Keywords: nerve regeneration, myelin growth inhibitors, myelin-associated glycoprotein, MAG-Fc, cell culture, receptors for myelin-associated glycoprotein, neuro-2a cell line, RhoA/ROCK signaling pathways, neurite outgrowth, neural regeneration
Abstract
Myelin-associated glycoprotein (MAG) inhibits the growth of neurites from nerve cells. Extraction and purification of MAG require complex operations; therefore, we attempted to determine whether commercially available MAG-Fc can replace endogenous MAG for research purposes. Immunofluorescence using specific antibodies against MAG, Nogo receptor (NgR) and paired immunoglobulin-like receptor B (PirB) was used to determine whether MAG-Fc can be endocytosed by neuro-2a cells. In addition, neurite outgrowth of neuro-2a cells treated with different doses of MAG-Fc was evaluated. Enzyme linked immunosorbent assays were used to measure RhoA activity. Western blot assays were conducted to assess Rho-associated protein kinase (ROCK) phosphorylation. Neuro-2a cells expressed NgR and PirB, and MAG-Fc could be endocytosed by binding to NgR and PirB. This activated intracellular signaling pathways to increase RhoA activity and ROCK phosphorylation, ultimately inhibiting neurite outgrowth. These findings not only verify that MAG-Fc can inhibit the growth of neural neurites by activating RhoA signaling pathways, similarly to endogenous MAG, but also clearly demonstrate that commercial MAG-Fc is suitable for experimental studies of neurite outgrowth.
Introduction
Myelin-associated glycoprotein (MAG, Siglec-4) is a protein of the Siglec family and a functional ligand of Nogo-66 receptors. MAG is a membranous glycoprotein produced by Schwann cells in the peripheral nervous system and by oligodendrocytes in the central nervous system and is selectively localized in myelin near the surface of axons (Quarles, 2007; Akbik et al., 2012). The special location of MAG on cell membranes indicates the functional interaction of glia and axons both in the peripheral nervous system and the central nervous system. Many published studies on MAG have focused on its ability to inhibit axonal regeneration (Fujita et al., 2011; Geoffroy and Zheng, 2014; He et al., 2016). Notably, a study by Liu et al. (2002) demonstrated that the inhibitory effect of MAG on neuritogenesis is mediated by its binding to Nogo receptors (NgR).
MAG and two other proteins, oligodendrocyte myelin glycoprotein and neurite outgrowth inhibitor (Nogo), are known as major myelin-associated inhibitors (Filbin, 2006). Although these three inhibitors have distinct structures, all act via binding to NgRs or receptor complexes (Quarles, 2009). It has been verified that NgR receptor complexes include NgR, p75, Lingo-1, and trisialoganglioside GT1b (Cao et al., 2010, 2016). Recently, paired immunoglobulin-like receptor B (PirB), another membranous protein located on neuronal membranes, was also found to contain a binding motif of MAG. The signals generated from the binding of MAG and NgR complexes are mainly transduced through p75 by activating RhoA and related downstream effectors, eventually leading to changes in the actin cytoskeleton that underlies growth cone collapse or repulsion (Niederöst et al., 2002). Therefore, the activation of RhoA and its downstream signaling pathway is critical for the inhibitory action of MAG on the growth of neuronal processes. Commercially available MAG-Fc is a recombinant chimeric protein of MAG and the Fc of IgG. Because of the Fc fragment, it is not clear whether MAG-Fc has the same properties as endogenous MAG. Therefore, we tested the actions of MAG-Fc in neuro-2a cells, a cell line originating from a mouse neuroblastoma, to verify the availability of MAG-Fc in vitro. We also studied the effect and mechanism of MAG-Fc on neuritogenesis.
Materials and Methods
Cell culture
Neuro-2a cells, purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China), were plated in 94-well plates at a density of 1 × 10–4 cells/well and in 6-well plates at a density of 1 × 10–6 cells/well. The cells were cultured in Dulbecco's Modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (ExCell Bio, Auckland, New Zealand), 1% penicillin/streptomycin (Solarbio, Beijing, China), and 2 mM glutamine at 37°C in a humidified 5% CO2 incubator (Thermo, Waltham, MA, USA). The medium was refreshed every three days, and cells were passaged at 80% confluency using 0.25% trypsin (TransGen, Beijing, China).
Immunofluorescence
Immunofluorescence staining of MAG, NgR, PirB, and Rho-associated protein kinase (ROCK) was performed. Briefly, neuro-2a cells in 96-well plates were harvested after culturing for 1 day. Then, the cells were rinsed twice with 0.01 M chilled phosphate buffered saline (PBS), followed by fixation with 4% paraformaldehyde for 30 minutes. The cells were then incubated for 10 minutes with 0.1% TritonX-100 in PBS for membrane permeability. Afterwards, 10% normal serum was used to block non-specific staining. The cells were incubated with primary antibodies for MAG (rabbit anti-MAG, 1:400; Millipore Corporation, Billerica, MA, USA), NgR (rabbit anti-NgR, 1:500; Millipore Corporation), PirB (goat anti-PirB, 1:200; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) or ROCK (rabbit anti-ROCKII, 1:500; Abcam Inc., Cambridge, MA, USA) at 4°C overnight. After washing, donkey anti-rabbit or donkey anti-goat IgG conjugated with Alexa Fluorescence-488 or -594 (1:500; Life Technologies, Carlsbad, CA, USA) was applied to the cells and incubated for 1 hour at room temperature in the dark. The cells were mounted with ProLong-Gold antifade reagent containing DAPI (Invitrogen). Images were captured under an inverted fluorescence microscope (IX71, Olympus, Tokyo, Japan). Six wells were used for each primary antibody. Negative controls incubated with antibody diluents instead of the primary antibody were also included.
Visualization of MAG and its intervention on neurite outgrowth
Commercially available recombinant rat MAG-Fc chimera (R&D System, Minneapolis, MN, USA), which shows the same bioactivity as natural MAG (Vinson et al., 2001), was used as a source of exogenous MAG. The expression and semi-quantification of MAG-Fc in neuro-2a cells were verified using anti-MAG immunofluorescence (mouse anti-MAG monoclonal antibody, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). MAG-Fc was diluted to different working concentrations (5, 10, 20, 30 and 40 nM) with DMEM. MAG-Fc was added to the medium when most of the neuro-2a cells were attached to the well bottom, which occurred by 4 hours after plating.
To detect cytoplasmic internalization of MAG-Fc, cells were harvested at 5, 10, 20, 30, 40, and 50 minutes after addition of MAG-Fc to cells. Immunofluorescence of MAG was quantified by integrated optical density (IOD) using NIH ImageJ software (https://imagej.nih.gov/ij/).
For measurement of neurite outgrowth of neuro-2a cells after MAG treatment, a neurite was defined as a process extending from the cell body that was at least longer than the length of the cell. After 24, 48, and 72 hours of MAG-Fc chimera treatment, neuro-2a cells were fixed with 4% paraformaldehyde and then incubated with a rabbit anti-βIII-tubulin primary antibody (1:1000; Boster Biotechnologies, Inc., Wuhan, China) overnight, at 4°C. Cells were then incubated with a secondary antibody (donkey anti-rabbit IgG conjugated with Alexa Fluorescence-594, 1:500; Life Technologies, Carlsbad, CA, USA) for 1 hour at room temperature to visualize neurite morphology. Neurite outgrowth was measured from immunofluorescence images using ImageJ software. The measurements included the total length of the neurites, the percentage of cells with neurites relative to the total number of cells in each image. Fifteen to twenty images were included for each MAG concentration group. In addition, the best MAG-Fc concentration was chosen upon neurite measurement.
The quantification of neurite outgrowth, the percentage of cells with neurites, and the strength of target protein immunofluorescence were analyzed using ImageJ software (https://imagej.nih.gov/ij/).
Detection of RhoA activity
Detection of RhoA activity was measured by enzyme-linked immunosorbent assay (ELISA). Briefly, neuro-2a cells were plated into 24-well plates, and 20 nM MAG-Fc was added to the medium, as described above. After 5, 10, 20, or 40 minutes of MAG treatment, the whole cell protein was extracted with radioimmunoprecipitation assay (RIPA) buffer, which contains 1% protease inhibitor (freshly added) and 1% phosphatase inhibitor. Protein concentration was measured by the Braford method (Bradford, 1976). The activity of RhoA was measured using a commercial RhoA activity assay ELISA kit (Jingma Bioscientifics, Inc., Shanghai, China). The procedure was followed according to the manufacturer's instructions. The activity of RhoA is expressed as U/g.
Detection of ROCK activation induced MAG-Fc by western blot assay
The function of ROCK after MAG-Fc treatment was evaluated by its phosphorylation and translocation in the cytoplasm of neuro-2a cells. The phosphorylation of ROCK was detected by the appearance of phospho-ROCK bands via western blot assays. The level of ROCK phosphorylation was calculated from the IOD value. Translocation of RhoA/Rho kinase and pathway activation, indicating ROCK activation (Zhang and Jin, 2017), was detected by strong immunofluorescence of ROCK translocating from areas deep in the cytoplasm close to the nucleus toward areas adjacent to the cell membrane.
For sodium dodecyl sulfate-polyacrylamide gel electrophoresis and western blot assays, whole cell protein of neuro-2a cells incubated with 20 nM MAG-Fc (for 5, 10, 20, and 40 minutes) was extracted with RIPA buffer. Thirty micrograms of protein from each group was loaded onto 10% gels and separated by electrophoresis. After transferring the protein from the gel to a polyvinylidene fluoride membrane (Immobilon-P, EMD Millipore Corporation, Billenca, MA USA), the membrane was incubated with a rabbit anti-phospho-ROCK antibody (1:500; Abcam) overnight at 4°C, then with a goat-anti-rabbit IgG secondary antibody labeled with horseradish peroxidase. The membranes were then stained with EasySee Western Blot Kit Luminous fluid (TransGen Biotech, Beijing, China). Positive phospho-ROCK bands were quantified with ImageJ software. The immunofluorescence of phospho-ROCK was assessed as described above.
Statistical analysis
All data are presented as the mean ± SE. Statistical significance was determined using GraphPad Prism 5.0 (Graph-Pad software, La Jolla, CA, USA), using one-way analysis of variance followed by t-test. P < 0.05 was regarded as statistically significant.
Results
Visualization and intracellular distribution of exogenous MAG in cultured neuro-2a cells
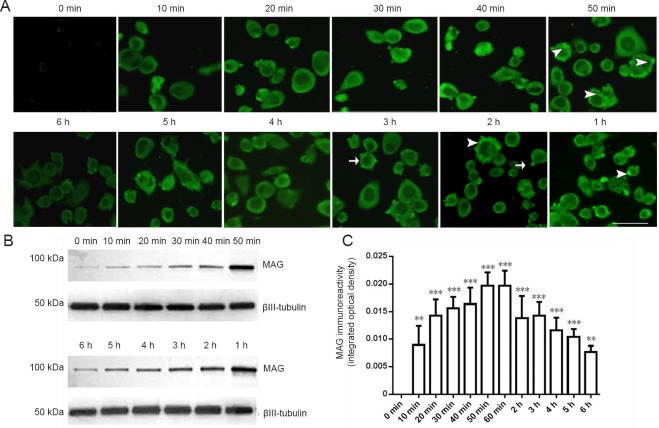
Cultured neuro-2a cells express extremely low levels of MAG (Figure 1). Intracellular MAG immunofluorescence was observed when 20 nM MAG-Fc was added to the cell medium while plating the cells. The cytoplasmic distribution of MAG was clearly identified after incubation with MAG-Fc for minutes to hours (10 minutes to 6 hours). Remarkable MAG staining was observed after 10 minutes of exogenous MAG-Fc treatment. The cytoplasmic MAG immunofluorescence tended to increase with MAG-Fc incubation time. Peak IOD of MAG immunoreactivity was reached 50 and 60 minutes of MAG-Fc treatment (P < 0.05). The IOD value of MAG at 50 and 60 minutes was twice that of controls (P < 0.001). However, longer MAG incubation times did not increase intracellular MAG immunofluorescence further, and MAG staining even decreased for incubation times approaching 6 hours (Figure 1).
Figure 1.
Immunofluorescence of MAG in neuro-2a cells treated with 20 nM MAG-Fc for 10 minutes to 6 hours.
(A) Intracellular level and distribution of MAG detected by immunofluorescence staining (scale bar: 50 μm). Arrowheads: Positive MAG dots in the cytoplasm; arrows: positive MAG dots near the plasma membrane (green: Alexa Fluorescence-488). (B) MAG levels detected by western blotting. (C) MAG immunoreactivity. Data are expressed as the mean ± SE (one-way analysis of variance followed by t-test). **P < 0.01, ***P < 0.001, vs. 0 min (control group). MAG: Myelin-associated glycoprotein; min: minute(s); h: hour(s).
MAG was primarily distributed along cell membranes in a circular manner. As time progressed, the membranous circular appearance of MAG positivity broadened and increased in intensity, and was particularly distinctive after 50 to 60 minutes of MAG treatment. A few localized, strong immunofluorescent MAG-positive dots of various sizes were observed both on the surface of cell membranes and in the cytoplasm (Figure 1). The cytoplasmic visualization of MAG in neuro-2a cells by addition of MAG-Fc to the medium indicated that MAG-Fc was possibly endocytosed through specific MAG receptors on the plasma membrane of neuro-2a cells.
Expression NgR and PirB and their colocalization with MAG
The immunofluorescence of both NgR and PirB was evaluated to verify whether neuro-2a cells expressed NgR and PirB after incubation with MAG-Fc for one hour. The results revealed that neuro-2a cells were positive for both NgR and PirB. Neuro-2a cells cultured for 4 days exhibited relatively weak NgR immunofluorescence and moderate PirB immunoreactivity. In addition, NgR staining was mainly distributed along the plasma membrane and also appeared as strong immunofluorescent foci along the membrane. However, the appearance of PirB staining was different from that of NgR; it was primarily cytoplasmic and diffuse (Figure 2).
Figure 2.

Expression of NgR and PirB in neuro-2a cells with or without MAG treatment.
(A) Cultured neuro-2a cells showed NgR-positive immunofluorescence staining, which mainly appeared along the cell membrane. (B) There was increased NgR expression when MAG-Fc was added to the medium at cell plating. NgR staining was clearly observed in the cytoplasm. (C) Double labeling of NgR and MAG was observed in most of the NgR-positive cells. (D) Cultured neuro-2a cells also exhibited positive PirB immunofluorescence staining. In contrast to NgR, PirB immunoreactivity was primarily distributed in the cytoplasm. (E) The level of PirB staining did not increase further with MAD incubation, and was similar to the pattern observed without MAG-Fc treatment. (F) A few cells were double-labeled for PirB and MAG. Scale bars: 50 μm. Green: Anti-NgR or anti-PirB labeled with Alexa Fluorescence-488; red: anti-MAG labeled with Alexa Fluorescence-594; blue: DAPI counterstaining for nuclei; arrowhead: location of double-labeling for NgR/MAG or PirB/MAG. w/o: Without; MAG: myelin-associated glycoprotein; PirB: paired immunoglobulin-like receptor B; NgR: Nogo receptor.
Incubation of cells with MAG-Fc (20 nM) for 1 hour enhanced the expression of NgR. Co-localization of NgR and MAG was clearly observed both on plasma membranes and in the cytoplasm. Furthermore, the addition of MAG-Fc did not alter the expression of PirB, although there appeared to be more double labeling of PirB and MAG based on immunofluorescence (Figure 2).
These findings indicated that MAG-Fc was endocytosed into neuro-2a cells via binding to its receptor, either NgR or PirB. Endocytosis of MAG-Fc upregulated the expression of NgR but not PirB in neuro-2a cells, or increased the rafting/clustering of NgR on the plasma membrane. Therefore, exogenous MAG may affect neuritogenesis by interacting with NgR and its relevant intracellular signaling pathways.
Effect of MAG-Fc on RhoA activity and phosphorylation of ROCK
Neuro-2a cells displayed a basal level of RhoA activation during culture. After 20 nM MAG-Fc was added to cells for 5, 10, 20, or 40 minutes, the activity of RhoA was increased. After 20 minutes of MAG-Fc treatment, the activity of RhoA was increased to nearly 1.5 times that of the 0 nM MAG-Fc group (P < 0.01). However, RhoA activity dropped to the level observed after 5 minutes of treatment when the MAG-Fc incubation time reached 40 minutes. This result indicated that activation of RhoA by MAG-NgR/PirB binding was a short-acting intracellular response; however, other intracellular events should proceed after RhoA activation.
Similar to the altered activation of RhoA, levels of phosphorylated ROCKII (p-ROCKII) were also increased after treatment with MAG-Fc for 5, 10 and 20 minutes. Moreover, p-ROCK levels were also increased after 40 minutes of MAG treatment, as observed for RhoA, although no significant difference was found (Figure 3). In addition, the immunofluorescence of p-ROCKII also increased in the cytoplasm after MAG-Fc treatment compared with controls. In addition to the increase in p-ROCK immunofluorescence, the staining was also translocated from deep within the cytoplasm toward the cytoplasmic area adjacent to the membrane. Some stronger p-ROCK immunopositive foci were found at random locations adjacent to the membrane (Figure 4). In summary, treatment of neuro-2a cells with exogenous MAG activated RhoA and the phosphorylation of its downstream signaling protein, ROCKII.
Figure 3.

Effects of exogenous MAG treatment on RhoA activity in neuro-2a cells (ELISA assay).
Data are expressed as the mean ± SE (n = 6 per group; one-way analysis of variance followed by t-test). **P < 0.01, ***P < 0.001. ELISA: Enzyme-linked immunosorbent assay; MAG: myelin-associated glycoprotein; ns: not significant; min: minutes.
Figure 4.
Effects of exogenous MAG-Fc on the phosphorylation of ROCK II in neuro-2a cells.
(A, B) Western blot assay showed that after MAG-Fc was administered, increased p-ROCK II levels were observed compared to the control (*P < 0.05, vs. control group), but the increased level dropped after MAG treatment for 40 minutes, although the level remained higher than controls. Data are expressed as the mean ± SE (one-way analysis of variance followed by t-test). (C) Immunofluorescence of cytoplasmic p-ROCK II in the MAG treatment group was generally stronger than that in controls. Some p-ROCK II foci were clearly identified in the cytoplasm, especially in the area adjacent to the membrane (arrows). (a–e) DAPI counterstaining; (f–g) anti-p-ROCK; (k–o) merged images of DAPI (blue) and anti-p-ROCK (green). Scale bar: 50 μm. Green color: Alexa Fluorescence-488; blue color: DAPI counterstaining of nuclei. MAG: myelin-associated glycoprotein; p-ROCK II: phosphorylated Rho-associated protein kinase II; DAPI: 4′,6-diamidino-2-phenylindole; min: minutes.
Effect of exogenous MAG-Fc on neuritogenesis in neuro-2a cells
Because MAG-specific receptors were present in neuro-2a cells, their functional effect on neurite outgrowth was investigated. All neurites were visualized by the immunofluorescence of βIII tubulin. Linear concentrations of MAG-Fc (5, 10, 20 and 40 nM) were added to the medium, and cells were incubated for 24, 48, or 72 hours. The neurite lengths were measured, revealing that neurite outgrowth was inhibited by MAG-Fc treatment. The inhibition of neurite growth also exhibited a linear pattern. The maximum inhibition of neurite growth appeared after 24 hours of treatment with 40 nM MAG-Fc (37.63 ± 1.08 μm), which was 20% shorter than the neurites of the control group (47.03 ± 1.30 μm). Moreover, further incubation with MAG-Fc for 48 hours or 72 hours did not induce any further inhibition on neurite outgrowth (Figure 5B).
Figure 5.
Linear dose effects of MAG-Fc on neurite outgrowth from neuro-2a cells.
(A) Neuro-2a cells treated with a series of MAG-Fc concentrations from 5–40 nM for 24–72 hours showed dose-dependent inhibition of neurite growth based on anti-βIII tubulin (red) immunofluorescence staining (Alexa Fluorescence-594 labeling). Scale bar: 50 μm. (B) The total neurite length of neuro-2a cells was decreased at 24 hours by 5–40 nM MAG treatment; the decrease in neurite length also appeared to be dose-dependent, although there was no significant difference with incubation time. (C) Adding MAG-Fc to neuro-2a cells also decreased the percentage of cells with neurites. The drop in the percentage was clearly observed at 48 and 72 hours of MAG treatment. Data are expressed as the mean ± SE (one-way analysis of variance followed by t-test). *P < 0.05, **P < 0.01, ***P < 0.001, vs. 0 nM group. MAG: Myelin-associated glycoprotein; h: hours.
MAG-Fc treatment also affects neuritogenesis because the number of neurites on neuro-2a cells was decreased upon MAG-Fc treatment. This decrease was seen at all time points of MAG-Fc incubation (Figure 5C). The percentage of cells with neurites began to reduce after treatment for 24 hours with 5 nM and 10 nM MAG-Fc, but a statistically significant decrease was not observed until 24 hours of treatment with 20 or 40 nM MAG-Fc (P < 0.05). Furthermore, at the final time points of MAG-Fc incubation (48 and 72 hours), a percentage decrease in cells with neurites was observed for all MAG-Fc concentrations (Figure 5C).
All of these functional effects of MAG-Fc on neurite outgrowth indicate that neuro-2a cells are susceptible to MAG-Fc. The endocytosis of MAG-Fc was mediated through the same specific receptors as endogenous MAG.
Discussion
The neuro-2a cell line was originally derived from mouse neuroblastoma cells from a spontaneous tumor in the A strain of white mice (Klebe and Ruddle, 1969; LePage et al., 2005). The cells are a type of brain tumor cell, but they carry many inherent morphological and physiological properties that resemble neural stem cells in neuronal development. However, neuronal processes appeared in only a few cultured neuro-2a cells under regular medium supplemented with 10% fetal bovine serum. Moreover, no study has shown that these cells contain the necessary machinery for MAG-Fc interaction, such as a membrane specific receptor for MAG and the related intracellular signaling proteins. Neuro-2a cells act differently to primary cerebellar neurons when used in in vitro cell models (Niederöst et al., 2002). Because of several features under culture conditions, such as the ability to replicate, monolayer growth, homogeneous cellular constituents, and minimal interference with others cells, neuro-2a cells are versatile and more economical than primary neuronal cultures. Therefore, many researchers have chosen neuro-2a cells as a substitute for primary neuron cultures. There are hundreds of publications regarding the use of these cells for different purposes, such as studies of neuronal survival, cytotoxicity, mechanisms of neuronal metabolic dysfunctions, neuronal differentiation, and ligand-receptor interactions (Eom et al., 2015; Nicolas et al., 2015; Wesley et al., 2015; Grimm et al., 2016). However, whether the cell line is susceptible to myelin-associated nerve growth inhibitors has not yet been verified.
Myelin-associated inhibitors of nerve growth include three major molecules: MAG, Nogo-A (Nogo-66), and oligodendrocyte myelin glycoprotein (Filbin, 2003). MAG was the first myelin-derived growth inhibitory protein to be identified, and its inhibitory activity on nerve growth was elucidated in 1994 independently by both the Filbin lab and the McKerracher lab using cell-based and biochemical techniques, respectively (McKerracher and Rosen, 2015). MAG is a transmembrane glycoprotein produced by myelinating glial cells (including oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system) with a higher expression level in the central nervous system (Mukhopadhyay et al., 1994). MAG has been widely used as an inhibitory substrate for neurite growth assays using postnatal and adult neurons (Quarles, 2007). The MAG-Fc used in this study is a commercial product formed by the fusion of MAG with the Fc moiety of human immunoglobulin (Li et al., 2009). Because of its increase in molecular weight and the structural difference between MAG and MAG-Fc fragment, their bioactive properties might not be identical. Here, we show that MAG-Fc imitated the activity of MAG and has the same inhibitory effect on neurite out-growth as MAG. It also interacted mainly with NgR, a receptor complex for MAG, which consists of NgR, LINGO1/TROY, P75NTR and ganglioside GT1b (Domeniconi et al., 2002; Saha et al., 2014). The increased immunofluorescence of NgR after incubation with MAG-Fc for 1 hour may be an effect of MAG on NgR expression, or facilitation of NgR raft/clustering on the membrane.
Both NgR and PirB are specific receptors of MAG (Domeniconi et al., 2002; Wang et al., 2002; Atwal et al., 2008; Filbin, 2008). The binding of MAG and NgR is key to initiating intracellular signaling pathways (Skaper, 2005) and MAG-Fc was able to magnify intracellular signaling strongly associated with the specific receptor complex. Many studies have shown that RhoA/ROCK is one of the main intracellular signaling pathways for NgR (Linseman and Loucks, 2008; Palandri et al., 2015). RhoA is a guanine triphosphatase. The binding of MAG to the NgR complex enables RhoA-GDP to convert to the activated form, RhoA-guanine triphosphatase. Activated RhoA subsequently initiates the phosphorylation of Rho kinase, resulting in growth cone collapse and axonal growth repulsion (Yamashita and Tohyama, 2003). The inhibitory signal from MAG binding to PirB may also be transduced through the Rho-ROCK signaling pathway (Wang et al., 2012). The present study confirmed the existence of the specific receptors of myelin-associated inhibitors on neuro-2a cells. Meanwhile, the maximal endocytosis of MAG-Fc was observed after 50 minutes of adding MAG-Fc. The treatment of neuro-2a cells with MAG-Fc activated RhoA/ROCK signaling and displayed obvious inhibition on neurite outgrowth. The high expression levels of NgR and PirB in neuro-2a cells also provide the structural basis by which exogenous MAG-Fc affects neurite outgrowth in these cells. However, the results from this study on the role of commercial MAG-Fc on neurite growth and its effect on the relevant intracellular signal pathways need to be verified further.
In summary, MAG-Fc can be used as a substitute for endogenous MAG to investigate the intracellular molecular events of myelin-associated inhibitors.
Additional file: Open peer review report 1 (6.3KB, pdf) .
Footnotes
Conflicts of interest: There are no conflicts of interest regarding financial interests, authorship, and copyright.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 81171178. The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Hiroyuki Tanaka, Osaka University of Graduate School of Medicine, Japan.
Funding: This study was supported by the National Natural Science Foundation of China, No. 81171178.
(Copyedited by Yu J, Li CH, Qiu Y, Song LP, Zhao M)
References
- 1.Akbik F, Cafferty WB, Strittmatter SM. Myelin associated inhibitors: a link between injury-induced and experience-dependent plasticity. Exp Neurol. 2012;235:43–52. doi: 10.1016/j.expneurol.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 3.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Dong YX, Xu J, Chu GL, Yang ZH, Liu YM. Spatiotemporal expression of Nogo-66 receptor after focal cerebral ischemia. Neural Regen Res. 2016;11:132–136. doi: 10.4103/1673-5374.175059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Z, Gao Y, Deng K, Williams G, Doherty P, Walsh FS. Receptors for myelin inhibitors: Structures and therapeutic opportunities. Mol Cell Neurosci. 2010;43:1–14. doi: 10.1016/j.mcn.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Eom HS, Park HR, Jo SK, Kim YS, Moon C, Jung U. Ionizing radiation induces neuronal differentiation of Neuro-2a cells via PI3-kinase and p53-dependent pathways. Int J Radiat Biol. 2015;91:585–595. doi: 10.3109/09553002.2015.1029595. [DOI] [PubMed] [Google Scholar]
- 7.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 8.Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci. 2006;361:1565–1574. doi: 10.1098/rstb.2006.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Filbin MT. PirB, a second receptor for the myelin inhibitors of axonal regeneration Nogo66, MAG, and OMgp: implications for regeneration in vivo. Neuron. 2008;60:740–742. doi: 10.1016/j.neuron.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Fujita Y, Endo S, Takai T, Yamashita T. Myelin suppresses axon regeneration by PIR-B/SHP-mediated inhibition of Trk activity. EMBO J. 2011;30:1389–1401. doi: 10.1038/emboj.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geoffroy CG, Zheng B. Myelin-associated inhibitors in axonal growth after CNS injury. Curr Opin Neurobiol. 2014;27:31–38. doi: 10.1016/j.conb.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grimm MO, Regner L, Mett J, Stahlmann CP, Schorr P, Nelke C, Streidenberger O, Stoetzel H, Winkler J, Zaidan SR, Thiel A, Endres K, Grimm HS, Volmer DA, Hartmann T. Tocotrienol affects oxidative stress, cholesterol homeostasis and the amyloidogenic pathway in neuroblastoma cells: consequences for Alzheimer's disease. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He H, Deng K, Siddiq MM, Pyie A, Mellado W, Hannila SS, Filbin MT. Cyclic AMP and polyamines overcome inhibition by myelin-associated glycoprotein through eIF5A-mediated increases in p35 expression and activation of Cdk5. J Neurosci. 2016;36:3079–3091. doi: 10.1523/JNEUROSCI.4012-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klebe RJ, Ruddle FH. Neuroblastoma: Cell culture analysis of a differentiating stem cell system. J Cell Biol. 1969;43:69A. [Google Scholar]
- 15.LePage KT, Dickey RW, Gerwick WH, Jester EL, Murray TF. On the use of neuro-2a neuroblastoma cells versus intact neurons in primary culture for neurotoxicity studies. Crit Rev Neurobiol. 2005;17:27–50. doi: 10.1615/critrevneurobiol.v17.i1.20. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Fu QL, Jing XL, Liao XX, Zeng AH, Xiong Y, Liao XX. Myelin-associated glycoprotein inhibits the neuronal differentiation of neural progenitors. Neuroreport. 2009;20:708–712. doi: 10.1097/WNR.0b013e32832aa942. [DOI] [PubMed] [Google Scholar]
- 17.Linseman DA, Loucks FA. Diverse roles of Rho family GTPases in neuronal development, survival, and death. Front Biosci. 2008;13:657–676. doi: 10.2741/2710. [DOI] [PubMed] [Google Scholar]
- 18.Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 19.McKerracher L, Rosen KM. MAG, myelin and overcoming growth inhibition in the CNS. Front Mol Neurosci. 2015;8:51. doi: 10.3389/fnmol.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 21.Nicolas J, Bovee TF, Kamelia L, Rietjens IM, Hendriksen PJ. Exploration of new functional endpoints in neuro-2a cells for the detection of the marine biotoxins saxitoxin, palytoxin and tetrodotoxin. Toxicol In Vitro. 2015;30:341–347. doi: 10.1016/j.tiv.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Niederöst B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22:10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palandri A, Salvador VR, Wojnacki J, Vivinetto AL, Schnaar RL, Lopez PH. Myelin-associated glycoprotein modulates apoptosis of motoneurons during early postnatal development via NgR/p75(NTR) receptor-mediated activation of RhoA signaling pathways. Cell Death Dis. 2015;6:e1876. doi: 10.1038/cddis.2015.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quarles RH. Myelin-associated glycoprotein (MAG): past, present and beyond. J Neurochem. 2007;100:1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 25.Quarles RH. A hypothesis about the relationship of myelin-associated glycoprotein's function in myelinated axons to its capacity to inhibit neurite outgrowth. Neurochem Res. 2009;34:79–86. doi: 10.1007/s11064-008-9668-y. [DOI] [PubMed] [Google Scholar]
- 26.Saha N, Kolev M, Nikolov DB. Structural features of the Nogo receptor signaling complexes at the neuron/myelin interface. Neurosci Res. 2014;87:1–7. doi: 10.1016/j.neures.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Skaper SD. Neuronal growth-promoting and inhibitory cues in neuroprotection and neuroregeneration. Ann N Y Acad Sci. 2005;1053:376–385. doi: 10.1196/annals.1344.032. [DOI] [PubMed] [Google Scholar]
- 28.Vinson M, Strijbos PJ, Rowles A, Facci L, Moore SE, Simmons DL, Walsh FS. Myelin-associated glycoprotein interacts with ganglioside GT1b. A mechanism for neurite outgrowth inhibition. J Biol Chem. 2001;276:20280–20285. doi: 10.1074/jbc.M100345200. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Xiong Y, Mu D. PirB restricts neuronal regeneration in developing rat brain following hypoxia-ischemia. Mol Med Rep. 2012;6:339–344. doi: 10.3892/mmr.2012.907. [DOI] [PubMed] [Google Scholar]
- 30.Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 31.Wesley UV, Hatcher JF, Dempsey RJ. Sphingomyelin synthase 1 regulates Neuro-2a cell proliferation and cell cycle progression through modulation of p27 expression and Akt signaling. Mol Neurobiol. 2015;51:1530–1541. doi: 10.1007/s12035-014-8829-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamashita T, Tohyama M. The p75 receptor acts as a displacement factor that releases Rho from Rho-GDI. Nat Neurosci. 2003;6:461–467. doi: 10.1038/nn1045. [DOI] [PubMed] [Google Scholar]
- 33.Zhang M, Jin F. 1alpha, 25-Dihydroxyvitamin D3 ameliorates seawater aspiration-induced lung injury by inhibiting the translocation of NF-kappaB and RhoA. Inflammation. 2017;40:832–839. doi: 10.1007/s10753-017-0527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.