Keywords: cerebrovascular disease, stroke, cerebral microbleeds, cognitive impairment, fronto-subcortical circuits, small vessel disease, white matter hyperintensities, lacunar infarct, magnetic resonance imaging, subcortical ischemic vascular disease
Abstract
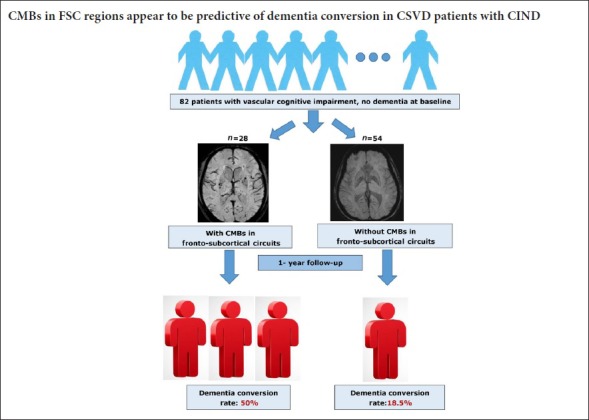
Cerebral small vessel disease (CSVD) is a common etiology of vascular cognitive impairment with no dementia (V-CIND). Studies have revealed that cerebral microbleeds (CMBs), a feature of CSVD, contribute to cognitive impairment. However, the association between CMBs and dementia conversion in individuals with V-CIND is still unclear. Here, we analyzed the predictive role of CMBs in the conversion from V-CIND to dementia in CSVD patients. We recruited and prospectively assessed 85 patients with CSVD and V-CIND. V-CIND was evaluated using a series of comprehensive neuropsychological scales, including the Chinese version of the Montreal Cognitive Assessment and the Clinical Dementia Rating. MRI assessments were used to quantify lacunar infarcts, white matter hyperintensities, CMBs, and medial temporal lobe atrophy. Eighty-two of the 85 patients completed the assessment for dementia conversion at a 1-year follow-up assessment. Multivariate logistic regression analyses were conducted to examine independent clinical and MRI variables associated with dementia conversion. Twenty-four patients (29.3%) had converted to dementia at the 1-year follow-up, and these individuals had significantly more CMBs in the fronto-subcortical circuits. Multivariate logistic regression analyses revealed that the patients with CMBs in the fronto-subcortical circuits (odds ratio = 4.4; 95% confidence interval: 1.602–12.081, P = 0.004) and 5 or more CMBs overall (odds ratio = 17.6, 95% confidence interval: 3.23–95.84, P = 0.001) had a significantly increased risk of dementia at the 1-year follow-up. These findings indicate that CMBs in the fronto-subcortical circuits may be predictive of dementia conversion in CSVD patients with V-CIND, and thus extend the clinical significance of CMBs. This trial was registered with the Chinese Clinical Trial Registry (registration number: ChiCTR1800017077). Protocol version: 1.0.
Introduction
Dementia is a major global health challenge. In China, the overall prevalence of dementia in older adults is approximately 6.0%, where dementia comprises Alzheimer's disease (AD) (4.8%) and vascular dementia (1.2%) (Zhang et al., 2005). In recent years, the concept of vascular cognitive impairment (VCI), particularly “vascular cognitive impairment, no dementia (V-CIND)”, has gained increasing attention (Gorelick et al., 2011). V-CIND and vascular dementia are both associated with increased rates of mortality and institutionalization in early stroke survivors (Brodaty et al., 2010). In addition, early identification and treatment of V-CIND is considered to be important in the prevention of vascular dementia (Gorelick et al., 2011).
V-CIND is associated with an increased risk of dementia, and the rate of dementia conversion in individuals with V-CIND is almost 10-fold higher than that in healthy participants (Mitchell and Shiri-Feshki, 2009). Cerebral small vessel disease (CSVD), which is characterized by lacunar infarcts, moderate-to-severe white matter hyperintensities (WMHs), and cerebral microbleeds (CMBs), is a major contributing factor of VCI (Pantoni, 2010; Blair et al., 2017). Several studies have examined the correlates of V-CIND-related dementia conversion, and found them to include advanced age, lower education level, WMHs, larger infarct volume, and cortical atrophy (Sachdev et al., 2009; Meguro et al., 2012; Mohd Zulkifly et al., 2016).
CMBs are a common feature of CSVD and cerebral amyloid angiopathy (Greenberg et al., 2009). The prevalence of CMBs is 5–6.8% in older adults and 25–30% in patients with ischemic stroke (Jeerakathil et al., 2004). Apart from an increased risk of intracranial hemorrhage, patients with CMBs have a higher risk of stroke recurrence, cognitive impairment, depression, and a decreased quality of life (Werring et al., 2004; Tang et al., 2011a, 2014; Wilson et al., 2016). However, as most previous studies have been cross-sectional, the associations between CMBs and other variables including cognitive impairment are tentative. A 12-month prospective study involving 143 post-stroke V-CIND patients found that the absence of CMBs was significantly associated with cognitive remission (Tang et al., 2011b). However, the rate of dementia conversion of V-CIND patients has not been examined.
Given the multi-factorial etiology of V-CIND, such as large artery atherosclerosis, small vessel disease, and cardioembolism, it may be critical to study CSVD in a homogeneous group of patients. Additionally, the T2*-weighted gradient echo imaging (GRET2*-WI) used in most previous studies has relatively low sensitivity compared with susceptibility-weighted imaging (SWI). Specifically, SWI has a 2/3 higher detection rate for CMBs compared with GRET2*-WI (Nandigam et al., 2009). Most previous studies have not examined independent associations between the location or severity of CMBs and dementia conversion. However, the occurrence of CMBs in specific cognition-related circuits, e.g. the fronto-subcortical circuits (FSC) (Cummings, 1993), may be associated with cognitive decline.
Therefore, we hypothesized that the presence of CMBs in specific cognition-related circuits can predict conversion to dementia. To assess this, we prospectively analyzed the association between CMBs and dementia conversion in patients with CSVD-V-CIND.
Participants and Methods
Participants
In this prospective observational study, inpatients and outpatients were consecutively screened and recruited from the Department of Neurology of Dongguan People's Hospital, Dongguan, China between July 1st, 2015 and August 31st, 2016. The inclusion criteria were as follows: (1) aged 40–85 years; (2) a diagnosis of CSVD according to the recommended criteria (occurrence of a symptomatic lacunar stroke or moderate-to-severe WMHs with or without lacunar infarcts on MRI; Jokinen et al., 2009); (3) completion of a MRI (including SWI) examination at admission or within the 14 days prior to joining the study; (4) modified Rankin Scale (mRS) score of ≤ 2 (Wilson et al., 2002); and (5) met the diagnostic criteria for V-CIND (Gorelick et al., 2010). The exclusion criteria included: (1) dementia as defined by a Clinical Dementia Rating (CDR) score ≥ 1 (Morris, 1997); (2) a recent stroke within 1 month; (3) moderate-to-severe intracranial arterial stenosis or any infarct with a diameter over 15 mm; (4) a history of dementia, depression, or schizophrenia; and (5) a malignant tumor or severe dysfunction of the heart, lung, liver, or kidney.
Patient demographics, clinical characteristics, and past treatments were recorded on standard data collection forms. The study protocol was approved by the Ethics Committee of Dongguan People's Hospital with approval No. 2015012601. All participants provided written informed consent.
Screening and cognitive assessment at baseline
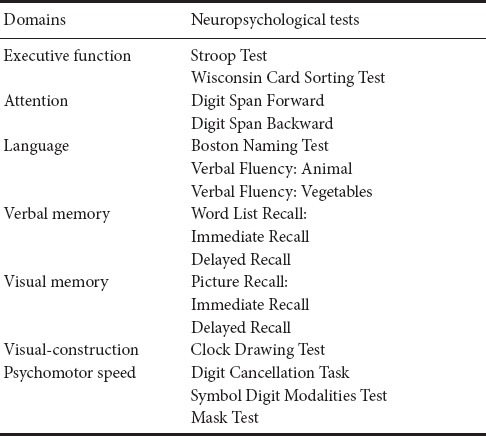
The Chinese version of the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005; Wong et al., 2009) was used to screen CSVD patients for V-CIND, and the CDR was used to exclude those with dementia. Participants with a MoCA total score < 26 and a CDR score < 1 were assessed with the modified version of the Vascular Dementia Battery (Tang et al., 2011b) with seven domains, including executive function, attention, language, verbal memory, visual memory, visual construction, and psycho-motor speed (Table 1). In each test, the cutoff value for “abnormality” was scored as < 1.5 standard deviations (SD) below the mean score (Tang et al., 2011b). Impairment was acknowledged if half or more of the abovementioned tests had one cognitive domain rated as “abnormal” (Tang et al., 2011b). V-CIND was defined as the presence of cognitive impairment affecting at least one cognitive domain, but not meeting the diagnostic criteria of dementia (Gorelick et al., 2011).
Table 1.
Neuropsychological assessment using the modified version of the Vascular Dementia Battery
Cognitive assessment at a 1-year follow-up
We used the same neuropsychological battery described above to measure cognitive performance at a 1-year follow-up assessment. Dementia conversion was defined as cognitive impairment in at least two cognitive domains affecting the activities of daily living, as well as a CDR score ≥ 1 during the follow-up assessments. The primary outcome measure was the dementia conversion of V-CIND at 1-year follow-up. Death and occurrence of strokes during the study period were also recorded.
MRI assessments
MRI assessments, including T1-weighted image (T1WI), T2- weighted image (T2WI), FLAIR, and susceptibility-weighted imaging (SWI), were performed with a 3.0T system (Sonata, Siemens Medical, Erlangen, Germany). Axial SE T1 (repetition time (TR)/ echo time (TE)/excitation = 488/15/1, field of view (FOV) = 230 mm, slice thickness/gap = 6 mm/1 mm, matrix = 256 × 256, time of acquisition = 1 minute and 24.8 seconds) and TSE T2 (TR/TE/excitation = 3992/110/2, turbo factor of 15, FOV = 230 mm, slice thickness/gap = 6 mm/1 mm, matrix of 512 × 512, time of acquisition = 1 minute and 55.8 seconds) images were obtained. SWI was performed using 3D balanced fast field echo (3D-BFFE), with TR/TE/excitation = 27/20/1, FOV = 173 mm × 230 mm, slice thickness/gap = 1.5 mm/0.3 mm, matrix = 256 × 256, time of acquisition = 3 minutes and 44 seconds (3:44), and turbo factor = 15°.
A neurologist (YKC) who was supervised by a neuroradiologist (XWF) assessed the MRI variables as follows: (1) Number of lacunar infarcts. Lacunar infarcts were defined as infarcts with a maximum diameter less than 15 mm on the MRI, with hypointensities on T1WI and hyperintensities on T2WI and FLAIR images. (2) White matter hyperintensities (WMHs). Deep white matter hyperintensities (DWMH) and periventricular hyperintensities (PVH) were scored on FLAIR images separately, based on the 4-point scale reported by Fazekas et al. (1987).(3) Medial temporal lobe atrophy (MTLA). MTLA was measured using Schelten's scale, which consists of standard images reflecting different severities of MTLA on the coronary section of the MRI. The severities range from 0–4 (no atrophy to severe atrophy) (Scheltens et al., 1992). (4) CMBs. CMBs were defined as small (2–10 mm) hypointense lesions with a clear margin in the SWI sequence, excluding symmetric basal ganglia calcification, cavernous malformation, and flow void artifacts generated by pial blood vessels (Cordonnier et al., 2007). CMBs were localized based on the standard atlas of the Microbleed Anatomical Rating Scale (MARS) (Gregoire et al., 2009). CMBs in the FSC regions were defined as CMBs in the frontal lobe, frontal white matter, thalamus, and basal ganglion (Tang et al., 2011c). Examples of CMBs in the FSC regions are shown in Figure 1. The intra-rater reliability of the MRI measurements was good to excellent; the kappa value was 0.81 and 0.83 for WMLs and CMBs, respectively, and the intra-class correlation coefficient was 0.81 for the number of lacunar infarcts.
Figure 1.
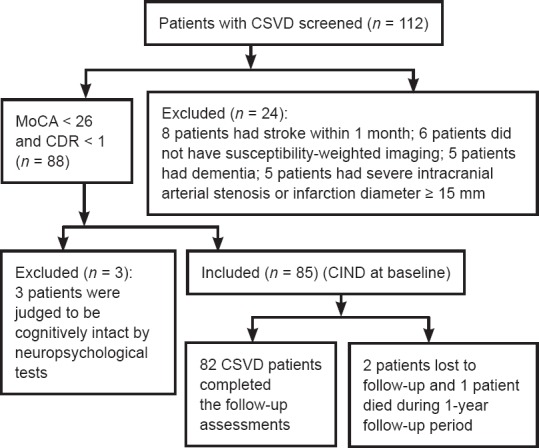
Flow chart of the study.
CSVD: Cerebral small vessel disease; MoCA: Montreal Cognitive Assessment; CIND: cognitive impairment with no dementia; CDR: Clinical Dementia Rating.
Statistical analysis
Data analyses were conducted using SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Demographic, clinical, and MRI variables at baseline were compared between patients with and without dementia conversion using the chi-square test, two independent samples t-test, and Mann-Whitney U test, as appropriate. We conducted multivariate logistic regressionanalyses with a stepwise method to examine the independent contributing factors of dementia conversion at the 1-year follow-up. CMBs and the variables that significantly differed in the univariate analyses served as independent variables. The significance level was set at 0.05 (two-sided).
Results
Quantitative analysis of V-CIND patients
A flowchart of the study design is shown in Figure 1. At baseline, 85 participants with V-CIND were recruited, but 2 participants subsequently dropped out and 1 died at 4 months after recruitment. Finally, 82 eligible participants were included in the analyses.
Univariate comparisons of variables between participants with and without dementia conversion
At the 1-year follow-up assessment, 24 participants with V-CIND (29.3%) had developed dementia. Compared with those who did not develop dementia, these individuals were more likely to smoke (Figure 2 and Table 2), and had a higher number of CMBs in the cortico-subcortical and deep regions of the brain (P < 0.05) (Table 3). They also had more frequent CMBs in the FSCs, including a greater number of CMBs in the FSCs (P < 0.01; Table 3).
Figure 2.
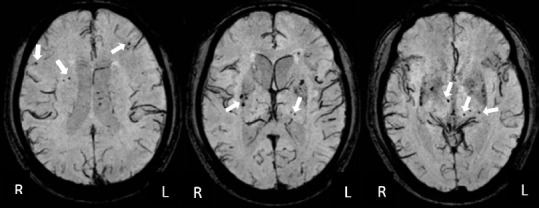
Different sections of susceptibility-weighted images showing CMBs in the FSC region.
A 61-year-old man with a 6-year history of hypertension had CSVD-V-CIND at baseline and had developed dementia at the 1-year follow-up. CMBs (white arrows) in the FSC regions were defined as CMBs within the frontal lobe, frontal white matter, thalamus, and basal ganglion. The total number of FSC CMBs in this patient was 21. The three images represent different MRI sections of the brain. CMBs: Cerebral microbleeds; FSC: fronto-subcortical circuits; CSVD: cerebral small vessel disease; V-CIND: vascular cognitive impairment, but no dementia; R: right; L: left.
Table 2.
Comparison of baseline demographic and clinical variables between patients with and without dementia conversion
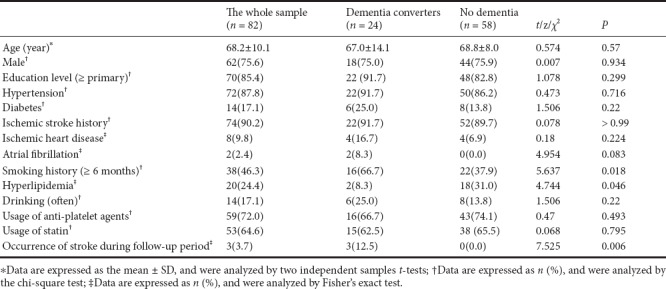
Table 3.
Comparison of MRI variables between patients with and without dementia conversion
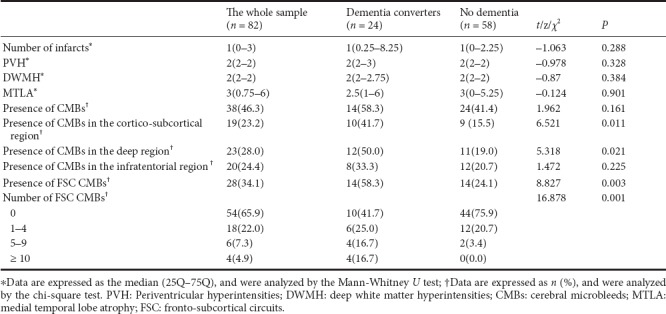
Multivariate logistic regression analysis of dementia conversion
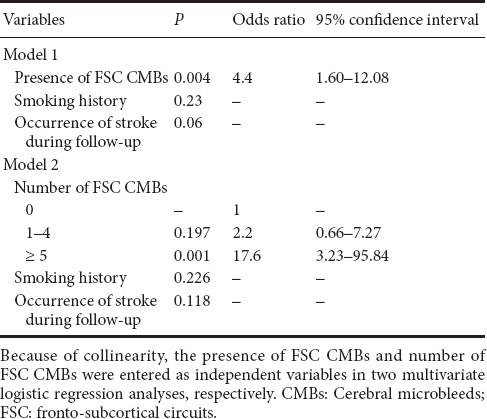
We included the presence of CMBs in the FSC region, rather than in the cortico-subcortical and deep regions, in the further regression models together with the significant clinical variables as it had a small P value (0.003) and a large χ2 value (8.827). Because of collinearity between the presence and number of FSC CMBs, we conducted two multiple logistic regression models separately. In model 1, the presence of FSC CMBs, smoking history, and occurrence of stroke during the follow-up period were entered as independent variables. The result of this model indicated that the presence of FSC CMBs was the only significant contributing factor to dementia conversion (P = 0.004, odds ratio = 4.4, 95% confidence interval: 1.60–12.08). In model two, the number of FSC CMBs, smoking history, and occurrence of stroke during the follow-up period were entered as independent variables. The result of this model indicated that 5 or more CMBs significantly contributed to dementia conversion (P = 0.001, odds ratio = 17.6, 95% confidence interval: 3.23–95.84) (Table 4). The adjusted odds ratio of the presence of FSC CMBs or the number of FSC CMBs did not change when age, sex, and educational level were adjusted.
Table 4.
Logistic regression analysis of dementia conversion at 1-year follow-up
Discussion
In this prospective study, we found that approximately one-third of participants with V-CIND had developed dementia at a 1-year follow-up assessment, and the presence of FSC CMBs and severity of CMBs were predictive of dementia conversion. To the best of our knowledge, this is the first study to explore the association between FSC CMBs and dementia conversion.
Our findings further indicate that the presence of CMBs may have heightened clinical importance. As CMBs are always concomitant with WMHs and lacunar infarction (Greenberg et al., 2009), the independent contribution of CMBs to cognitive impairment is difficult to confirm. Our findings suggest that among patients with a similar WMH severity and number of lacunar infarcts, the presence and severity of CMBs in FSC regions can predict cognitive decline over time. The common features of these circuits are that they originate in the frontal cortex, project to the striatum (caudate, putamen, and ventral striatum), and connect to the globus pallidus and substantia nigra and then to the thalamus (Cummings, 1993). The FSC is associated with emotion and affective behaviors (Tekin and Cummings, 2002). Some studies have found that patients with FSC infarction are more likely to have post-stroke depression (odds ratio = 2.6) (Tang et al., 2011c). In addition, functions of the frontal lobe, involving executive function, attention, and psychomotor speed, are also mediated by the FSC (Cummings, 1993). Thus, FSC infarctioncan lead to both cognitive impairment and psychiatric symptoms. Patients with cognitive impairment related to CSVD exhibit some common neuropsychiatric features, including frontal lobe dysfunction and depression (Prins et al., 2005, Lawrence et al., 2015). A cross-sectional study by Werring et al. (2004) first discovered the association between executive dysfunction and CMBs in the frontal region and the basal ganglia. Another longitudinal study involving post-stroke V-CIND also found an association between CMBs and cognitive changes (Tang et al., 2011b), which is consistent with our findings that CMBs could predict dementia conversion in V-CIND patients. Furthermore, the presence of CMBs at baseline is usually associated with increased numbers of CMBs in the future (Lee et al., 2011). Findings from the present study reveal that the severity of CMBs (more than 5) in the FSC contributes to dementia conversion with an odds ratio of 17.6, in comparison with individuals without CMBs in the FSC. The observed cut-off value of 5 or more CMBs, which reflects a moderate-to-severe level, might serve as useful threshold for signaling a high risk for subsequent dementia in clinical practice.
The strengths of this study include the prospective design and the homogenous sample. Additionally, the SWI used to assess CMBs in this study is more sensitive than the GRET2*-WI. However, there are also some limitations to this study. First, the sample size was relatively small, which may limit the statistical power. Second, some potential factors related to cognitive function, such as emotional symptoms, were not recorded in this study. Third, the follow-up period was relatively short, and the long-term effect of CMBs on dementia conversion was not examined. Therefore, further prospectivestudies with larger sample sizes and longer follow-up durations are warranted.
In conclusion, our data indicate that CMBs in the FSC contribute significantly to the risk of dementia conversion in CSVD-V-CIND patients. Regular assessment and early treatment of CMBs may reduce the risk of dementia and improve clinical outcomes in patients with CSVD.
Additional file: Open peer review report 1 (5KB, pdf) .
Footnotes
Conflicts of interest: None declared.
Financial support: This work was supported by the Medical Scientific Research Foundation of Guangdong Province, China (No. A2015160). The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, or in the decision to submit the paper for publication.
Institutional review board statement: The study was approved by the Ethics Committee of Dongguan People's Hospital (approved number: 2015012601). This study was performed in strict accordance with the Declaration of Helsinki.
Declaration of patient consent: The authors certify that they obtained all appropriate patient consent forms. In the form, the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials have not been published and due efforts have be made to conceal their identity, but anonymity cannot be guaranteed.
Reporting statement: This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Biostatistics statement: The statistical methods of this study were reviewed by the biostatisticians of Dongguan People's Hospital (Affiliated Dongguan Hospital, South Medical University), China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Individual participant data that underlie the results reported in this article after deidentification (text, tables, figures, and appendices) will be shared. Study protocol, informed consent form and clinical study report will be promulgated within 6 months after the completion of the trial. Results will be disseminated through presentations at scientific meetings and/or by publication in a peer-reviewed journal. Anonymized trial data will be available indefinitely at www.figshare.com.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer review reports:
Reviewer 1: Lei Xiao, UT Southwestern Medical Center at Dallas, USA.
Reviewer 2: Manju Bhaskar, National Institute of Neurological Disorders and Stroke, USA.
Comments to authors: Overall it is well written and addresses a significant predictive assessment in the conversion of CSVD to CIND. The clinical study is well designed as well as the statistical analysis.
Funding: This work was supported by the Medical Scientific Research Foundation of Guangdong Province, China (No. A2015160).
References
- 1.Blair GW, Hernandez MV, Thrippleton MJ, Doubal FN, Wardlaw JM. Advanced neuroimaging of cerebral small vessel disease. Curr Treat Options Cardiovasc Med. 2017;19:56. doi: 10.1007/s11936-017-0555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodaty H, Altendorf A, Withall A, Sachdev PS. Mortality and institutionalization in early survivors of stroke: the effects of cognition, vascular mild cognitive impairment, and vascular dementia. J Stroke Cerebrovasc Dis. 2010;19:485–493. doi: 10.1016/j.jstrokecerebrovasdis.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain. 2007;130:1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 4.Cummings JL. Frontal-subcortical circuits and human behavior. Arch Neurol. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- 5.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 6.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, Warach S, Launer LJ, Van Buchem MA, Breteler MM Microbleed Study Group. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jager HR, Werring DJ. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- 9.Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, Massaro JM, DeCarli C. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2004;35:1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- 10.Jokinen H, Kalska H, Ylikoski R, Madureira S, Verdelho A, van der Flier WM, Scheltens P, Barkhof F, Visser MC, Fazekas F, Schmidt R, O’Brien J, Waldemar G, Wallin A, Chabriat H, Pantoni L, Inzitari D, Erkinjuntti T, LADIS group Longitudinal cognitive decline in subcortical ischemic vascular disease--the LADIS Study. Cerebrovasc Dis. 2009;27:384–391. doi: 10.1159/000207442. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence AJ, Brookes RL, Zeestraten EA, Barrick TR, Morris RG, Markus HS. Pattern and rate of cognitive decline in cerebral small vessel disease: A Prospective Study. PLoS One. 2015;10:e0135523. doi: 10.1371/journal.pone.0135523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SH, Lee ST, Kim BJ, Park HK, Kim CK, Jung KH, Roh JK. Dynamic temporal change of cerebral microbleeds: long-term follow-up MRI study. PLoS One. 2011;6:e25930. doi: 10.1371/journal.pone.0025930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meguro K, Akanuma K, Meguro M, Kasai M, Ishii H, Yamaguchi S. Prognosis of vascular mild cognitive impairment includes vascular dementia onset and death by cardiovascular disease: reanalysis from the Osaki-Tajiri project. J Stroke Cerebrovasc Dis. 2012;21:607–611. doi: 10.1016/j.jstrokecerebrovasdis.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell AJ, Shiri-Feshki M. Rate of progression of mild cognitive impairment to dementia--meta-analysis of 41 robust inception cohort studies. Acta Psychiatr Scand. 2009;119:252–265. doi: 10.1111/j.1600-0447.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 15.Mohd Zulkifly MF, Ghazali SE, Che Din N, Singh DK, Subramaniam P. A Review of risk factors for cognitive impairment in stroke survivors. ScientificWorldJournal. 2016;2016:3456943. doi: 10.1155/2016/3456943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr. 1997;9(Suppl 1):173–178. doi: 10.1017/s1041610297004870. [DOI] [PubMed] [Google Scholar]
- 17.Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, Greenberg SM, Dickerson BC. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009;30:338–343. doi: 10.3174/ajnr.A1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 19.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 20.Prins ND, van Dijk EJ, den Heijer T, Vermeer SE, Jolles J, Koudstaal PJ, Hofman A, Breteler MM. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128:2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 21.Sachdev PS, Chen X, Brodaty H, Thompson C, Altendorf A, Wen W. The determinants and longitudinal course of post-stroke mild cognitive impairment. J Int Neuropsychol Soc. 2009;15:915–923. doi: 10.1017/S1355617709990579. [DOI] [PubMed] [Google Scholar]
- 22.Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, Kuiper M, Steinling M, Wolters EC, Valk J. Atrophy of medial temporal lobes on MRI in “probable” Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang WK, Chen Y, Liang H, Chu WC, Mok VC, Ungvari GS, Wong KS. Cerebral microbleeds as a predictor of 1-year outcome of poststroke depression. Stroke. 2014;45:77–81. doi: 10.1161/STROKEAHA.113.002686. [DOI] [PubMed] [Google Scholar]
- 24.Tang WK, Chen YK, Lu J, Ahuja AT, Chu WC, Mok VC, Ungvari GS, Xiang YT, Wong KS. Cerebral microbleeds and quality of life in acute ischemic stroke. Neurol Sci. 2011a;32:449–454. doi: 10.1007/s10072-011-0571-y. [DOI] [PubMed] [Google Scholar]
- 25.Tang WK, Chen YK, Lu JY, Wong A, Mok V, Chu WC, Ungvari GS, Wong KS. Absence of cerebral microbleeds predicts reversion of vascular ‘cognitive impairment no dementia’ in stroke. Int J Stroke. 2011b;6:498–505. doi: 10.1111/j.1747-4949.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 26.Tang WK, Lu JY, Chen YK, Chu WC, Mok V, Ungvari GS, Wong KS. Association of frontal subcortical circuits infarcts in poststroke depression: a magnetic resonance imaging study of 591 Chinese patients with ischemic stroke. J Geriatr Psychiatry Neurol. 2011c;24:44–49. doi: 10.1177/0891988710392375. [DOI] [PubMed] [Google Scholar]
- 27.Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res. 2002;53:647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- 28.Werring DJ, Frazer DW, Coward LJ, Losseff NA, Watt H, Cipolotti L, Brown MM, Jäge HR. Cognitive dysfunction in patients with cerebral microbleeds on T2*-weighted gradient-echo MRI. Brain. 2004;127:2265–2275. doi: 10.1093/brain/awh253. [DOI] [PubMed] [Google Scholar]
- 29.Wilson D, Charidimou A, Ambler G, Fox ZV, Gregoire S, Rayson P, Imaizumi T, Fluri F, Naka H, Horstmann S, Veltkamp R, Rothwell PM, Kwa VI, Thijs V, Lee YS, Kim YD, Huang Y, Wong KS, Jager HR, Werring DJ. Recurrent stroke risk and cerebral microbleed burden in ischemic stroke and TIA: A meta-analysis. Neurology. 2016;87:1501–1510. doi: 10.1212/WNL.0000000000003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson JT, Hareendran A, Grant M, Baird T, Schulz UG, Muir KW, Bone I. Improving the assessment of outcomes in stroke: use of a structured interview to assign grades on the modified Rankin Scale. Stroke. 2002;33:2243–2246. doi: 10.1161/01.str.0000027437.22450.bd. [DOI] [PubMed] [Google Scholar]
- 31.Wong A, Xiong YY, Kwan PW, Chan AY, Lam WW, Wang K, Chu WC, Nyenhuis DL, Nasreddine Z, Wong LK, Mok VC. The validity, reliability and clinical utility of the Hong Kong Montreal Cognitive Assessment (HK-MoCA) in patients with cerebral small vessel disease. Dement Geriatr Cogn Disord. 2009;28:81–87. doi: 10.1159/000232589. [DOI] [PubMed] [Google Scholar]
- 32.Zhang ZX, Zahner GE, Roman GC, Liu J, Hong Z, Qu QM, Liu XH, Zhang XJ, Zhou B, Wu CB, Tang MN, Hong X, Li H. Dementia subtypes in China: prevalence in Beijing, Xian, Shanghai, and Chengdu. Arch Neurol. 2005;62:447–453. doi: 10.1001/archneur.62.3.447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


