Abstract
Differentiation of hematopoietic stem cells into distinct cell types was thought to occur through a series of discrete, stable progenitor states. Work from Velten et al. (2017) now shows that hematopoietic cells differentiate via a mechanism of continuous lineage priming and thus represent a CLOUD-HSPC.
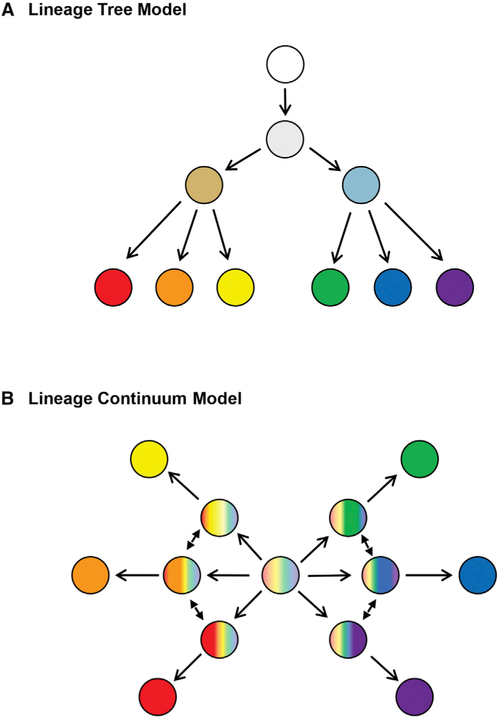
Traditional models of lineage progression from stem cells to their differentiated progeny are often thought of as hierarchical trees involving successive binary fate decisions as oligo-potent cells differentiate toward mature cell types with distinct functions (Figure 1A). Many of these hierarchies have been proposed based on phenotypic analysis of pre-defined subpopulations of cells within stem cell compartments (niches) or differentiated progeny. However, a recent study in Nature Cell Biology by Velten and coworkers (2017) has taken the approach of analyzing single cells within human bone marrow and has found that hematopoietic stem and progenitor cells (HSPCs) exhibit characteristics of multiple lineages and are thus likely to undergo direct lineage commitment to generate distinct blood cell types, as opposed to transitioning through a series of discrete and stable progenitors (Figure 1B).
Figure 1. Generic Models of Lineage Commitment.
(A) Lineage tree model: The most oligo-potent cell is depicted in white, and transitions via binary fate decisions to ultimately generate distinct cell lineages are depicted in vibrant, distinct colors. (B) Lineage continuum model: All oligo-potent cells are highly transcriptionally diverse and undergo direct lineage commitment to generate distinct cell types, as opposed to transitioning through a series of discrete and stable progenitors.
Velten et al. (2017) performed single-cell immunophenotyping and transcriptional and functional analyses on two sets of lineage-negative (Lin−; i.e.,non-differentiated) cells: (1) Lin−CD34+ CD38− cells, thought to contain HSCs, multi-potent progenitors (MPPs), and multi-lymphoid progenitors (MLPs), and (2) Lin−CD34+CD38+, thought to contain lineage-restricted progenitors. The samples were taken from the bone marrow of two young adults (male and female). These analyses revealed that the Lin− CD34+CD38− compartment does not contain stable clusters of cell types; rather, the HSPCs are highly interconnected and represent a continuum of low-primed, undifferentiated (CLOUD)-HSPCs. Based on the authors’ analysis, the HSCs in the center of the CLOUD are transcriptionally diverse and the least “primed” toward a specific lineage. These cells gradually acquire continuous lineage priming that nudges them toward either of two major hematopoietic branches, lymphoid/myeloid or megakaryocytic/erythroid. However, a clear separation into single lineages was only observed among cells in the Lin−CD34+CD38+ compartment, when differentiation has progressed to the level of restricted progenitors.
These findings are consistent with the hypothesis that Conrad Waddington, the father of epigenetics, put forth in The Strategy of the Genes in 1957. He suggested that mechanisms are in place during embryonic development to allow a population of cells to inherit particular traits independently from “standard” genetics. In Waddington’s model, cells possessing all of the genes necessary to make multiple phenotypic choices are promoted toward a particular phenotype by environmental stimuli. Strikingly, such thinking is now supported by results stemming from the latest technologies such as the single-cell sequencing that was employed by Velten and coworkers (2017). These studies open the door for further investigation of the environmental stimuli and epigenetic mechanisms regulating the progressive priming of CLOUD-HSPCs toward distinct hematopoietic lineages.
This idea of lineage continuum may apply to other types of stem and progenitor cell populations, and perhaps to the differentiation process of somatic cell types that acquire progressively more specialized phenotypes and functions. For example, when vascular endothelial cells are generated during blood vessel formation, they possess a uniform underlying phenotype. However, in the absence of particular micro-RNAs, they acquire distinct phenotypes and functions and are more susceptible to environmental factors affecting blood vessel development (Kasper et al., 2017). Thus, epigenetic regulation is critical for defining specific endothelial cell phenotype and function. It is interesting to speculate that primordial endothelial cells in the embryo, and perhaps capillary endothelial cells in adult tissues, exist in a “primed” state, ready to generate specialized phenotypes as dictated by environmental needs. In fact, a small subset of endothelial cells is specified to become hemogenic endothelium that gives rise to HSPCs during embryonic development (Marcelo et al., 2013). Thus, both HSPCs and the endothelial cells that generate them may exist in an even broader CLOUD.
Although having lots of choices is a good thing, there may be associated risks. In the study by Velten et al. (2017), classically defined HSCs, MPPs, and MLPs were found to be highly transcriptionally diverse, which was previously underappreciated. Given that transcriptional activity is enabled by chromatin accessibility, these findings are consistent with other recent studies showing that chromatin composition (e.g., the balance between euchromatin and heterochromatin) and spatial organization of the genome are not different between HSCs and MPPs (Ugarte et al., 2015). However, both populations have significantly more euchromatin than differentiated hematopoietic cells. The higher level of open chromatin and transcriptional activity of the collective CLOUD-HSPCs may therefore be a factor that contributes to hematological disorders. There is a growing awareness that DNA damage during transcription, in addition to replication errors, can contribute to the genesis of somatic mutations (Lodato et al., 2015) and that some regions of open chromatin in particular can exhibit very high mutational rates (Makova and Hardison, 2015). Human HSCs are reported to develop 0.13 ± 0.02 exonic mutations per year of life (Welch et al., 2012), and it has been estimated that by age 50 an individual would acquire five random coding gene mutations within each HSC (Wong et al., 2015). Given that the numbers of HSCs in a human adult are ~104, this situation provides a near mathematical certainty that mutated driver genes will arise in a small number of HSCs in middle-aged individuals. Such mutations could confer a proliferative advantage to HSPCs, setting the stage for a clonal hematopoietic event. Clonal hematopoiesis is commonly observed in the elderly, and it is considered to be a harbinger of hematological malignancies and, potentially, chronic diseases that contribute to overall mortality (Jaiswal et al., 2014; Fuster et al., 2017). Thus, while the “phenotypic continuum” of CLOUD-HSPCs may provide phenotypic flexibility during hematopoiesis, the mechanisms that afford this flexibility may contribute to the acquisition of pathological HSPC mutations.
REFERENCES
- Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, et al. (2017). Science 355, 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. (2014). N. Engl. J. Med 371, 2488–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper DM, Moro A, Ristori E, Narayanan A, Hill-Teran G, Fleming E, Moreno-Mateos M, Vejnar CE, Zhang J, Lee D, et al. (2017). Dev. Cell 40, 552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodato MA, Woodworth MB, Lee S, Evrony GD, Mehta BK, Karger A, Lee S, Chittenden TW, D’Gama AM, Cai X, et al. (2015). Science 350, 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makova KD, and Hardison RC (2015). Nat. Rev. Genet 16, 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelo KL, Sills TM, Coskun S, Vasavada H, Sanglikar S, Goldie LC, and Hirschi KK (2013). Dev. Cell 27, 504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarte F, Sousae R, Cinquin B, Martin EW, Krietsch J, Sanchez G, Inman M, Tsang H, Warr M, Passegué E, et al. (2015). Stem Cell Reports 5, 728–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velten L, Haa SF, Raffel S, Blaszkiewicz S, Islam S, Hennig BP, Hirche C, Lutz C, Buss EC, Nowak D, et al. (2017). Nat. Cell Biol Published online March 20, 2017. 10.1038/ncb3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, Wartman LD, Lamprecht TL, Liu F, Xia J, et al. (2012). Cell 150, 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, Lamprecht TL, Shen D, Hundal J, Fulton RS, et al. (2015). Nature 518, 552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]