female senescence-accelerated mouse prone 8 (SAMP8) mice were housed for 3 months in an enriched environment or a standard environment. These mice were then subcutaneously injected in the abdomen with 14 mg/kg MPTP four times at 2-hour intervals. Morris water maze testing demonstrated that learning and memory abilities were better in the enriched environment group than in the standard environment group. Reverse-transcription polymerase chain reaction, immunohistochemistry and western blot assays showed that mRNA and protein levels of GAP-43 in the substantia nigra were higher after MPTP application in the enriched environment group compared with the standard environment group. These findings indicate that an enriched environment can increase GAP-43 expression in SAMP8 mice. The upregulation of GAP-43 may be a mechanism by which an enriched environment protects against MPTP-induced neuronal damage.
Introduction
Parkinson's disease (PD) is a common neurodegenerative disease in the elderly. Its main feature is gradual loss of dopaminergic neurons in the substantia nigra, accompanied by motor symptoms and cognitive impairments and sometimes dementia (Wirdefeldt et al., 2011). PD affects 1–2% of the population over the age of 60 years (Ohashi et al., 2006; Wood et al., 2010; Pringsheim et al., 2014). Motor impairments and cognitive dysfunction are usual symptoms of PD (Wood et al., 2010) that result from extended death of dopaminergic neurons in the substantia nigra pars compacta and a 70–80% reduction in dopamine levels in the striatum (Bergman and Deuschl, 2002; Galvan and Wichmann, 2008). PD is considered a multi-factor disorder that results from the combined effects of multiple factors, including genetic and environmental factors and aging (Elbaz et al., 2016), with aging being the most prominent risk factor (Collier et al., 2007).
The senescence-accelerated mouse prone 8 (SAMP8) inbred mouse line has early onset senility and a short life span (Flood and Morley, 1998) and is characterized by learning and memory impairments, and affective disturbance during the aging process (Kawamata et al., 1997; Takeda et al., 1997). In our previous studies, we have used a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-SAMP8 model of PD (Liu et al., 2008; Yuan et al., 2008), which has non-motor symptoms (Liu et al., 2010).
With an aging population, the number of people with PD is increasing (LaHue et al., 2016). Although several studies have suggested that combinations of pharmacological and non-pharmacological therapies can alleviate PD symptoms (Mo et al., 2015; Chang et al., 2016), all therapeutic options to date have failed to halt the progression of degeneration (Kakkar and Dahiya, 2015). Some experimental data indicate that the environment can change behavior and molecular indicators in some animal models (Laviola et al., 2008). These changes can induce plastic brain changes and promote beneficial effects on the progression of neuronal impairment related to PD (Campêlo et al., 2017). Physical activity and experience of novelty have been shown to improve both motor and cognitive functions in PD patients (Benka Wallén et al., 2015; Hindle et al., 2016).
A previous study has shown that enriched environments (EEs) can protect dopaminergic neurons from MPTP-induced neuronal injury (Yuan et al., 2009a). What are the underlying mechanisms for this effect? In the past few years, many studies have focused on neural plasticity. Growth associated protein-43 (GAP-43) is strongly associated with neurite growth and axon regeneration during neural development (Aigner and Caroni, 1993). GAP-43 is also regarded as a molecular marker of neural plasticity (Gorup et al., 2015). To investigate the possible protective effects of EEs, this study examined the cognitive ability of MPTP-treated SAMP8 mice in the Morris water maze (MWM), and determined mRNA and protein levels of GAP-43 in the substantia nigra pars compacta.
Materials and Methods
Animals
Ninety-six 3-month-old female SAMP8 mice weighing 20–23 g were obtained from the Animal Center of Hebei Medical University of China (No. SCXK (Ji) 2008-1-003). All mice were randomly and equally assigned to EE and standard environment (SE) groups. The EE cages (52 cm × 37 cm × 22 cm) consisted of a platform, several tunnels, two running wheels, a variety of toys and nesting material. There were 10–12 mice per cage. The SE cages (32 cm × 20 cm × 15 cm) only contained nesting material and 5–6 mice per cage. Mice were housed in a room at 22–25°C with 55 ± 5% humidity and a 12-hour light/dark cycle. Mice had free access to water and food and these conditions were maintained for 3 months. All experimental procedures were performed according to the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China. This study was approved by the Animal Ethics Committee of the First Hospital of Hebei Medical University of China (approval No. 20131001).
MPTP injection
Mice were equally and randomly divided into an MPTP group and a 0.9% normal saline solution (NS) group. The MPTP mice were subcutaneously injected in the abdomen with MPTP (14 mg/kg) four times from 8 am at 2-hour intervals. The mice in the NS group received an equal volume (14 mL/kg) of 0.9% saline solution at the same time point. MWM experiments were conducted for 5 days. The following day, mice were euthanized for immunohistochemistry, and reverse-transcription polymerase chain reaction (RT-PCR) and western blot assays.
MWM test
The day after MPTP and NS injection, 96 mice were tested in the MWM for 5 consecutive days using apparatus from Anhui Huaibei Zhenghua Biological Equipment (Anhui, China). The swimming pool, 50 cm in height and 120 cm in diameter, had four quadrants. A hidden platform, 20 cm in height and 14 cm in diameter, was fixed 1.5 cm under the surface of the water and was defined as the target quadrant. The water temperature was maintained at 22 ± 2°C and was made opaque with black non-toxic dye to prevent the mice from seeing the platform. A place navigation test was performed for four days. Mice were put into the pool facing the wall of the pool and allowed to swim randomly until the platform was found or for up to 120 seconds. If a mouse could not find the platform, they were guided to the platform. All mice were allowed to stay on the platform for 10 seconds. Escape latency (the time taken to find the platform) was recorded. This was repeated three more times with the mice being placed in a different quadrant each time. On day 5, the probe test was performed; the hidden platform was removed and each mouse was allowed to swim for 120 seconds. The swim paths were automatically recorded with a video tracking system (Anhui Huaibei Zhenghua Biological Equipment) and the time spent in each quadrant was recorded.
RT-PCR
The day after the MWM test, eight mice from each group were euthanized. The substantia nigra was dissected on ice from each mouse. Total RNA was purified from fresh tissue, and cDNA was prepared for RT-PCR. β-Actin served as the internal reference. Primers were synthesized by Shanghai Bioasia Daily Chemical Co., Ltd. (Shanghai, China). The primers used are listed in Table 1.
Table 1.
Primers for reverse-transcription polymerase chain reaction

For amplification of β-actin and GAP-43, the number of cycles was 27 and 30, respectively. Amplification conditions were as follows: predenaturation at 95°C for 5 minutes, 30 cycles of 95°C for 30 seconds, 54°C for 45 seconds and 72°C for 45 seconds, followed by a final extension at 72°C for 10 minutes. After electrophoresis, gel images were scanned and data were analyzed with a JEDA 801E Gel image analyzer (Nanjing University Company). PCR products were quantitatively analyzed. Results are expressed as the ratio of the absorbance value of GAP-43 to that of β-actin.
Immunohistochemistry staining
The day after the MWM test, eight mice from each group were anesthetized with 10% chloral hydrate, perfused through the left ventricle with saline, and the brains were embedded in paraffin. According to The Mouse Brain Atlas in Stereotaxic Coordinates, the substantia nigra was sectioned in to consecutive 5-μm sections. Every fifth section was selected for immunostaining. Sections were incubated in 10% normal goat serum for 1 hour at 37°C and then incubated overnight at 4°C with rabbit anti-GAP-43 antibody (1:100; Chemicon Biotechnology, Temecula, CA, USA). Sections were then washed in 0.1 M PBS and incubated sequentially with biotinylated goat anti-rabbit IgG (1:100; Zhongshan Golden Bridge Biotechnology, Beijing, China) for 30 minutes at 37°C, horseradish peroxidase-conjugated streptavidin (1:100; Zhongshan Golden Bridge Biotechnology) for 30 minutes at 37°C, and then briefly in diaminobenzidine. The cytoplasm of cells located in the substantia nigra pars compacta was positive for GAP-43, visible as a brownish yellow stain. Pictures were taken at 200× magnification using a BX53 microscope (Olympus, Tokyo, Japan) and analyzed using the Motic Med 6.0 digital medical image analysis system. Three sections were selected from each mouse, and a visual field on one side of the substantia nigra compacta was randomly selected from each section. The corrected optical density value of GAP43-ir was determined and the mean value obtained.
Western blotting
The day after the MWM test, eight mice of each group were euthanized. Protein from fresh substantia nigra tissue was extracted. For each sample, 60 µg of protein were subjected to polyacrylamide gel electrophoresis, and then transferred to a polyvinylidene fluoride membrane. Membranes were incubated with rabbit anti-GAP-43 polyclonal antibody (1:500; Chemicon Biotechnology, Temecula, CA, USA), or rabbit anti-β-actin polyclonal antibody (1:200; Zhongshan Golden Bridge Biotechnology) at room temperature for 1 hour, followed by horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 2 hours at room temperature. The products were developed with Super ECL Plus luminescence fluid (Applygen Technologies Inc., Beijing, China). Following film scanning, visualized bands were analyzed using Quantity One-4.6.2 software (Bio-rad, Hercules, CA, USA). β-Actin was used as the internal reference to confirm equal loading of the samples.
Statistical analysis
Data, presented as the mean ± SD, were analyzed with SPSS 11.5 software (SPSS, Chicago, IL, USA). The escape latency in the place navigation test of the MWM task was analyzed by two-way repeated measures analysis of variance followed by the least significant difference post hoc test. All other data analyses were performed by one-way analysis of variance followed by the least significance difference test. P values < 0.05 were considered statistically significant.
Results
EE improved the learning and memory abilities of MPTP-treated SAMP8 mice
Learning acquisition
Compared with the SE + NS group, escape latencies were significantly shorter in the EE + NS group (P < 0.01), but significantly longer in the SE + MPTP group (P < 0.01). Compared with the EE + NS group, escape latencies were longer in the EE + MPTP group (P < 0.01). Compared with the SE + MPTP group, escape latencies were shorter in the EE + MPTP group over four consecutive days (P < 0.01). The escape latency of each group shortened significantly with increasing number of trials, illustrating learning acquisition (Figure 1).
Figure 1.
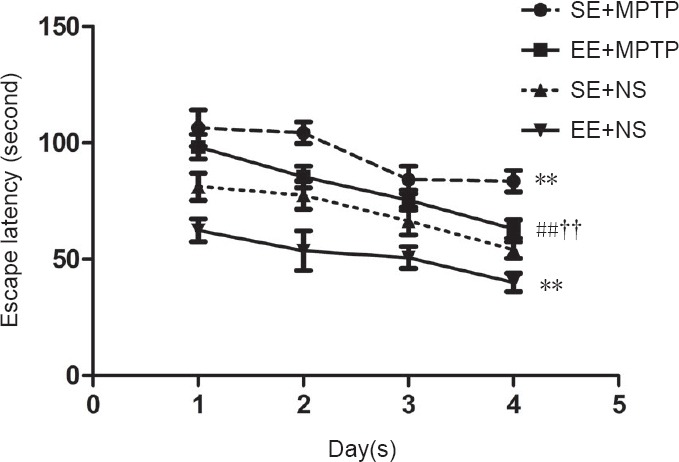
Effects of EE and MPTP on the average escape latency of SAMP8 mice in the place navigation test of the Morris water maze task.
Data are shown as the mean ± SD (two-way repeated measures analysis of variance followed by the least significant difference post hoc test). **P < 0.01, vs. SE + NS group; ##P < 0.01, vs. EE + NS group; ††P < 0.01, vs. SE + MPTP group. EE: Enriched environment; SE: standard environment; MPTP: 1-methyl-4-phenyl-1,,2,3,6-tetrahydropyridine; NS: normal saline; SAMP8: senescence-accelerated mouse prone 8.
Memory retention
The percentage of time spent in the target quadrant was significantly longer (P < 0.001) and the percentage of time spent in the opposite quadrant was significantly shorter (P < 0.01) in the EE + NS group compared with the SE + NS group. After MPTP injection, the percentage of time spent in the target quadrant was significantly shorter (P < 0.001) and the percentage of time spent in the opposite quadrant was significantly longer (P < 0.05) in the EE + MPTP group compared with the EE + NS group. Moreover, the percentage of time spent in the target quadrant was significantly shorter (P < 0.05) and the percentage of time spent in the opposite quadrant was significantly longer (P < 0.05) in the SE + MPTP group compared with the SE + NS group. However, compared with the SE + MPTP group, the percentage of time spent in the target quadrant was significantly longer (P < 0.05) and the percentage of time spent in the opposite quadrant was significantly shorter (P < 0.05) in the EE + MPTP group (Figure 2).
Figure 2.
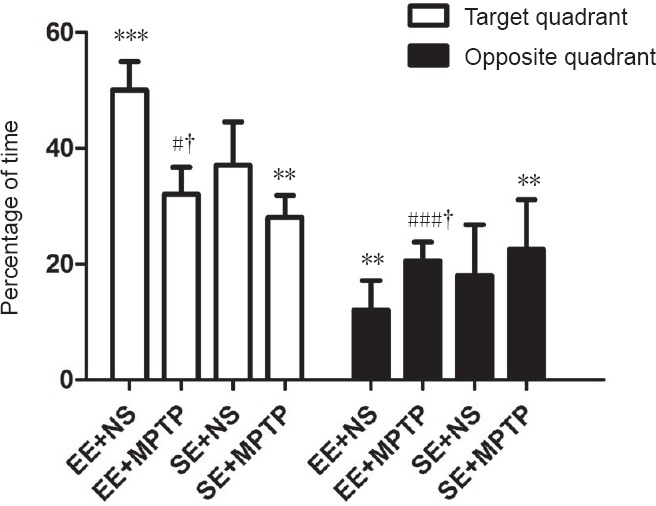
Effects of EE and MPTP on the swim time of SAMP8 mice in the spatial probe experiment of the Morris water maze task.
Each mouse was allowed to swim for 120 seconds and the time spent in each quadrant was recorded with a video tracking system. Data are shown as the mean ± SD (one-way analysis of variance followed by the least significant difference test). **P < 0.01, ***P < 0.001, vs. SE + NS group; #P < 0.05, ###P < 0.001, vs. EE + NS group; †P < 0.05, vs. SE + MPTP group. EE: Enriched environment; SE: standard environment; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NS: normal saline; SAMP8: senescence-accelerated mouse prone 8.
Effect of EE on the levels of GAP-43 mRNA in the substantia nigra of MPTP-treated mice
Compared with the SE + NS group, GAP-43 mRNA levels were significantly increased in the EE + NS group (P < 0.05). MPTP resulted in a significant increase in GAP-43 mRNA levels both in the EE + MPTP group (P < 0.01) and the SE + MPTP group (P < 0.01). Compared with the SE + MPTP group, mRNA levels of GAP-43 were higher in the EE + MPTP group (P < 0.01; Figure 3).
Figure 3.
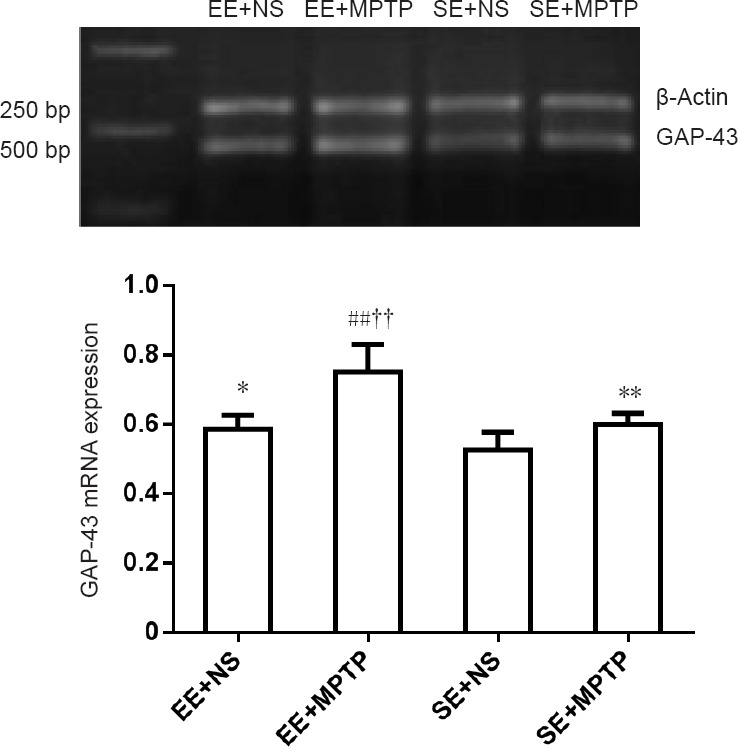
Effects of EE and MPTP on the relative level of GAP-43 mRNA in the substantia nigra of SAMP8 mice.
GAP-43 mRNA levels are expressed as the ratio of the absorbance value of GAP-43 mRNA to that of β-actin mRNA. Data are shown as the mean ± SD (one-way analysis of variance followed by the least significant difference test). *P < 0.05, **P < 0.01, vs. SE + NS group; ##P < 0.01, vs. EE + NS group; ††P < 0.01, vs. SE + MPTP group. EE: Enriched environment; SE: standard environment; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NS: normal saline; SAMP8: senescence-accelerated mouse prone 8.
Effect of EE on GAP-43 immunoreactivity (GAP-43-ir) in the substantia nigra of MPTP-treated mice
Compared with the SE + NS group, GAP-43-ir was significantly increased in the EE + NS group (P < 0.01). GAP-43-ir was significantly greater in the EE + MPTP group compared with the EE + NS group (P < 0.01) and was significantly greater in the SE + MPTP group compared with the SE + NS group (P < 0.05). Compared with the SE + MPTP group, GAP-43-ir was greater in the EE + MPTP group (P < 0.01; Figure 4).
Figure 4.
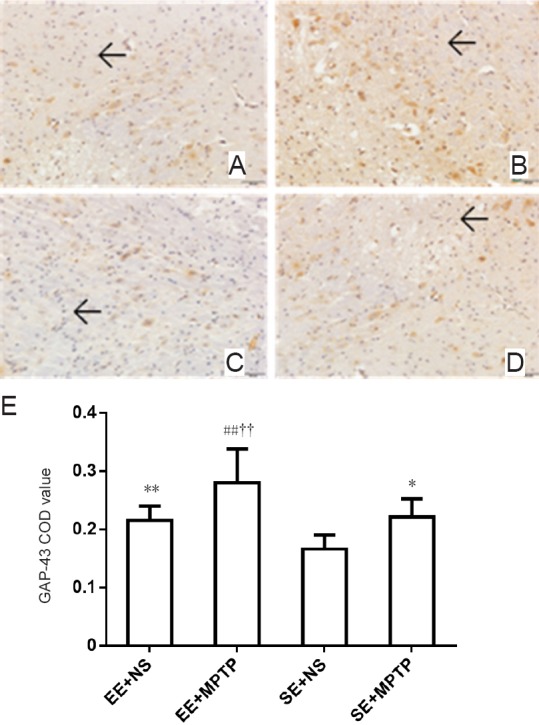
Effects of EE and MPTP on GAP-43-ir in the substantia nigra of SAMP8 mice.
(A–D) GAP-43-ir in the substantia nigra (original magnification, 200×). (A) EE + NS group, (B) EE + MPTP group, (C) SE + NS group, (D) SE + MPTP group. GAP-43-ir was intense in the EE + NS group compared with the SE + NS group. GAP-43-ir was higher in the EE + MPTP group compared with the SE + MPTP group. GAP-43-ir was higher in the EE + MPTP group than in the EE + NS group and GAP-43-ir was greater in the SE + MPTP group than in the SE + NS group. Arrows indicate GAP-43-ir. Scale bar: 50 µm. (E) Quantitation of GAP-43-ir. Data are expressed as the mean ± SD (one-way analysis of variance followed by the least significant difference test). **P < 0.01, vs. SE + NS group; ##P < 0.01, vs. EE + NS group; ††P < 0.01, vs. SE + MPTP group. COD: Corrected optical density; EE: enriched environment; SE: standard environment; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NS: normal saline; SAMP8: senescence-accelerated mouse prone 8; GAP-43-ir: growth associated protein-43 immunoreactivity.
Effect of EE on GAP-43 protein levels in the substantia nigra of MPTP-treated mice
Compared with the SE + NS group, GAP-43 protein levels were significantly higher in the EE + NS group (P < 0.05). Compared with the EE + NS group, GAP-43 protein levels were significantly higher in the EE + MPTP group (P < 0.01). Compared with the SE + NS group, GAP-43 protein levels were significantly greater in the SE + MPTP group (P < 0.05). Compared with the SE + MPTP group, GAP43 protein levels were significantly increased in the EE + MPTP group (P < 0.01; Figure 5).
Figure 5.
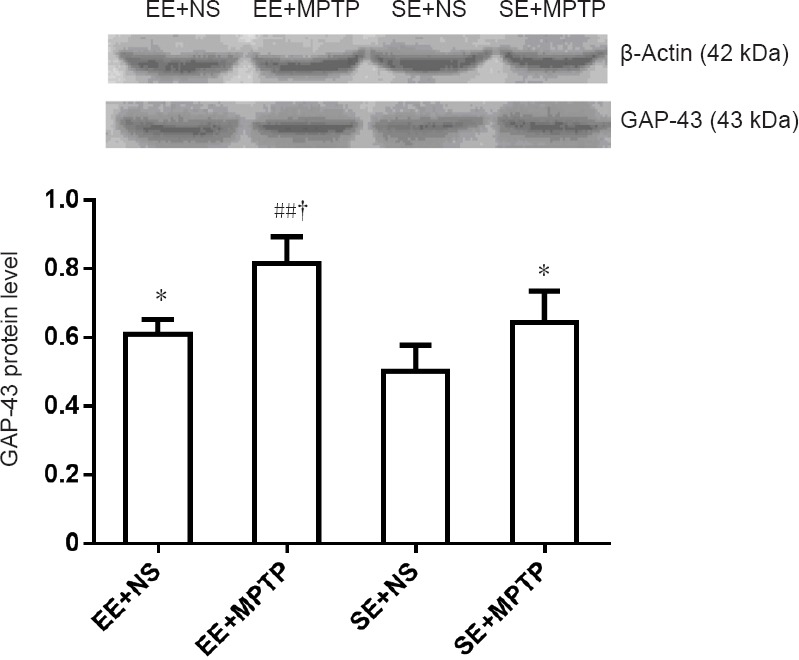
Effects of EE and MPTP on GAP-43 protein levels in the substantia nigra of SAMP8 mice.
GAP-43 protein levels are expressed as the ratio of the absorbance value of GAP-43 to that of β-actin. Data are expressed as the mean ± SD (one-way analysis of variance followed by the least significant difference test). *P < 0.05, vs. SE + NS group; ##P < 0.01, vs. EE + NS group; †P < 0.05, vs. SE + MPTP group. EE: Enriched environment; SE: standard environment; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; NS: normal saline; SAMP8: senescence-accelerated mouse prone 8; GAP-43: growth associated protein-43.
Discussion
With the number of PD patients increasing, an effective treatment method is desperately needed. Several studies have focused on new neuroprotective agents that may prevent dopaminergic cell loss (Reglódi et al., 2006, 2017; Song et al., 2012). Alternatively, there is evidence that exposure to EE improves neuroprotective effects (Young et al., 1999; Beauquis et al., 2010; Obiang et al., 2011), improves plasticity (Hosseiny et al., 2015; Mahati et al., 2016), reduces anxiety (Yuan et al., 2009b; Lach et al., 2016), ameliorates memory impairments (Yuan et al., 2013; Takuma et al., 2014; Wang et al., 2016; Bhagya et al., 2017), improves cognitive effects (Schreiber et al., 2014), and improves recuperation in animal models of PD (Requejo et al., 2018). EE refers to a living environment in which animals are kept in a large cage with running wheels, platforms, tunnels, and toys that provide increased opportunities for enhanced motor, cognitive and sensory stimulation (White et al., 2015; Huang et al., 2016). EE can dramatically induce behavioral and biochemical changes (van Praag et al., 2000; Kazlauckas et al., 2011), and influence the development and function of neural circuits (O’Connor et al., 2014). Physical exercise and sport can attenuate neuroinflammation (Spielman et al., 2016) and appear to be protective later in life (Lamotte et al., 2015; Shih et al., 2016). Appropriate environmental stimuli may help in the treatment of brain and behavioral dysfunction related to imbalances in the brain (Tomas et al., 2015). Although perinatal asphyxia and hypoxia can lead to permanent brain injuries (Placha et al., 2016; Rainaldi and Perlman, 2016), EE in early life can rescue dopaminergic cells after treatment of adult rats with 6-OHDA (Jungling et al., 2017).
Our previous study showed that EE can upregulate the mRNA and protein levels of brain-derived neurotrophic factor in the hippocampus (Yuan et al., 2012). The present study provides evidence that EE can improve cognitive ability and also increase the expression of GAP-43 in the substantia nigra of SAMP8 mice. This study assessed the cognitive ability of SAMP8 mice in different environments using the MWM test. Compared with the SE + NS group, escape latencies were shorter in the EE + NS group. Compared with the SE + MPTP group, escape latencies were shorter in the EE + MPTP group over four consecutive days. In the memory retention test, the percentage of time spent in the target quadrant was longer and the percentage of time spent in the opposite quadrant was shorter in the EE + NS group compared with the SE + NS group. Compared with the SE + MPTP group, the percentage of time spent in the target quadrant was longer and in the opposite quadrant was shorter in the EE + MPTP group. All these behavioral results showed that learning and retention performance was better in the EE group compared with the SE group, which indicated that EE might improve the cognitive abilities of mice in the water maze environment (Garthe et al., 2016). Compared with the SE group, greater space in the EE offered more opportunities for activity and more animals in a cage provided more social stimulation and mice also had enhanced opportunities for voluntary exercise on the running wheels. EE has a wide impact at the behavioral level and on molecular networks in the animal brain. Neuroplasticity changes are strongly associated with neurotrophic factors, neurotransmitters, neuronal patterns, brain network characteristics, and behavioral changes in cognition (Kotloski and Sutula, 2015).
The present study examined mRNA and protein levels of GAP-43, and GAP-43-ir. Compared with the SE + NS group, GAP-43 mRNA levels were increased in the EE + NS group. Compared with the SE + MPTP group, GAP-43 mRNA levels were higher in the EE + MPTP group. Compared with the SE + NS group, GAP-43-ir was intense in the EE + NS group. Compared with the SE + MPTP group, corrected optical density values of GAP-43-ir were significantly increased in the EE + MPTP group. Furthermore, compared with the SE + NS group, relative GAP43 protein levels were higher in the EE + NS group. Compared with the SE + MPTP group, GAP-43 protein levels were higher in the EE + MPTP group. All these results showed that EE upregulated the expression of GAP43 in the substantia nigra. Development of the brain and learning is accompanied by changes in the morphological structure of neurons and related molecular pathways. GAP-43 is a marker of neuronal elongation and synaptic formation that is highly expressed in the early stage of nervous system development (Rosskothen-Kuhl and Illing, 2014). Upregulation of GAP-43 and IGF-1 was found following treadmill running (Tsai et al., 2013). Voluntary exercise increased the levels of GAP-43 and enhanced recovery of cognitive function induced by traumatic injury (Gu et al., 2014). GAP-43 expression is strongest and most widespread during development (Jacobson et al., 1986), when the nervous system structure is subjected to extensive changes and rearrangements. The induction of GAP-43 was identified as an early event of neurogenesis (Chabot et al., 2013), and is strongly implicated in memory performance (Baumgartel and Mansuy, 2012).
In summary, EE improved learning and memory performance of SAMP8 mice and up-regulated GAP-43 mRNA and protein levels in the substantia nigra. This study provides a theoretical foundation of GAP-43 up-regulation being part of the mechanism for EE protection against MPTP-induced neuronal damage. However, the expression of GAP-43 in the striatum, hippocampus and cortex was not determined in the current study, and we will explore the expression of GAP-43 in different brain regions in future investigations.
Footnotes
Conflicts of interest: None declared.
Financial support: This study was supported by a grant from the Health Department of Hebei Province of China, No. 20120056 and No. 20140314; the Funding Project for Introduced Abroad Study Personnel of Hebei Province of China, No. C2011003039. The conception, design, execution, and analysis of experiments, as well as the preparation of and decision to publish this manuscript, were made independent of any funding organization.
Institutional review board statement: All animal experiments were approved by Animal Ethics Committee of Hebei Medical University in China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by a grant from the Health Department of Hebei Province of China, No. 20120056, 20140314; the Funding Project for Introduced Abroad Study Personnel of Hebei Province of China, No. C2011003039.
(Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Aigner L, Caroni P. Depletion of 43-kD growth-associated protein in primary sensory neurons leads to diminished formation and spreading of growth cones. J Cell Biol. 1993;123:417–429. doi: 10.1083/jcb.123.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgartel K, Mansuy IM. Neural functions of calcineurin in synaptic plasticity and memory. Learn Mem. 2012;19:375–384. doi: 10.1101/lm.027201.112. [DOI] [PubMed] [Google Scholar]
- 3.Beauquis J, Roig P, De Nicola AF, Saravia F. Short-term environmental enrichment enhances adult neurogenesis, vascular network and dendritic complexity in the hippocampus of type 1 diabetic mice. PLoS One. 2010;5:e13993. doi: 10.1371/journal.pone.0013993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benka Wallén M, Franzén E, Nero H, Hagströmer M. Levels and patterns of physical activity and sedentary behavior in elderly people with mild to moderate Parkinson disease. Phys Ther. 2015;95:1135–1141. doi: 10.2522/ptj.20140374. [DOI] [PubMed] [Google Scholar]
- 5.Bergman H, Deuschl G. Pathophysiology of Parkinson's disease: from clinical neurology to basic neuroscience and back. Mov Disord. 2002;17(Suppl 3):S28–40. doi: 10.1002/mds.10140. [DOI] [PubMed] [Google Scholar]
- 6.Bhagya VR, Srikumar BN, Veena J, Shankaranarayana Rao BS. Short-term exposure to enriched environment rescues chronic stress-induced impaired hippocampal synaptic plasticity, anxiety, and memory deficits. J Neurosci Res. 2017;95:1602–1610. doi: 10.1002/jnr.23992. [DOI] [PubMed] [Google Scholar]
- 7.Campêlo CLC, Santos JR, Silva AF, Dierschnabel AL, Pontes A, Cavalcante JS, Ribeiro AM, Silva RH. Exposure to an enriched environment facilitates motor recovery and prevents short-term memory impairment and reduction of striatal BDNF in a progressive pharmacological model of parkinsonism in mice. Behav Brain Res. 2017;328:138–148. doi: 10.1016/j.bbr.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 8.Chabot A, Meus MA, Hertig V, Duquette N, Calderone A. The neurogenic response of cardiac resident nestin(+) cells was associated with GAP43 upregulation and abrogated in a setting of type I diabetes. Cardiovasc Diabetol. 2013;12:114. doi: 10.1186/1475-2840-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang WH, Park CH, Kim DY, Shin YI, Ko MH, Lee A, Jang SY, Kim YH. Cerebrolysin combined with rehabilitation promotes motor recovery in patients with severe motor impairment after stroke. BMC Neurol. 2016;16:31. doi: 10.1186/s12883-016-0553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collier TJ, Lipton J, Daley BF, Palfi S, Chu Y, Sortwell C, Bakay RA, Sladek JR, Jr, Kordower JH. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol Dis. 2007;26:56–65. doi: 10.1016/j.nbd.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbaz A, Carcaillon L, Kab S, Moisan F. Epidemiology of Parkinson's disease. Rev Neurol (Paris) 2016;172:14–26. doi: 10.1016/j.neurol.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev. 1998;22:1–20. doi: 10.1016/s0149-7634(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 13.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garthe A, Roeder I, Kempermann G. Mice in an enriched environment learn more flexibly because of adult hippocampal neurogenesis. Hippocampus. 2016;26:261–271. doi: 10.1002/hipo.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorup D, Bohacek I, Milicevic T, Pochet R, Mitrecic D, Kriz J, Gajovic S. Increased expression and colocalization of GAP43 and CASP3 after brain ischemic lesion in mouse. Neurosci Lett. 2015;597:176–182. doi: 10.1016/j.neulet.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 16.Gu YL, Zhang LW, Ma N, Ye LL, Wang de X, Gao X. Cognitive improvement of mice induced by exercise prior to traumatic brain injury is associated with cytochrome c oxidase. Neurosci Lett. 2014;570:86–91. doi: 10.1016/j.neulet.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Hindle JV, Hurt CS, Burn DJ, Brown RG, Samuel M, Wilson KC, Clare L. The effects of cognitive reserve and lifestyle on cognition and dementia in Parkinson's disease--a longitudinal cohort study. Int J Geriatr Psychiatry. 2016;31:13–23. doi: 10.1002/gps.4284. [DOI] [PubMed] [Google Scholar]
- 18.Hosseiny S, Pietri M, Petit-Paitel A, Zarif H, Heurteaux C, Chabry J, Guyon A. Differential neuronal plasticity in mouse hippocampus associated with various periods of enriched environment during postnatal development. Brain Struct Funct. 2015;220:3435–3448. doi: 10.1007/s00429-014-0865-y. [DOI] [PubMed] [Google Scholar]
- 19.Huang J, Wu FF, He X, Li JP, Yan XX, Pan AH, Li ZY. Effects of enriched environment on immature neurons in piriform cortex of a rat model of vascular dementia. Zhongguo Zuzhi Gongcheng Yanjiu. 2016;20:4006–4012. [Google Scholar]
- 20.Jacobson RD, Virag I, Skene JH. A protein associated with axon growth, GAP-43, is widely distributed and developmentally regulated in ratc CNS. J Neurosci. 1986;6:1843–1855. doi: 10.1523/JNEUROSCI.06-06-01843.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jungling A, Reglodi D, Karadi ZN, Horvath G, Farkas J, Gaszner B, Tamas A. Effects of postnatal enriched environment in a model of Parkinson's disease in adult rats. Int J Mol Sci. 2017;18:E406. doi: 10.3390/ijms18020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakkar AK, Dahiya N. Management of Parkinsons disease: Current and future pharmacotherapy. Eur J Pharmacol. 2015;750:74–81. doi: 10.1016/j.ejphar.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Kawamata T, Akiguchi I, Yagi H, Irino M, Sugiyama H, Akiyama H, Shimada A, Takemura M, Ueno M, Kitabayashi T, Ohnishi K, Seriu N, Higuchi K, Hosokawa M, Takeda T. Neuropathological studies on strains of senescence-accelerated mice (SAM) with age-related deficits in learning and memory. Exp Gerontol. 1997;32:161–169. doi: 10.1016/s0531-5565(96)00063-0. [DOI] [PubMed] [Google Scholar]
- 24.Kazlauckas V, Pagnussat N, Mioranzza S, Kalinine E, Nunes F, Pettenuzzo L, Souza DO, Portela LV, Porciúncula LO, Lara DR. Enriched environment effects on behavior, memory and BDNF in low and high exploratory mice. Physiol Behav. 2011;102:475–480. doi: 10.1016/j.physbeh.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Kotloski RJ, Sutula TP. Environmental enrichment: evidence for an unexpected therapeutic influence. Exp Neurol. 2015;264:121–126. doi: 10.1016/j.expneurol.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Lach G, Bicca MA, Hoeller AA, Santos EC, Costa AP, de Lima TC. Short-term enriched environment exposure facilitates fear extinction in adult rats: The NPY-Y1 receptor modulation. Neuropeptides. 2016;55:73–78. doi: 10.1016/j.npep.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 27.LaHue SC, Comella CL, Tanner CM. The best medicine? The influence of physical activity and inactivity on Parkinson's disease. Mov Disord. 2016;31:1444–1454. doi: 10.1002/mds.26728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamotte G, Rafferty MR, Prodoehl J, Kohrt WM, Comella CL, Simuni T, Corcos DM. Effects of endurance exercise training on the motor and non-motor features of Parkinson's disease: a review. J Parkinsons Dis. 2015;5:21–41. doi: 10.3233/JPD-140425. [DOI] [PubMed] [Google Scholar]
- 29.Laviola G, Hannan AJ, Macri S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol Dis. 2008;31:159–168. doi: 10.1016/j.nbd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Wang YY, Liu L, Wang QD, Yuan ZY, Zhang ZX, Gu P, Wang MW. Damage to the nigrostriatal system in the MPTP-treated SAMP8 mouse. Neurosci Lett. 2008;448:184–188. doi: 10.1016/j.neulet.2008.10.070. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, Wang MW, Gu P, Ma QY, Wang YY, Geng Y, Yuan ZY, Cui DS, Zhang ZX, Ma L, Zhang BH, Zhou MG, Zhu AP. Microglial activation and age-related dopaminergic neurodegeneration in MPTP-treated SAMP8 mice. Brain Res. 2010;1345:213–220. doi: 10.1016/j.brainres.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 32.Mahati K, Bhagya V, Christofer T, Sneha A, Shankaranarayana Rao BS. Enriched environment ameliorates depression-induced cognitive deficits and restores abnormal hippocampal synaptic plasticity. Neurobiol Learn Mem. 2016;134(Pt B):379–391. doi: 10.1016/j.nlm.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Mo C, Hannan AJ, Renoir T. Environmental factors as modulators of neurodegeneration: insights from gene-environment interactions in Huntington's disease. Neurosci Biobehav Rev. 2015;52:178–192. doi: 10.1016/j.neubiorev.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor AM, Burton TJ, Leamey CA, Sawatari A. The use of the puzzle box as a means of assessing the efficacy of environmental enrichment. J Vis Exp. 2014:e52225. doi: 10.3791/52225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obiang P, Maubert E, Bardou I, Nicole O, Launay S, Bezin L, Vivien D, Agin V. Enriched housing reverses age-associated impairment of cognitive functions and tPA-dependent maturation of BDNF. Neurobiol Learn Mem. 2011;96:121–129. doi: 10.1016/j.nlm.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Ohashi S, Mori A, Kurihara N, Mitsumoto Y, Nakai M. Age-related severity of dopaminergic neurodegeneration to MPTP neurotoxicity causes motor dysfunction in C57BL/6 mice. Neurosci Lett. 2006;401:183–187. doi: 10.1016/j.neulet.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Placha K, Luptakova D, Baciak L, Ujhazy E, Juranek I. Neonatal brain injury as a consequence of insufficient cerebral oxygenation. Neuro Endocrinol Lett. 2016;37:79–96. [PubMed] [Google Scholar]
- 38.Pringsheim T, Jette N, Frolkis A, Steeves TD. The prevalence of Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2014;29:1583–1590. doi: 10.1002/mds.25945. [DOI] [PubMed] [Google Scholar]
- 39.Rainaldi MA, Perlman JM. Pathophysiology of birth asphyxia. Clin Perinatol. 2016;43:409–422. doi: 10.1016/j.clp.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Reglódi D, Tamás A, Lengvári I, Toth G, Szalontay L, Lubics A. Comparative study of the effects of PACAP in young, aging, and castrated males in a rat model of Parkinson's disease. Ann N Y Acad Sci. 2006;1070:518–524. doi: 10.1196/annals.1317.072. [DOI] [PubMed] [Google Scholar]
- 41.Reglódi D, Renaud J, Tamas A, Tizabi Y, Socias SB, Del-Bel E, Raisman-Vozari R. Novel tactics for neuroprotection in Parkinson's disease: Role of antibiotics, polyphenols and neuropeptides. Prog Neurobiol. 2017;155:120–148. doi: 10.1016/j.pneurobio.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Requejo C, Ruiz-Ortega JA, Cepeda H, Sharma A, Sharma HS, Ozkizilcik A, Tian R, Moessler H, Ugedo L, Lafuente JV. Nanodelivery of cerebrolysin and rearing in enriched environment induce neuroprotective effects in a preclinical rat model of Parkinson's disease. Mol Neurobiol. 2018;55:286–299. doi: 10.1007/s12035-017-0741-x. [DOI] [PubMed] [Google Scholar]
- 43.Rosskothen-Kuhl N, Illing RB. Gap43 transcription modulation in the adult brain depends on sensory activity and synaptic cooperation. PLoS One. 2014;9:e92624. doi: 10.1371/journal.pone.0092624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schreiber S, Lin R, Haim L, Baratz-Goldstien R, Rubovitch V, Vaisman N, Pick CG. Enriched environment improves the cognitive effects from traumatic brain injury in mice. Behav Brain Res. 2014;271:59–64. doi: 10.1016/j.bbr.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 45.Shih IF, Liew Z, Krause N, Ritz B. Lifetime occupational and leisure time physical activity and risk of Parkinson's disease. Parkinsonism Relat Disord. 2016;28:112–117. doi: 10.1016/j.parkreldis.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song JX, Sze SC, Ng TB, Lee CK, Leung GP, Shaw PC, Tong Y, Zhang YB. Anti-Parkinsonian drug discovery from herbal medicines: what have we got from neurotoxic models? J Ethnopharmacol. 2012;139:698–711. doi: 10.1016/j.jep.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 47.Spielman LJ, Little JP, Klegeris A. Physical activity and exercise attenuate neuroinflammation in neurological diseases. Brain Res Bull. 2016;125:19–29. doi: 10.1016/j.brainresbull.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 48.Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of senescence. Exp Gerontol. 1997;32:105–109. doi: 10.1016/s0531-5565(96)00036-8. [DOI] [PubMed] [Google Scholar]
- 49.Takuma K, Maeda Y, Ago Y, Ishihama T, Takemoto K, Nakagawa A, Shintani N, Hashimoto H, Baba A, Matsuda T. An enriched environment ameliorates memory impairments in PACAP-deficient mice. Behav Brain Res. 2014;272:269–278. doi: 10.1016/j.bbr.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Tomas D, Prijanto AH, Burrows EL, Hannan AJ, Horne MK, Aumann TD. Environmental modulations of the number of midbrain dopamine neurons in adult mice. J Vis Exp. 2015:52329. doi: 10.3791/52329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai SW, Tung YT, Chen HL, Shen CJ, Chuang CH, Tang TY, Chen CM. Treadmill running upregulates the expression of acetylcholine receptor in rat gastrocnemius following botulinum toxin A injection. J Orthop Res. 2013;31:125–131. doi: 10.1002/jor.22180. [DOI] [PubMed] [Google Scholar]
- 52.van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Chen A, Wu H, Ye M, Cheng H, Jiang X, Wang X, Zhang X, Wu D, Gu X, Shen F, Shan C, Yu D. Enriched environment improves post-stroke cognitive impairment in mice by potential regulation of acetylation homeostasis in cholinergic circuits. Brain Res. 2016;1650:232–242. doi: 10.1016/j.brainres.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 54.White JH, Bartley E, Janssen H, Jordan LA, Spratt N. Exploring stroke survivor experience of participation in an enriched environment: a qualitative study. Disabil Rehabil. 2015;37:593–600. doi: 10.3109/09638288.2014.935876. [DOI] [PubMed] [Google Scholar]
- 55.Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol. 2011;26(Suppl 1):S1–58. doi: 10.1007/s10654-011-9581-6. [DOI] [PubMed] [Google Scholar]
- 56.Wood LD, Neumiller JJ, Setter SM, Dobbins EK. Clinical review of treatment options for select nonmotor symptoms of Parkinson's disease. Am J Geriatr Pharmacother. 2010;8:294–315. doi: 10.1016/j.amjopharm.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448–453. doi: 10.1038/7449. [DOI] [PubMed] [Google Scholar]
- 58.Yuan Z, Wang M, Yan B, Gu P, Jiang X, Yang X, Cui D. An enriched environment improves cognitive performance in mice from the senescence-accelerated prone mouse 8 strain: Role of upregulated neurotrophic factor expression in the hippocampus. Neural Regen Res. 2012;7:1797–1804. doi: 10.3969/j.issn.1673-5374.2012.23.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuan ZY, Gu P, Liu L. Effects of MPTP on spatial learning and memory ability and dopaminergic neurons in Nigra of senescence accelerated prone8 mice. Dier Junyi Daxue Xuebao. 2008;11:1337–1340. [Google Scholar]
- 60.Yuan ZY, Jiang XM, Gu P, Wang YX, Wang MW. Effects of different feeding environments on learning and memory ability and hippocampal synapsin I in senescence accelerated-prone mice. Zhongguo Laonian Xue Zazhi. 2013;33:4196–4199. [Google Scholar]
- 61.Yuan ZY, Gu P, Liu L, Cui DS, Liu J, Wang YY. Effect of enriched environment on emotional behaviour in senescence accelerated-prone mice 8. Disi Junyi Daxue Xuebao. 2009a;30:2231–2234. [Google Scholar]
- 62.Yuan ZY, Gu P, Liu L, Wang YY, Liu J, Cui DS, Geng Y, Zhang ZX, Zhu AP, Ma L, Wang MW. Neuroprotective effects of enriched environment in MPTP-treated SAMP8 mice. Neurosci Lett. 2009b;454:6–10. doi: 10.1016/j.neulet.2009.02.058. [DOI] [PubMed] [Google Scholar]


