Abstract
We report the synthesis of tungsten oxide (WO3) nanosheets using a simple yet efficient hydrothermal technique free of surfactantand template. The WO3 nano-sheets are self-assembled as well to form ordered one-dimensional chain nanostructure. A comprehensive microscopic characterization reveals that the nano-sheets have triangular and circular two different shape edges, dislocation and stacking faults are also observed, which should have implications for our understanding of catalytic activity of ceria. We also propose a growth mechanism for the nano-sheets. As a result of this unique morphology, this WO3 nano-sheets are found to show excellent gas-sensing properties which can use as promising sensor materials detecting ethanol with low concentration.
Keywords: crystal structure, defects, nanosheets, highly self-assembled, gas-sensitivity
Introduction
Tungsten trioxide WO3 nanomaterials are extensively applied in electrochromic device, gas sensor and photocatalysts filed (Hai et al., 2016; Zhang et al., 2018). Great effort has been devoted to the control the specific size and shape of WO3 nanoparticles which can significantly impact properties of materials. Up to now, the WO3 nanoparticles with avariety of morphologies (such as nano-wires, nano-rods, nano-plate, nano-spheres and so on) have been syhthesized successfully via high-intensity ultrasound, rapid microwave, hydrothermal synthesis and other methods (Chai et al., 2016, Hu et al., 2017a, Xu et al., 2017; Chen et al., 2018; Parthibavarman et al., 2018; Zhan et al., 2018). Among all of those applied methods, hydrothermal synthesis technique has been concerned due to the merits of simple operation, low energy consumption, the possibility for large-scale industrialization and so on.
Here, we report the WO3 nano-sheets with highly self-assembled architecture synthesized via the hydrothermal synthesis process. Defects are observed in the nano-sheets, which may play a key role in affecting properties of WO3 nano-sheets. In addtion, we also test the response and selectivity of the sensor fabricated from the WO3 nano-sheets.
Materials and methods
All the reagents were analytical grade and without further purification. We adopted a facile hydrothermal method to synthesize the nanostructures. First of all, 32 ml of deionized water and 8 ml glycerol (C3H8O3) were mixed into a mixture, then 1.6 nmol of sodium tungstate dihydrate (Na2WO4·2H2O) and 3 nmol oleic acid (C18H34O2) were dispersed into the mixture, and stirred for 15 min with a magnetic stirrer. Secondly, the pH of the mixture was adjusted to 1.25 by HCl. After stirring for 15 min, the solution was transferred into the Teflon-lined stainless steel autoclave and treated at 120°C during 12 h under autogenously pressure. Finally, the obtained particles were washed by deionized and alcohol to remove the unexpected ions by high-speed centrifugation and then dried at 60°C for 10 h in air.
The characterization of speciemen as our previous work (Chen et al., 2013, 2014; Hu et al., 2017b). The process of measuring the gas sensitivity of the prepared nanomaterials is described in the literature (Guo and Wang, 2016). Response of the sensors was defined as the ratio of Ra (resistances in air) to Rg (resistances in target gases).
Results
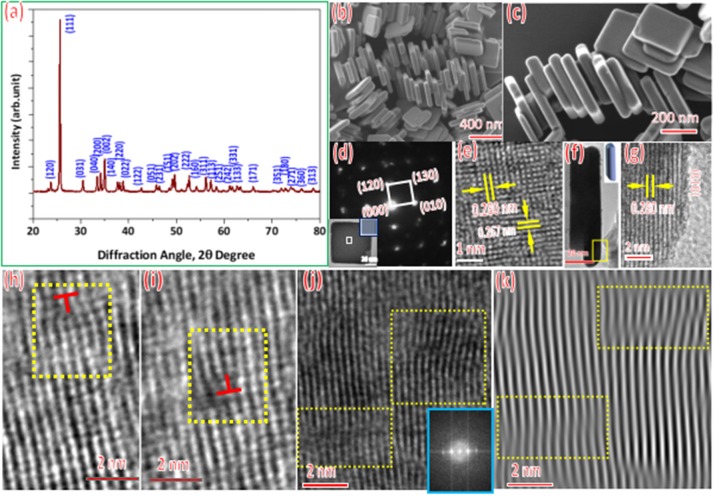
Figure 1a shows a typical XRD spectrum of the products, the diffraction peaks match well with those of a standard WO3·H2O with orthorhombic structure (JCPDS No. 84-0886). The WO3·H2O nano-particles exhibit rectangle shape with an average size of ~400 nm and thickness of 30nm (Figure 1b). In addtion, one-dimensional chain nanostructure is self-assembled by the quadrilateral faces on the both sides of the nano-sheets. The face of nano-sheet are flat (Figures 1b,c). The corresponding SADP identifies that the structure of WO3 nano-sheets is orthorhombic (Figures 1d). Figure 1e shows a high-resolution TEM (HRTEM) image taken around the corner of a nano-sheet. We determined the lattice spacing of the perpendicularlattice fringes to be ~0.280 and ~0.267 nm which are belong to the WO3 (010) and (120) planes respectively. Figure 1g shows a HRTEM image taken from the corner area of the nano-sheet Figure 1f from which lattice spacing is determined to be ~0.280 nm, in line with the distance between {010} planes of WO3. In addtion, the edges of the nano-sheets are in an arc shape (insert of Figure 1f). Two different direction edge dislocations: positive edge dislocation (Figure 1h) and negative edge dislocation (Figure 1i) are deteced by the HR-TEM, which should be critical for the properties of WO3. Apart from the defects on the surface, we also observed the stacking faults in nano-sheet (Figure 1j), which should impact the properties of WO3 nanoparticles as well. The stacking fault was observed obviously by the one-dimensionally filtered HR-TEM images of the WO3 nano-sheet (Figure 1k). Those dislocations and stacking faults may affect the catalytic activity or other properties of nano material (Wang et al., 2011, 2014, 2016; Sun et al., 2015).
Figure 1.
Characterization of the samples prepared by hydrothermal method:(a) XRD spectrum, (b–c) SEM images, (d–g)TEM images, insert of (d) shows the TEM image of a WO3 nanoparticle, insert of (f) shows the model of the side of the nanosheet, (h–k) defects of samples, insert of (j) shows the Fast Fourier transform of the HRTEM image.
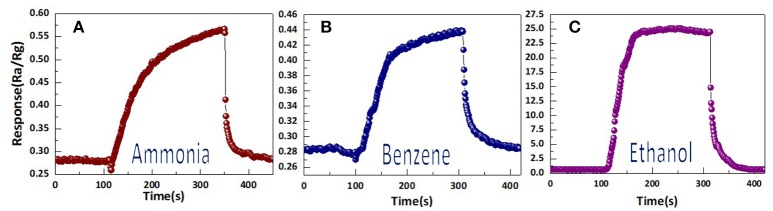
Figure 2 shows the gases (NH3, CH3OH, C6H6) response of the sensor based on WO3 nanosheets. All of the gases were tested at an operating temperature of 300°C with a concentration of 30 ppm. In Figure 2, the results indicate that the sensor exhibited little responses to NH3, C6H6, to indicated that it was insensitive to NH3, C6H6. For ethanol, the highest response of the sensor was 25.6, while the responses to NH3 and C6H6 were no >1.
Figure 2.
The response of highly self-assembled tungsten oxide nanosheets sensor toward 30 ppm of different testing gases: (a) ammonia, (b) benzene, (c) ethanol.
Discussion
In light of the aforementioned microstructural characterization, we propose a likely growth mechanism for the nanosheet. First, the Na2WO4·2H2O is ionized to . Then, the ion react with H+ which ionized by HCl, forming the H2WO4 suspension. The H2WO4 suspension decomposes, at the high temperature and pressure during hydrothermal process, resulting in to the nucleation of WO3. The oleic acid acts as a soft template and controls the growth rate of different crystal plane owing to its selective absorption and desorption behavior. Then most of WO3 nano-sheets with {010} exposure planes are self-assembled, forming one-dimensional chain nanostructures, due to the addition of oleic acid. The formation process can be described as follows:
| (1) |
| (2) |
| (3) |
| (4) |
We imply that the WO3 nano-sheets can act as an efficient gas-sensing material for selective detection of ethanol. Such the sensing performance due to the fact that the diffusion of ethanol and its oxidation with O− or O2− are very rapidly in nanoplates structures (Xiao et al., 2017). When sensor prepared by the WO3 nanosheets is exposed in air, the resistance of the WO3 nanosheets is increased by oxygen molecules which adsorbed on the surfaces of the WO3 nanosheets, trapping electrons in the conduction band and forming oxygen species (O−, O2−). As ethanol is introduced to the sensor, the oxygen species (O−, O2−) react with ethanol molecules on the surface of the WO3 nanosheets, which will release trapped electrons to conduction band and resistance of the WO3 nanosheets is decreased (Ahmad et al., 2013).
Conclusions
We have adopted the hydrothermal technique to synthesize highly self-assembled WO3 nano-sheets using the tungsten resource Na2WO4·2H2O and the soft template oleic acid. We demonstrated that the WO3 nano-sheets are mainly exposed with {010} planes and crystal defects such as edge dislocations and stacking faults exist in single crystalline nano WO3 by microscopic investigations, which may be important for the catalytic activity of WO3. We indicate that the WO3 nano-sheets could be used as promising sensor material for detecting CH3OH with low concentration.
Author contributions
LH synthesized and characterized the microstructure of highly self-assembled tungsten oxide nanosheets and wrote the manuscript. PH and ZL tested the response and selectivity of the sensor fabricated from the WO3 nano-sheets. YC and CQ directed the experiment. All authors read and approved the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
YC thanks the financial support by the Construct Program of the Key Discipline of Hunan province (Education Department of Hunan Province Bulletin grant no.[2014]85). ZL thanks the financial support by the Project of Research Study and Creativity Experiment Plan for College Students of the Hunan province (Education Department of Hunan Province Bulletin grant no.[2016]283).
References
- Ahmad M. Z., Sadek A. Z., Ou J. Z., Yaacob M. H., Latham K., Wlodarski W. (2013). Facile synthesis of nanostructured WO3 thin films and their characterization for ethanol sensing. Mater. Chem. Phys. 141, 912–919. 10.1016/j.matchemphys.2013.06.022 [DOI] [Google Scholar]
- Chai Y., Ha F. Y., Yam F. K., Hassan Z. (2016). Fabrication of tungsten oxide nanostructure by sol-gel method. Proc. Chem. 19, 113–118. 10.1016/j.proche.2016.03.123 [DOI] [Google Scholar]
- Chen G. C., Chua X. F., Qiao H. B., Ye M. F., Chen J., Gao C., et al. (2018). Thickness controllable single-crystal WO3 nanosheets: highly selective sensor for triethylamine detection at room temperature. Mater. Lett. 226, 59–62. 10.1016/j.matlet.2018.05.022 [DOI] [Google Scholar]
- Chen Y, Liu T. M., Chen C. L., Guo W. W., Sun R., Lv S., et al. (2013). Synthesis and characterization of CeO2 Nano-rods. Ceram. Int. 39, 6607–6610. 10.1016/j.ceramint.2013.01.096 [DOI] [Google Scholar]
- Chen Y, Lv S. H., Chen C. L., Qiu C. J., Fan X. F., Wang Z. C. (2014). Controllable synthesis of ceria nanoparticles with uniform reactive {100} exposure planes. J. Phys. Chem. C 118, 4437–4443. 10.1021/jp410625n [DOI] [Google Scholar]
- Guo W. W., Wang Z. C. (2016). Composite of ZnO spheres and functionalized SnO2 nanofibers with an enhanced ethanol gas sensing properties. Mater. Lett. 169, 246–249. 10.1016/j.matlet.2016.01.118 [DOI] [Google Scholar]
- Hai G., Huang J., Jie Y., Li J., Cao L., Zhang G., et al. (2016). Influence of octadecylamine on the phase composition and the photocatalytic property of the tungsten oxide. Mater. Lett. 174, 134–137. 10.1016/j.matlet.2016.03.049 [DOI] [Google Scholar]
- Hu P. F., Chen Y., Chen Y., Lin Z. H., Wang Z. C. (2017a). Hydrothermal synthesis and photocatalytic properties of WO3 nanorods by using capping agent SnCl4·5H2O. Phys. E 92, 12–16. 10.1016/j.physe.2017.05.004 [DOI] [Google Scholar]
- Hu P. F., Chen Y., Sun R., Chen Y., Yin Y. R., Wang Z. C., et al. (2017b). Synthesis, characterization and frictional wear behavior of ceria hybrid architectures with {111} exposure planes. Appl. Surf. Sci. 401, 100–105. 10.1016/j.apsusc.2017.01.005 [DOI] [Google Scholar]
- Parthibavarman M., Karthik M., Prabhakaran S. (2018). Facile and one step synthesis of WO3 nanorods and nanosheets as an efficient photocatalyst and humidity sensing material. Vacuum 155, 224–232. 10.1016/j.vacuum.2018.06.021 [DOI] [Google Scholar]
- Sun R., Wang Z. C., Saito M., Shibata N., Ikuhara Y. (2015). Atomistic mechanisms of nonstoichiometry-induced twin boundary structural transformation in titanium dioxide. Nat. Commun. 6:7120. 10.1038/ncomms8120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. C., Saito M., McKenna K. P., Fukami S., Sato H., Ikeda S., et al. (2016). Atomic-scale structure and local chemistry of CoFeB-MgO magnetic tunnel junctions. Nano Lett. 16, 1530–1536. 10.1021/acs.nanolett.5b03627 [DOI] [PubMed] [Google Scholar]
- Wang Z. C., Saito M., McKenna K. P., Gu L., Tsukimoto S., Shluger A. L., et al. (2011). Atom-resolved imaging of ordered defect superstructures at individual grain boundaries. Nature 479, 380–383. 10.1038/nature10593 [DOI] [PubMed] [Google Scholar]
- Wang Z. C., Saito M., McKenna K. P., Ikuhara Y. (2014). Polymorphism of dislocation core structures at the atomic scale. Nate Commun. 5:3239 10.1038/ncomms4239 [DOI] [PubMed] [Google Scholar]
- Xiao J., Song C. W., Dong W., Chen L., Yanyan Y. (2017). Synthesis, characterization, and gas sensing properties of WO3 nanoplates. Rare Metal Mater. Eng. 46, 1241–1244. 10.1016/S1875-5372(17)30144-3 [DOI] [Google Scholar]
- Xu L., Gu D. X., Chang X. T., Chai L. G., Li Z., Jin X., et al. (2017). Rare-earth-doped tungsten oxide microspheres with highly enhanced photocatalytic activites. Ceram. Int. 43, 10263–10269. 10.1016/j.ceramint.2017.05.055 [DOI] [Google Scholar]
- Zhan Y, Liu Y. L., Liu Q. Q., Liu Z. M., Yang H., Lei B., et al. (2018). Size-controlled synthesis of fluorescent tungsten oxide quantum dots via one-pot ethanol-thermal strategy for ferric ions detection and bioimaging. Sens. Actuats B Chem. 255, 290–298. 10.1016/j.snb.2017.08.043 [DOI] [Google Scholar]
- Zhang J., Fu X., Hao H., Gan W. (2018). Facile synthesis 3D flower-like Ag@WO3 nanostructures and applications in solar-light photocatalysis. J. Alloys Compd. 757, 134–141. 10.1016/j.jallcom.2018.05.068 [DOI] [Google Scholar]