ABSTRACT
Streptococcus pneumoniae is a major human bacterial pathogen responsible for millions of deaths each year and significantly more illnesses worldwide. With over 90 different serotypes, providing effective vaccine programs has been a continuing challenge. Since 1983, the world has been introduced to four different pneumococcal vaccines (PPSV23, PCV7, PCV10, and PCV13) each with their own complications and successes. Since vaccination programs began, a decrease in the overall rate of pneumococcal pneumonia and associated diseases has been observed, notably in higher risk populations. However, with a decrease in incidence of vaccine type pneumococcal serotypes, increases in non-vaccine serotypes of the bacteria have been observed along with serotype switching. Additionally, a rise in antibiotic resistant strains of S. pneumoniae is noted. Here we discuss both the positive and negative clinical manifestations of pneumonia vaccine programs and discuss the challenges in pneumococcal vaccine design.
KEYWORDS: capsular polysaccharide, conjugate vaccines, pneumonia, serotype replacement, serotype, Streptococcus pneumoniae
Characteristics of the bacterium
Streptococcus pneumoniae is a gram-positive coccus bacterium.1 It most often colonizes in the nasopharynx of its host.2 Colonization with this bacterium in the nasopharynx is exceptionally common, with 40–95% of infants and 1–10% of adults being colonized at any time.3 There are over 90 distinctive serotypes of this bacterium, which vary in geographical prevalence.4 Different serotypes either cause mucosal colonization or invasive disease. For example, serotypes 1 and 3 are most often isolated from the lung, whereas serotypes 6,10, and 23 are regularly associated with meningitis.5 Approximately 23 serotypes are responsible for 80–90% of invasive pneumococcal disease (IPD).5 Each serotype is distinguished by its unique capsular polysaccharide (CPS), which decorates the outside surface of the bacterium. The CPS is a major virulence factor, significantly contributing to disease. Other virulence factors include the cell wall, pneumolysin, and pneumococcal surface protein A.6
Virulence of the bacterium differs with the composition of the CPS, making it one of the primary virulence factors. The CPS allows the bacterium to evade phagocytosis by the host cells.7 Its antigenicity allows the host to make antibodies against specific serotypes.
Diseases associated with S. pneumoniae
S. pneumoniae is responsible for many invasive infections including meningitis, sepsis, and bacteremia, as well as more common bacterial infections such as community acquired pneumonia (CAP) and otitis media. On average, there are 4,100 cases of meningitis,8 12,000 of bacteremia,9 500,000 of pneumonia, and 7 million cases of otitis media annually in the United States.10,11 The bacterium most commonly affects pediatric, elderly, and immunocompromised hosts. S. pneumoniae is the leading cause of pneumonia in children worldwide and is responsible for 30% of adult pneumonia cases.12 This bacterium is particularly troublesome in at-risk populations, including diabetics, asthmatics, and the HIV positive. In fact, coinfection with influenza or HIV leads to increased S. pneumoniae carriage, which leads to a significantly higher risk of pneumococcal infection and mortality.3 S. pneumoniae and H. influenzae coinfection is the eighth leading cause of death in the United States, with a death toll of over 50,000 people in 2014.13,14 An average of 1.2 million young children worldwide die due to pneumococcal pneumonia and meningitis.10 In developed countries, the mortality rate for pneumococcal pneumonia can reach 11–40%.12
Pneumococcal vaccines
In 1983, Pneumovax® PPSV23, a vaccine against 23 prevalent serotypes of S. pneumoniae was introduced in the United States (Table 1).5 This vaccine showed evidence of protection against invasive disease in adults.12,15 There is also some evidence to suggest the vaccine protects the elderly, which is a high-risk population for pneumococcal infections.16 However, the vaccine still has many shortcomings, including poor immunogenicity in children, especially those under the age of two.15 There is also no strong evidence showing protection in immunocompromised patients.5 The effectiveness of PPSV23 seems to decrease with age of the patient and time since administration.5 The majority of these factors can be attributed to the short-lived IgM antibodies elicited from the T-cell independent response, no affinity maturation, and lack of IgG class switching of antibodies.17 The solution for these issues came with the introduction of the T cell dependent conjugate vaccines in the 2000s.
Table 1.
Description of past and present pneumococcal vaccines. Table was adapted from references 4 and 9.
| Vaccine | Year Introduced | Carrier Protein | Serotypes | Impacts |
|---|---|---|---|---|
| PSV23 | 1983 | N/A | 1,2,3,4,5,6B,7F,8,9N,9V,10A,11A,12F,14,15B | -Reduced invasive disease in adults |
| 17F,18C,19A,19F,20,22F,23F,and33F | -Not protective in children | |||
| PCV7 | 2000 | CRM197 | 4,6B,9V,14,18C,19F, and 23F | -Reduced invasive disease |
| -More protective in children | ||||
| -Increase 19A and 7F infections | ||||
| PCV10 | 2011 (Not in US) | NTHi protein D; tetanus toxoid; diphtheria toxoid | 1,4,5,6B,7F,9V,14,18C,19F, and 23F | -Decrease in otitis media infection |
| PCV13 | 2010 | CRM197 | 1,3,4,5,6A,6B,7F,9V,14,18C,19A,19F, and 23F | -Reduced invasive disease-Protection in all age/risk groups-Increase in 35B infections |
In 2000, PCV7 was introduced in the United States. This vaccine contained the CPS of seven highly virulent serotypes of S. pneumoniae covalently conjugated to the carrier protein, non-toxic diphtheria toxin mutant CRM197 (Table 1).18 While this conjugate vaccine showed evidence of greater protection in children and herd effect among the population,19 the effectiveness in adults was not well studied.5 In 2010, a new conjugate vaccine was released in the United States, Prevnar® PCV13. Much like PCV7, PCV13 contains the CPS of 13 virulent serotypes conjugated to CRM197 (Table 1).15 PCV13 showed promising results for protecting against invasive pneumonia infection as well as otitis media in young children.5 Additionally, a 2015 study on PCV13 found it to be protective against CAP in elderly patients.15
Populations affected
Pneumococcal pneumonia infections are prevalent among three population groups: children, elderly, and the immunocompromised. Children under the age of two are affected the most. This is most likely due to their immature immune systems, which makes administering vaccinations at this age critical. Children aged two to five are also considered at risk. It is estimated that worldwide pneumococcal disease causes 11% of all deaths in children less than five.19 A study looking at the effects of the PCV7 vaccine noted the risk in this group and the importance of implementing the PCV7 vaccine as PPSV23 was not considered effective in children.20
Adults over the age of 65 are considered a major risk group. The high risk of pneumococcal infection mostly stems from altered immunity and increased carriage.3 While PPSV23 vaccine was not found effective in children, it did offer limited immunity in adults. The introduction of PCV7 saw a great reduction in pneumococcal pneumonia cases in children; however, its impact on adult disease was not well studied.5,15 With the introduction of PCV13, a greater impact of protection was observed in adults, specifically 65 and older.15
Lastly, patients who are immunocompromised are at a greater risk of infection. This includes patients who are HIV positive and patients who suffer from chronic diseases.5 S. pneumoniae is the most common cause of pneumonia, sepsis, and meningitis among the immunocompromised.3 A more detailed look into how the vaccines have affected these populations is explored in the subsequent section.
Vaccine impact on disease
The overall impact of vaccines on pneumococcal disease has been a major success. The bulk of post vaccination data comes from PCV7 as it was released in 2000; data on PCV13 effectiveness is still being observed. After the introduction of PCV7 in the United States in 2000, a 77% reduction in pneumonia in children under the age of five was observed.21 Additionally, in 2001 there was a 69% decrease in IPD in young children compared to 1998–1999 (Figs. 1 and 2).20,22 The overall rate of vaccination in the United States was between 68–69%.21 A reduction in IPD among immunocompromised patients was also seen as an effect of PCV7 introduction.5
Figure 1.
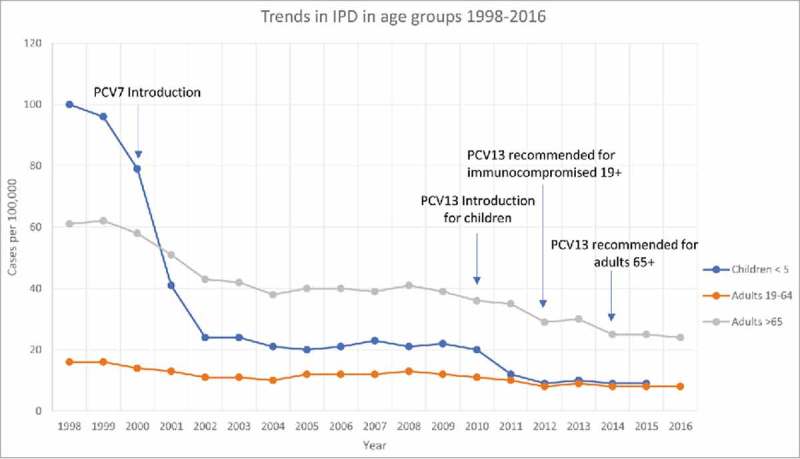
Trends in overall rate of IPD (invasive pneumonia disease) in different age groups between the years 1998–2016 in the United States. Blue line represents children under five, orange line adults ages 19–64, gray line adults over the age of 65. Important dates for vaccine introduction and recommendations are pinpointed. Data for children less than five was only available through 2015. Figure was constructed with data from the CDC ABCs bacterial surveillance program, 2016 reference 22.
Figure 2.
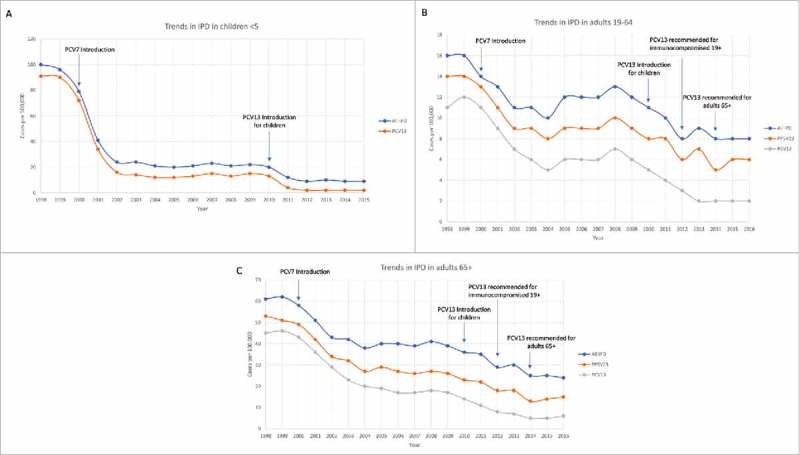
Trends in overall rate of IPD (invasive pneumonia disease), disease caused by PPSV23 included serotypes, and disease caused by PCV13 serotypes in different age groups between the years 1998–2016 in the United States. A) Children under 5, with only overall and PCV13 data available through 2015. B) Adults ages 19–64 C) Adults over 65. Figure was constructed with data from the CDC ABCs bacterial surveillance program, 2016 reference 22.
There has also been reported data of effectiveness in Europe. Specifically, in the Netherlands there was a 35% reduction of disease in children under the age of two and vaccine-type caused IPD decreased by 67% after the introduction of PCV7.21 However, this was lower than the results seen in the United States despite the Netherlands having an increased rate of vaccination among children (94.4%).21 The Netherlands also used PCV10, a conjugate vaccine containing all the serotypes of PCV7 plus three more (Table 1) and observed a 34% reduction in the first episode of otitis media.5 Similar results were seen throughout Europe.
From a major study done on the impact of the PCV13 vaccine, we can see the first effects of this vaccine.15 In 2008 it was reported that 68.4% of invasive pneumonia incidents in adults 65 or older were caused by PCV13 serotypes and 49.7% by PCV7 serotypes.15 In 2013, after the introduction of PCV13, these numbers decreased to 42.3% by PCV13 serotypes and 6% by PCV7 serotypes (Fig. 2).15,22 Additionally, PCV13 has reduced the incidence of IPD in young children by 36% (Fig. 1).5,22
However, with the great many positives observed from the introduction of pneumococcal vaccines there are still areas of concern. In 2006 it was reported that between 23–30% of children still carried pneumococci with no evidence of a decline in carriage prevalence.18,23 Additionally, while PCV7 reduced the incidence of IPD by 97% for serotypes included in the vaccine in children under the age of five, infection from non-vaccine type serotypes increased by 22%.5 This was also observed in the Netherlands, where IPD rates for non-vaccine serotypes increased by 13%.21 The rate of IPD eventually leveled off despite the huge reduction seen in PCV7 serotypes because an emergence in infections caused by serotypes 19A and 7F was observed (Figs 1 and 2).21,22 It was also noted that the rate of IPD in adults 65 and older caused by 19A was 5.3% in 2008 but increased to 11.4% by 2013.15 Large increases in non-vaccine serotypes were also observed for HIV/AIDs positive adults.24 Further insights into serotype replacement and the consequences of pneumonia vaccines is discussed in the following sections.
Challenges associated with pneumococcal conjugate vaccines
The negative consequences observed from pneumococcal pneumonia vaccines are not solely attributed to the vaccines themselves. While there are long-standing problems associated with conjugate vaccine production, such as cost effectiveness and empirical conjugation chemistries, more uncontainable factors are at play.
A major factor in variable responses from the vaccines is that serotypes are not the same geographically. For example, the serotypes contained in PCV7 covered 80% of the most prominent serotypes seen in the US, and 60% of those in Europe.21 The most common serotypes seen in Europe are 1, 3, 7F, 14, and 19A.5 However, even with this general consensus, large differences are seen country to country. Serotypes 1, 5, 6A, 6B, 14, 19F, and 23F have been isolated in at least 50% of infected individuals worldwide.5
Additionally, serotype distribution changes between age groups and diseases. The most common serotypes seen in children are 6A, 14,19A, and 19F,25 while the most prevalent in adults are 3, 6A, 7F, and 19A.5 Among pneumococcal diseases, serotypes 1, 2, 4, 6A, 6B, 7F, 8, 9V, 14, 18C, 19F, and 23F are dominantly associated with IPD worldwide.5 However, serotypes 6, 10, and 23 are frequently linked with meningitis and serotypes 14 and 19F with otitis media.26 One can see how producing a vaccine to capture these many serotype factors would be challenging. Furthermore, immunity is limited to the serotypes contained within the vaccines, with current conjugation chemistries controlling the number of capsules that can be incorporated.
A major challenge with conjugate immunization is carrier-specific immune suppression. This is the idea that pre-existing immunity to the carrier protein due to vaccination suppresses the immune response to the carbohydrate linked to the same carrier protein in conjugate vaccines.27 A possible mechanism for this occurring within the host is currently existing antibodies to the carrier protein may prevent or hinder anti-carbohydrate B cells from interacting with their epitopes, thereby favoring anti-carrier protein B cell responses. There have been studies that found that pre-existing immunity to the carrier protein TT interferes with antibody responses to conjugate vaccines.27 However, challenges with carrier-specific immune suppression have been reduced with the use of CRM197 as a carrier protein in pneumococcal conjugate vaccines.15,28 The idea of carrier-specific immune suppression and possible mechanisms by which it occurs is of particular importance to not only pneumococcal conjugate vaccines, but also all existing conjugate vaccines.
Lastly, an issue that is of rising concern is the increase in antibiotic-resistant strains of S. pneumoniae. After the introduction of the PCV7 vaccine there was a significant increase in strain 19A, a non-vaccine type strain.29 Importantly, the amount of antibiotic-resistance in this serotype also increased.30 Serotypes 6A, 6B, 9V, 14, 15A, 19F, and 23F have also shown the most antibiotic-resistance to penicillin and erythromycin, with many of these serotypes being included in the vaccines.25 A positive side to this is that many of these vaccine type serotypes, while showing antibiotic resistance, have decreased in frequency since the introduction of the conjugate vaccine. Importantly, the introduction of PCV13 has led to a 45% decrease in IPD caused by 19A in young children.5
Serotype replacement and serotype switching
A major element that contributes to the ineffectiveness of the vaccines is serotype replacement and serotype switching. Serotype replacement can be defined as the expanding of non-vaccine type serotypes within the population, while serotype (capsular) switching is a change in a serotype of a single clone by changing of its cps locus that synthesizes the CPS. These two events are not mutually exclusive, with capsular switch variants often expanding within the population.7
The post PCV7 vaccination era saw an increase in multiple non-vaccine type serotypes. During pre-vaccination years 17% of pneumonia was caused by non-vaccine serotypes in children under five, this increased to 88% by 2004.24 For adults 65 and older the same trend was witnessed. Between 1998 and 1999 the IPD rate by non-vaccine serotypes was 44%, which increased to 78% by 2004.24 There was a notable rise in strains 19A and 7F.18 The proportion of IPD caused by 19A increased from 0% in 1991–1994 to 18% in 2001–2003.30 Additionally, strains 3, 15, 22F and 33F increased in children under the age of five, however, these strains were also seen among adults.24 Slight increases in strain 19F, 6A, 16F, 23A, and 35 were also observed in adults.24,25,30 The increase in strains 19A and 3 is particularly alarming since these two serotypes are commonly associated with IPD. The post PCV7 era also witnessed an increase in non-vaccine type IPD in the elderly, with this increase being more pronounced than the increase that was observed in children under five.30 There was also an increase in non-vaccine type IPD in HIV adults. However, there was a 33% reduction in overall S. pneumoniae caused meningitis in the elderly.30 While data is still being acquired for the post PCV13 era, there has been a noteworthy increase in serotype 35B since introduction of the vaccine.12,31
Nonencapsulated S. pneumoniae
In addition to capsular switch variants and serotype replacement strains, it is important to consider nonencapsulated strains (NESp) of S. pneumoniae. As per their name, these strains do not produce a capsular polysaccharide, but NESp also include strains that express novel serotypes or capsules below detectable levels. These strains have been associated with causing conjunctivitis, IPD, otitis media, and carriage.32 Strains can be divided into two classes. Class I types contain the cps locus which normally codes for the CPS; however, mutations or disruptions prevent the bacteria from making a capsule. Class II types, on the other hand, completely lack the cps locus and genes required to produce a capsule, and instead have novel genes and virulence factors.32-34 Typically, the CPS is the primary virulence factor and without it strains cannot readily colonize or cause disease. However, NESp have been shown to colonize the nasopharynx.33 This is probably due to increased exposure of host cell receptors to surface proteins due to lack of capsule. The surface proteins of S. pneumoniae are important because they adhere to the hosts' cells. Many group II NESp have a novel surface protein, PspK.34 Strains that produce PspK have been shown to have increased colonization over strains lacking PspK.34 Additionally, NESp may be able to cause disease because many contain DltA and EndA proteins which have been shown to inhibit neutrophil extracellular traps, thus allowing them to evade bacterial clearance.33 Lastly, NESp create larger biofilms than encapsulated strains. This is again most likely due to increased exposure of the surface proteins, which adhere to the host cells.33 Larger biofilm formation also reduces the strains sensitivity to antibiotics and host response.
Discussion
Despite the current challenges with pneumococcal vaccine programs, there is no denying the effectiveness of the vaccines. Post vaccine era statistics on the decreases seen in pneumococcal pneumonia infections attest to this (Fig. 1). However, one important aspect of these vaccines that needs to be researched and addressed further is their production. Up to this point, conjugate vaccines have been constructed using poorly controlled conjugation chemistries to link carbohydrate and protein.35 These designs do not consider the mechanism of action within the body once administered, with gaps in knowledge of the required immune response to induce protection being a major scientific challenge.36 This leads to poorly-characterized, heterogeneous, and variably immunogenic conjugate vaccines,37,38 Recently, a mechanism for conjugate vaccine-triggered immune response has been proposed.39,40 In this model, uptake and processing of conjugate vaccines by an antigen-presenting cell yield the presentation of a carbohydrate epitope via the major histocompatibility complex class II. This in turn stimulates carbohydrate specific T-cells (Tcarbs) and leads to the production of high-affinity carbohydrate specific IgGs.39,41,42 Considering the mechanism of action in producing conjugate vaccines will help produce knowledge-based vaccines that are target specific, structurally better-defined, both immunogenic and protective, and produced at a much lower cost.40 This will allow for use on a more global scale to help control pneumococcal infections. Additionally, this will aid in designing vaccines targeted specifically for certain at-risk groups, as it is becoming increasingly clear that vaccination, particularly in adults, is not a one-size-fits-all concept. A recent study has found that adults aged 55–74, when previously vaccinated with PPSV23, showed reduced OPA response to PCV13.43 This is known as immune hyporesponsiveness, in which the patient shows poor immune response to PCV13 as a result of prior polysaccharide antigen administration in PPSV23.43 Similarly, a study found that children who had previously received doses of PCV7 when boosted with PPSV23 at 12 months exhibited immune hyporesponsiveness compared to children who did not receive the PPSV23 booster.44 Fortunately, by age 5–7 these children showed normal immune response to PCV13 and the hyporesponsiveness did not persist.44
Furthermore, while it is true that we see a rise in non-vaccine type serotypes post introduction of the vaccines, there is some evidence that points to this occurring naturally. In a study by Wyres et al., it was noted that capsular switching most likely arises naturally in pneumococcal strains as well as post vaccine introduction.7 While vaccination programs do induce a selective pressure on pneumococci, which contributes to the serotype epidemiology, these naturally occurring fluctuations among serotypes play a contributing role. An example of vaccine induced selective pressure and natural selection occurring simultaneously was observed in the post PCV7 era.18 Croucher et al., points out that a number of successful lineages that were vaccine-type prior to PCV7 introduction will likely persist through variants that have acquired non-vaccine type capsules through natural transformation from vaccine pressure, i.e., serotype switching.18 As was the circumstance after the introduction of PCV7, it is probable that non-vaccine type capsular switch variants will persist and emerge from the PCV13 vaccine program. Fortunately, the extent of serotype replacement and serotype switching on IPD and pneumonia rates has been minimal to this point.30
Awareness of NESp is important for a multitude of reasons. Firstly, there has been an increase in NESp prevalence as it was observed that strains increased from 1.5% in 2001 to 5.1% in 2006.32 However, at any point in time NESp frequency can be between 3–19% worldwide.33 This is important, as current conjugate vaccines do not protect against NESp. Additionally, as pointed out above, these strains are still able to cause diseases, albeit at a lower frequency than encapsulated strains.33 These strains are also found to be highly resistant to antibiotics. One study out of Portugal found 7% of carriage isolates to be NESp, with all NESp strains being resistant to one antibiotic and 89% of them being resistant to multiple.33,45 Another possibility is genetic exchange between NESp and encapsulated strains. The cps locus is considered a hotspot due to abnormally high horizontal gene transfer.23 NESp have been shown to acquire mutants at a higher rate than encapsulated strains.33 With the increase in nasopharynx colonization by NESp and non-vaccine type serotypes the likelihood of transfer between the two is great.34 This could transfer novel genes, such as pspK, and antibiotic resistance to encapsulated strains. NESp also contain novel virulence factors, which could potentially be transferred. Lastly, as the use of conjugate vaccines becomes more widespread, the increased pressure on S. pneumoniae strains to adapt becomes greater. Conjugate vaccines affect the niche that non-vaccine type and NESp can now exploit to expand. This leaves room for increased risk of IPD caused by NESp. Pressure from vaccines also forces the vaccine type serotypes to adapt in order to persist, leaving room for genetic exchange between them and NESp. Over all, the frequency of NESp and non-vaccine type strains will likely continue to grow as conjugate vaccines continue to target specific serotypes.
Lastly, when thinking about the rise in antibiotic-resistant strains of pneumococci, it is possible that a subsidizing factor in this is the increased use of antibiotics and broad-spectrum antibiotics, not just vaccine programs. There is evidence that the increase in disease caused by serotype 19A is also universally related to the increase in antibiotic use and therefore the trend of antibiotic resistance observed in this particular serotype.25,30 Moreover, there is a rise in infection from antibiotic-resistant strains seen in countries without a national pediatric vaccination program, leaving room for uncertainty whether the spike in 19A, and antibiotic-resistance in this strain, is solely from the introduction of PCV7.30 As noted in a 2013 study, rates of antibiotic-resistance tend to vary from region to region and are influenced by such factors as serotype epidemiology of that region, vaccination programs, and antibiotic usage.25 Additionally, the use of conjugate vaccines against S. pneumoniae has led to a reduction in antibiotic use to treat these infections, which hopefully lessens the pressure for resistance to evolve.46 This further corroborates the belief that both antibiotic-resistance and serotype replacement/switching are not solely influenced by vaccination programs, but act via natural and outside factors. However, with an estimated 700,000 deaths annually from antibiotic-resistant infections, and that number only expected to rise, this is an important issue to consider.46
In conclusion, numerous studies of the pneumococcal vaccines have all shown increases in non-vaccine type serotypes post vaccine era, the persistence of vaccine type serotypes, and the connection of antibiotic resistance with certain serotypes. However, the greater impact of these vaccines is undeniable. The overall percentage of pneumonia has decreased worldwide since the introduction of the conjugate vaccines. Multiple studies show evidence of reduction in carriage from vaccine type strains in those vaccinated and importantly even the unvaccinated are protected indirectly through herd immunity. This has been an added benefit of vaccination programs where vaccinated individuals are protected from disease and colonization, however, by preventing disease they also subsequently reduce transmission of the pathogen to individuals who are not/cannot be vaccinated.46 Reservations over serotype replacement, antibiotic resistance, and nonencapsulated strains should not dissuade the use of conjugate vaccines nor refute the fact that these vaccines have impeded serious illnesses among the population.
Funding Statement
This work was supported by funding from the National Institute of Health grant: 1R01AI123383.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank Ms. Cathryn Quinn for her editorial assistance and Mr. Dustin Middleton for his critical review.
References
- 1.Watson DA, Musher DM, Jacobson JW, Verhoef J. A brief history of the pneumococcus in biomedical research: a panoply of scientific discovery. Clin Infect Dis. 1993;17:913–24. doi: 10.1093/clinids/17.5.913. PMID:8286641. [DOI] [PubMed] [Google Scholar]
- 2.Griffith F. The Significance of Pneumococcal Types. J Hyg (Lond). 1928;27:113–59. doi: 10.1017/S0022172400031879. PMID:20474956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jochems SP, Weiser JN, Malley R, Ferreira DM. The immunological mechanisms that control pneumococcal carriage. PLoS Pathog. 2017;13:e1006665. doi: 10.1371/journal.ppat.1006665. PMID:29267378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geno KA, Gilbert GL, Song JY, Skovsted IC, Klugman KP, Jones C, Nahm MH. Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin Microbiol Rev. 2015;28:871–99. doi: 10.1128/CMR.00024-15. PMID:26085553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aliberti S, Mantero M, Mirsaeidi M, Blasi F. The role of vaccination in preventing pneumococcal disease in adults. Clin Microbiol Infect. 2014;20(Suppl)5:52–8. doi: 10.1111/1469-0691.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AlonsoDeVelasco E, Verheul AF, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol Rev. 1995;59:591–603. PMID:8531887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wyres KL, Lambertsen LM, Croucher NJ, McGee L, von Gottberg A, Linares J, Jacobs MR, Kristinsson KG, Beall BW, Klugman KP, et al.. Pneumococcal capsular switching: a historical perspective. J Infect Dis. 2013;207:439–49. doi: 10.1093/infdis/jis703. PMID:23175765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL, Harrison LH, Farley MM, Reingold A, Bennett NM, et al.. Bacterial meningitis in the United States, 1998–2007. N Engl J Med. 2011;364:2016–25. doi: 10.1056/NEJMoa1005384. PMID:21612470. [DOI] [PubMed] [Google Scholar]
- 9.Hamborsky J KA, Wolfe S. Pneumococcal Disease. In: Prevention CfDCa , ed. Epidemiology and Prevention of Vaccine-Preventable Diseases. Washington D.C.: Public Health Foundation; 2015. [Google Scholar]
- 10.Hausdorff WP, Bryant J, Kloek C, Paradiso PR, Siber GR. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis. 2000;30:122–40. doi: 10.1086/313609. PMID:10619741. [DOI] [PubMed] [Google Scholar]
- 11.Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 1997;46:1–24. [PubMed] [Google Scholar]
- 12.Daniels CC, Rogers PD, Shelton CM. A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Recommendations and Future Protein Antigens. J Pediatr Pharmacol Ther. 2016;21:27–35. PMID:26997927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;1098:962–70. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: Final Data for 2014. Natl Vital Stat Rep. 2016;65:1–122. PMID:27378572. [PubMed] [Google Scholar]
- 15.Bonten MJ, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, van Werkhoven CH, van Deursen AM, Sanders EA, Verheij TJ, et al.. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–25. doi: 10.1056/NEJMoa1408544. PMID:25785969. [DOI] [PubMed] [Google Scholar]
- 16.Feldman C, Anderson R. Review: current and new generation pneumococcal vaccines. J Infect. 2014;69:309–25. doi: 10.1016/j.jinf.2014.06.006. PMID:24968238. [DOI] [PubMed] [Google Scholar]
- 17.Sun L, Middleton DR, Wantuch PL, Ozdilek A, Avci FY. Carbohydrates as T-cell antigens with implications in health and disease. Glycobiology. 2016;26:1029–40. doi: 10.1093/glycob/cww062. PMID:27236197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croucher NJ, Finkelstein JA, Pelton SI, Mitchell PK, Lee GM, Parkhill J, Bentley SD, Hanage WP, Lipsitch M. Population genomics of post-vaccine changes in pneumococcal epidemiology. Nature Genet. 2013;45:656–63. doi: 10.1038/ng.2625. PMID:23644493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamaluba M, Kandasamy R, Ndimah S, Morton R, Caccamo M, Robinson H, Kelly S, Field A, Norman L, Plested E, et al.. A cross-sectional observational study of pneumococcal carriage in children, their parents, and older adults following the introduction of the 7-valent pneumococcal conjugate vaccine. Medicine (Baltimore). 2015;94:e335. doi: 10.1097/MD.0000000000000335. PMID:25569650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, et al.. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348:1737–46. doi: 10.1056/NEJMoa022823. PMID:12724479. [DOI] [PubMed] [Google Scholar]
- 21.Rodenburg GD, de Greeff SC, Jansen AG, de Melker HE, Schouls LM, Hak E, Spanjaard L, Sanders EA, van der Ende A. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis. 2010;16:816–23. doi: 10.3201/eid1605.091223. PMID:20409372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trends by Serotype Group, 1998–2015 In: National Center for Immunization and Respiratory Diseases DoBD , ed. Active Bacterial Core surveillance: Centers for Disease Control and Prevention. 2016. https://www.cdc.gov/abcs/reports-findings/survreports/spneu-types.html [Google Scholar]
- 23.Croucher NJ, Finkelstein JA, Pelton SI, Parkhill J, Bentley SD, Lipsitch M, Hanage WP. Population genomic datasets describing the post-vaccine evolutionary epidemiology of Streptococcus pneumoniae. Sci Data. 2015;2:150058. doi: 10.1038/sdata.2015.58. PMID:26528397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, Jackson D, Thomas A, Beall B, Lynfield R, et al.. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J Infect Dis. 2007;196:1346–54. doi: 10.1086/521626. PMID:17922399. [DOI] [PubMed] [Google Scholar]
- 25.Hackel M, Lascols C, Bouchillon S, Hilton B, Morgenstern D, Purdy J. Serotype prevalence and antibiotic resistance in Streptococcus pneumoniae clinical isolates among global populations. Vaccine. 2013;31:4881–7. doi: 10.1016/j.vaccine.2013.07.054. PMID:23928466. [DOI] [PubMed] [Google Scholar]
- 26.Rosenblut A, Napolitano C, Pereira A, Moreno C, Kolhe D, Lepetic A, Ortega-Barria E. Etiology of acute otitis media and serotype distribution of Streptococcus pneumoniae and Haemophilus influenzae in Chilean children <5 years of age. Medicine (Baltimore). 2017;96:e5974. doi: 10.1097/MD.0000000000005974. PMID:28178138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dagan R, Poolman J. Siegrist C-A. Glycoconjugate vaccines and immune interference: A review. Vaccine. 2010;28:5513–23. doi: 10.1016/j.vaccine.2010.06.026. PMID:20600514. [DOI] [PubMed] [Google Scholar]
- 28.Pichichero ME. Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum Vaccin Immunother. 2013;9:2505–23. doi: 10.4161/hv.26109. PMID:23955057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croucher NJ, Harris SR, Fraser C, Quail MA, Burton J, van der Linden M, McGee L, von Gottberg A, Song JH, Ko KS, et al.. Rapid pneumococcal evolution in response to clinical interventions. Science. 2011;331:430–4. doi: 10.1126/science.1198545. PMID:21273480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dagan R. Serotype replacement in perspective. Vaccine. 2009;27(Suppl)3:C22–4. doi: 10.1016/j.vaccine.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999–2011(1.). Emerg Infect Dis. 2013;19:1074–83. doi: 10.3201/eid1907.121830. PMID:23763847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park IH, Kim KH, Andrade AL, Briles DE, McDaniel LS, Nahm MH. Nontypeable pneumococci can be divided into multiple cps types, including one type expressing the novel gene pspK. MBio. 2012;3:e00035–12. doi: 10.1128/mBio.00035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller LE, Robinson DA, McDaniel LS. Nonencapsulated Streptococcus pneumoniae: Emergence and Pathogenesis. MBio. 2016;7:e01792. doi: 10.1128/mBio.01792-15. PMID:27006456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pipkins HR, Bradshaw JL, Keller LE, McDaniel LS. Increased virulence of an encapsulated Streptococcus pneumoniae upon expression of pneumococcal surface protein K. J Infect Dis. 2018;217:1637–44. doi: 10.1093/infdis/jiy058. PMID:29394357. [DOI] [PubMed] [Google Scholar]
- 35.Avci F, Kasper D, Paul W, Littman D, Yokoyama W. How Bacterial Carbohydrates Influence the Adaptive Immune System. Annu. Rev. Immunol., Vol 28 2010;28:107–30. doi: 10.1146/annurev-immunol-030409-101159. PMID:19968562. [DOI] [PubMed] [Google Scholar]
- 36.Sheerin D, Openshaw PJ, Pollard AJ. Issues in vaccinology: Present challenges and future directions. Eur J Immunol. 2017;47:2017–25. doi: 10.1002/eji.201746942. PMID:28861908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avci F. Novel Strategies for Development of Next-Generation Glycoconjugate Vaccines. Curr. Top. Med. Chem. 2013;13:2535–40. [DOI] [PubMed] [Google Scholar]
- 38.Costantino P, Rappuoli R, Berti F. The design of semi-synthetic and synthetic glycoconjugate vaccines. Expert Opin Drug Discov. 2011;6:1045–66. doi: 10.1517/17460441.2011.609554. PMID:22646863. [DOI] [PubMed] [Google Scholar]
- 39.Avci F, Li X, Tsuji M, Kasper D. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med. 2011;17:1602–U115. doi: 10.1038/nm.2535. PMID:22101769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Middleton DR, Sun L, Paschall AV, Avci FY. T Cell-Mediated Humoral Immune Responses to Type 3 Capsular Polysaccharide of Streptococcus pneumoniae. J Immunol. 2017;199:598–603. doi: 10.4049/jimmunol.1700026. PMID:28566369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avci F, Li X, Tsuji M, Kasper D. Isolation of carbohydrate-specific CD4(+) T cell clones from mice after stimulation by two model glycoconjugate vaccines. Nat Protoc. 2012;7:2180–92. doi: 10.1038/nprot.2012.138. PMID:23196974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avci FY, Li X, Tsuji M, Kasper DL. Carbohydrates and T cells: a sweet twosome. Semin Immunol. 2013;25:146–51. doi: 10.1016/j.smim.2013.05.005. PMID:23757291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jackson LA, El Sahly HM, George S, Winokur P, Edwards K, Brady RC, Rouphael N, Keitel WA, Mulligan MJ, Burton RL, et al.. Randomized clinical trial of a single versus a double dose of 13-valent pneumococcal conjugate vaccine in adults 55 through 74 years of age previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2018;36:606–14. doi: 10.1016/j.vaccine.2017.12.061. PMID:29279281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Licciardi PV, Toh ZQ, Clutterbuck EA, Balloch A, Marimla RA, Tikkanen L, Lamb KE, Bright KJ, Rabuatoka U, Tikoduadua L, et al.. No long-term evidence of hyporesponsiveness after use of pneumococcal conjugate vaccine in children previously immunized with pneumococcal polysaccharide vaccine. J Allergy Clin Immunol. 2016;137:1772–9 e11. doi: 10.1016/j.jaci.2015.12.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sa-Leao R, Nunes S, Brito-Avo A, Alves CR, Carrico JA, Saldanha J, Almeida JS, Santos-Sanches I, de Lencastre H, et al.. High rates of transmission of and colonization by Streptococcus pneumoniae and Haemophilus influenzae within a day care center revealed in a longitudinal study. J Clin Microbiol. 2008;46:225–34. doi: 10.1128/JCM.01551-07. PMID:18003797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jansen KU, Knirsch C, Anderson AS. The role of vaccines in preventing bacterial antimicrobial resistance. Nat Med. 2018;24:10–9. doi: 10.1038/nm.4465. PMID:29315295. [DOI] [PubMed] [Google Scholar]


