ABSTRACT
This Phase 1, randomized, double-blind, placebo-controlled study was conducted to evaluate the safety, tolerability and immunogenicity of different doses of ExPEC4V conjugate vaccine (4-16µg Polysaccharide [PS]/serotype) in healthy Japanese participants, stratified into younger (≥20 to <50 years) or older age groups (≥50 years). Within each age group, participants were randomized to a single vaccination with 1 of 3 dose levels of ExPEC4V (4, 8 and 16 µg PS/serotype) or placebo. Safety and tolerability were the primary objectives; immunogenicity was secondary. Of the 48 participants, 47 (98%) completed; one (2%) in the placebo group discontinued. A total of 48% participants had ≥1 AE (younger group: n = 13 [54%]; older group: n = 10 [41.7%]). Solicited and unsolicited AEs were reported in 44% and 8% participants, respectively in the combined ExPEC4V groups. Pain/tenderness (n = 11 [31%]) and redness (n = 9 [25%]) were the most frequently reported solicited local AEs, whereas fatigue (n = 4 [11%]), headache (n = 4 [11%]), muscle pain (n = 2 [6%]), and malaise (n = 5 [14%]) were the most common solicited systemic AEs in the combined ExPEC4V group. No serious AEs, deaths, or discontinuation due to AEs were reported. All doses were immunogenic with an increase in IgG (ELISA) geometric mean titers of at least 5-fold from baseline to Days 15 and 30 for all serotypes. Of participants vaccinated with ExPEC4V, 75% - 100% demonstrated an ELISA titer increase of ≥2-fold. Strong correlation observed between ELISA and OPK. ExPEC4V was well tolerated and elicited an immunogenic response at all dose levels (up to 16 µg PS/serotype) in healthy Japanese participants.
KEYWORDS: ExPEC4V, Immunogenicity, NCT02748967, Safety, Tolerability
Introduction
Extraintestinal pathogenic E. coli (ExPEC) cause infections outside the GI tract1 and are the most common cause of urinary tract infections (UTIs) and blood stream infections.2,3 ExPEC is also a leading cause of severe sepsis, which is the 10th overall cause of death and the 6th leading cause of hospitalization in the United States.4,5 In the US, it is estimated that up to 40,000 patients die annually due to invasive ExPEC disease (IED), in particular from E. coli sepsis. Overall case-fatality rates for ExPEC bacteremia range from 13% to 19%, but may be much higher (up to 60%) in the elderly with healthcare-associated infections.6,7
Until the late 1990s, the majority of the ExPEC isolates were susceptible to first line antibiotic classes including cephalosporins, fluoroquinolones, and trimethoprim-sulfamethoxazole. However, the management of ExPEC infections became more difficult worldwide during the 2000s due to increasing prevalence of (multi) drug-resistant (MDR) E. coli strains such as the MDR sequence type (ST) O25b:ST131.3,8 An increase in antibiotic resistance of this clone resulted in increased morbidity, mortality, and treatment cost.8,9 A broad distribution of antibiotic resistance among E. coli strains has also been demonstrated from human infection in Japan.10,11
The lipopolysaccharide (LPS) O-antigen, a surface antigen of E. coli, is essential for the pathogenesis of the bacteria as it protects the E. coli from opsonophagocytosis.2,12 Therefore, antibodies specific to O-antigen might offer effective protection against ExPEC infections. Preclinical and clinical studies have shown that multivalent conjugated E coli O-antigen vaccines are safe and immunogenic.13
ExPEC4V is a tetravalent O-polysaccharide conjugate vaccine being developed for active immunization for the prevention of IED, which is caused by vaccine O-serotypes in adults. It is composed of the O-antigens of the ExPEC serotypes O1A, O2, O6A and O25B bioconjugated to a detoxified variant of Exotoxin A from Pseudomonas aeruginosa (EPA). These O-antigens for the bioconjugated ExPEC4V vaccine were selected on the basis of epidemiological studies that showed these E. coli serotypes to be frequently associated with UTI and bacteremia infections.12 In preclinical research and in the first-in-human clinical study conducted in women with a history of recurrent UTI, ExPEC4V (4 µg Polysaccharide [PS]/serotype) was well tolerated and elicited a robust antibody response at Days 30 and 270 post-vaccination.2,12 Data from Japanese Nosocomial Infections Surveillance suggest that E.coli causing bacteremia is increasing in the Japanese population.14 Since antibiotic resistant ExPEC strains are also prevalent in the Japanese population,15 the Phase 1 study described herein was conducted to investigate the safety, tolerability and immunogenicity of 3 different dose levels of ExPEC4V vaccine in healthy adult Japanese participants. This study was conducted in a dose-ascending manner as there was no prior clinical experience in the Japanese population. The results of this study in Japanese subjects, along with a global Phase 2 study (manuscript under preparation), would be used to support the dose selection of ExPEC4V in future studies.
Results
Participants
Of the 48 randomized participants (younger age group: n = 24; older age group: n = 24), 47 completed the study. One participant from placebo group (younger age group) withdrew consent due to family reason. The median age of the younger group was 41.0 years (range: 20 to 49) and that of the older age group was 56.5 years (range: 50 to 70). A history of previous vaccination was reported in 71% of participants in the younger group, and 54% in the older group. In the younger group, 14 of 24 participants (58%) had ≥ 1 medical history abnormality, and the most commonly reported were rhinitis allergic (n = 4/24, 17%) and headache (n = 3/24,13%). In the older group, 18 of 24 participants (75%) had ≥1 medical history abnormality, with the most commonly reported being appendicitis and appendectomy (n = 6, 25%). There were no apparent differences in demographic and baseline characteristics between the treatment groups or across age groups (Table 1).
Table 1.
Demographics and baseline characteristics of participants by age group and treatment.
| Placebo | ExPEC4V |
|||
|---|---|---|---|---|
| N = 12 | 4:4:4:4 N = 12 | 8:8:8:8 N = 12 | 16:16:16:16 N = 12 | |
| All ages | ||||
| Sex, n (%) | ||||
| Male | 5 (42) | 6(50) | 6(50) | 5 (42) |
| Race, n (%) | ||||
| Asian | 12 (100) | 12 (100) | 12 (100) | 12 (100) |
| Age (years) | ||||
| Mean (SD) | 44.2 (16.57) | 44.6 (15.48) | 45.6 (10.97) | 51.9 (13.83) |
| Body-mass index (kg/m2) | ||||
| Mean (SD) | 22.94 (2.374) | 22.10 (1.817) | 22.17 (2.575) | 22.22 (2.832) |
| Childbearing potential for female patients, n (%) | ||||
| N | 7 | 6 | 6 | 7 |
| Yes | 4 (57) | 4 (67) | 3 (50) | 3 (43) |
| Cigarette smokers, n (%) | ||||
| N | 5 | 6 | 8 | 9 |
| Current | 1 (20) | 2 (33) | 3 (38) | 5 (56) |
| Former | 4 (80) | 4 (67) | 5 (63) | 4 (44) |
| Age: > = 20 to <50 years | ||||
| N | 6 | 6 | 6 | 6 |
| Sex, n (%) | ||||
| Male | 2 (33) | 3 (50) | 4 (67) | 3 (50) |
| Age (years) | ||||
| Mean (SD) | 30.3 (9.91) | 32.7 (13.11) | 37.3 (9.22) | 41.0 (9.17) |
| Body-mass index (kg/m2) | ||||
| Mean (SD) | 23.05 (3.078) | 21.77 (2.067) | 21.44 (3.120) | 21.16 (2.643) |
| Childbearing potential for female patients, n (%) | ||||
| N | 4 | 3 | 2 | 3 |
| Yes | 4 (100) | 3 (100) | 2 (100) | 3 (100) |
| Cigarette smokers, n (%) | ||||
| N | 2 | 3 | 6 | 5 |
| Current | 1 (50) | 1 (33) | 2 (33) | 4 (80) |
| Former | 1 (50) | 2 (67) | 4 (67) | 1 (20) |
| Age: > = 50 years | ||||
| N | 6 | 6 | 6 | 6 |
| Sex, n (%) | ||||
| Male | 3 (50) | 3 (50) | 2 (33) | 2 (33) |
| Age (years) | ||||
| Mean (SD) | 58.0 (6.84) | 56.5 (3.83) | 53.8 (4.07) | 62.8 (7.14) |
| Body-mass index (kg/m2) | ||||
| Mean (SD) | 22.83 (1.702) | 22.43 (1.651) | 22.91 (1.886) | 23.29 (2.815) |
| Childbearing potential for female patients, n (%) | ||||
| N | 3 | 3 | 4 | 4 |
| Yes | 0 | 1 (33) | 1 (25) | 0 |
| Cigarette smokers, n (%) | ||||
| N | 3 | 3 | 2 | 4 |
| Current | 0 | 1 (33) | 1 (50) | 1 (25) |
| Former | 3 (100) | 2 (67) | 1 (50) | 3 (75) |
Safety
A total of 23 (48%) participants experienced ≥1 AE (n = 13, 54% in younger age group; n = 10, 42% in older age group). Deaths, other SAEs or AEs leading to discontinuation were not reported in the study. Solicited AEs were reported in 16 (44%) participants who received ExPEC4V and in 6 (50%) participants who received placebo. A total 13 (54%) participants in the younger age group and 9 (38%) participants in the older age group reported solicited AEs. All solicited AEs were mild to moderate (Grade 1 or 2).
Solicited local AEs were reported more frequently in participants who received ExPEC4V (n = 12, 33%) compared to participants who received placebo (n = 1, 8%). The incidence of solicited local AEs was comparable between the younger (n = 6, 25%) and older (n = 7, 29%) age group. Overall, the most commonly reported solicited local AEs were pain/tenderness at the injection site (n = 12, 25%) and redness at the injection site (n = 9, 19%). In the younger age group, pain/tenderness was reported in 3 participants in the 8 μg PS/serotype group, 2 participants in the 16 μg PS/serotype group, and 1 participant in the placebo group. In the older age group, pain/tenderness was reported in 2 participants in the 8 μg PS/serotype group and 4 participants in the 16 μg PS/serotype group. The median (range) time to onset was 6.5 days (2.0–8.0 days) for pain/tenderness at the injection site and 7.0 days (5.0–8.0 days) for redness at the injection site. The median duration of pain/tenderness was 3.0 days (range: 1.0 to 6.0 days) for all participants with a median of 2.5 days in the younger age group and 3.0 days in the older age group. Solicited systemic AEs were reported in 8 (22%) participants who received ExPEC4V and 5 (42%) participants who received placebo. The most frequently reported solicited systemic AEs were malaise (n = 9, 19%), fatigue (n = 7, 15%), and headache (n = 6, 13%) (Table 2). The median (range) time to onset of malaise and headache was 1.0 day (1.0–6.0 days) and 2.0 days (1.0–8.0 days), respectively. Both malaise and headache had a similar median duration of 2.0 days (1.0–9.0 days). Whereas the median (range) time to onset of fatigue, tiredness, feeling weary was 3.0 days (1.0–5.0 days) with a median (range) duration 2.0 days (1.0–9.0 days).
Table 2.
Adverse events summary.
| Placebo | ExPEC4V |
||||
|---|---|---|---|---|---|
| N = 12 | 4:4:4:4 N = 12 | 8:8:8:8 N = 12 | 16:16:16:16 N = 12 | Combined N = 36 | |
| Any AE, n (%) | 7 (58) | 2 (17) | 6 (50) | 8 (67) | 16 (44) |
| Any AE leading to discontinuation of the study | 0 | 0 | 0 | 0 | 0 |
| Any serious AE | 0 | 0 | 0 | 0 | 0 |
| Any solicited AE, n (%) | 6 (50) | 2 (17) | 6 (50) | 8 (67) | 16 (44) |
| Any Grade 3 solicited AE, n (%) | 0 | 0 | 0 | 0 | 0 |
| Any solicited local AE, n (%) | 1 (8) | 0 | 6 (50) | 6 (50) | 12 (33) |
| Pain/Tenderness | 1 (8) | 0 | 5 (42) | 6 (50) | 11(31) |
| Redness | 0 | 0 | 4 (33) | 5 (42) | 9 (25) |
| Swelling/hard spot | 0 | 0 | 0 | 1 (8) | 1 (3) |
| Any solicited systemic AE, n (%) | 5 (42) | 2 (17) | 2 (17) | 4 (33) | 8 (22) |
| Fatigue/Tiredness/Feeling weary | 3 (25) | 1 (8) | 1 (8) | 2 (17) | 4 (11) |
| Headache | 2 (17) | 2 (17) | 0 | 2 (17) | 4 (11) |
| Nausea | 2 (17) | 0 | 0 | 0 | 0 |
| Muscle Pain | 0 | 1 (8) | 0 | 1 (8) | 2 (6) |
| Malaise | 4 (33) | 2 (17) | 1 (8) | 2 (17) | 5 (14) |
| Fever | 0 | 0 | 1 (8) | 0 | 1 (3) |
| Any solicited systemic AE thought to be related to vaccine, n (%) | 3 (25) | 2 (17) | 2 (17) | 4 (33) | 8 (22) |
| Any unsolicited AE, n (%) | 2 (17) | 0 | 2 (17) | 1 (8) | 3 (8) |
| Nasopharyngitis | 1 (8) | 0 | 0 | 0 | 0 |
| Laceration of left hand | 1 (8) | 0 | 0 | 0 | 0 |
| Lymph node pain | 0 | 0 | 1 (8) | 0 | 1 (8) |
| Lymphadenopathy | 0 | 0 | 1 (8) | 0 | 1 (8) |
| Myalgia | 0 | 0 | 1 (8) | 0 | 1 (8) |
| Increased WBC | 0 | 0 | 0 | 1 (8) | 1 (8) |
| Any Grade 3 unsolicited AE, n (%) | 0 | 0 | 0 | 0 | 0 |
| Any unsolicited AE thought to be related to vaccine, n (%) | 0 | 0 | 2 (17) | 1 (8) | 3 (8) |
Unsolicited AEs were reported in 3 (8%) participants who received ExPEC4V and 2 (17%) participants who received placebo (Table 2). Lymph node pain and lymphadenopathy were reported in 1 participant (8 μg PS/serotype) of the younger age group, while myalgia (8 μg PS/serotype) and increased WBC (16 μg PS/serotype) were reported in 2 participants of the older age group. Also, nasopharyngitis and laceration of the left hand that were reported in a placebo recipient was considered as an unsolicited AE. There were no clinically notable changes in the biochemical or hematology laboratory parameters and vital sign measurements over time in any of the treatment groups.
Immunogenicity
The ELISA IgG geometric mean titers (GMTs) of ExPEC4V recipients increased markedly (at least 5-fold) on Day 15 and Day 30, relative to baseline, for all dose levels and serotypes of ExPEC4V (Fig. 1) (Supplementary Table 1). For the 4 μg, 8 μg and 16 μg PS/serotype groups, Day 15 GM fold increases in IgG titer values relative to baseline were as follows, respectively: O1A, 5.2-, 9.4- and 21.8-fold; O2, 19.7-, 27.5- and 34.9-fold; O6A, 7.5-, 7.0- and 23.67-fold; O25B, 16.1-, 6.5- and 13.5-fold. IgG GMT values and GM fold increases on Day 15 and Day 30 were similar, indicating the vaccine-mediated immune response was at a maximum by Day 15. Placebo group exhibited little or no change in IgG GMT values through Day 30.
Figure 1.
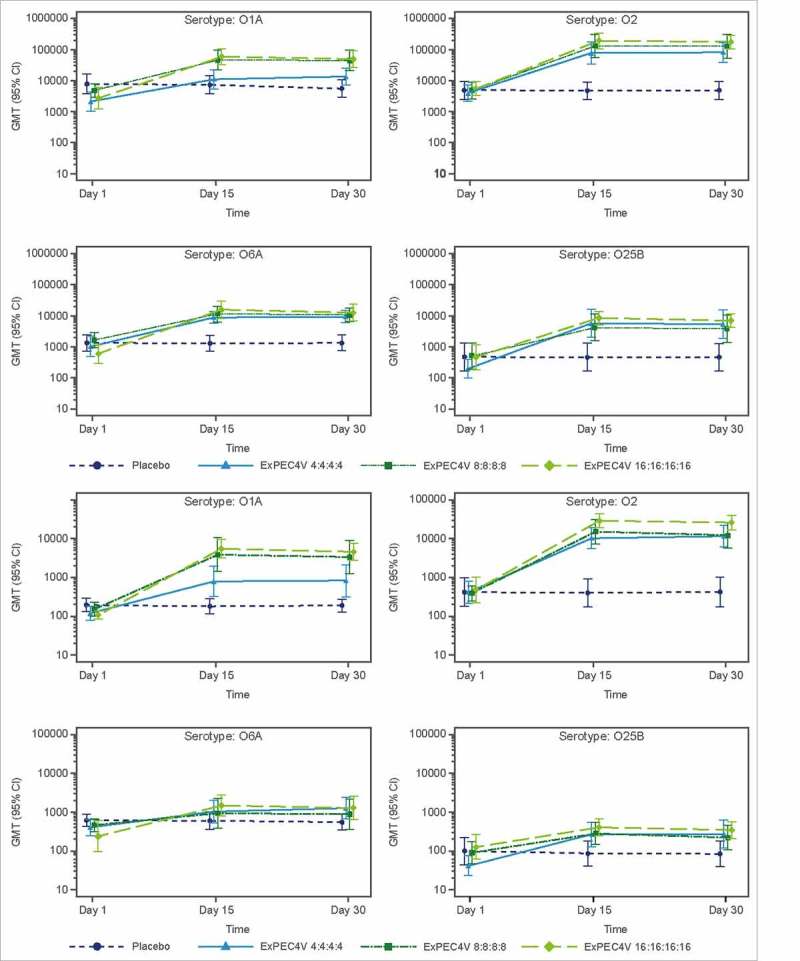
Postimmunization GMT at Day 15 and Day 30 as measured with ELISA (A) and OPK (B) assays.
The OPK functional antibody GMT values also demonstrated an immunogenic response on Day 15 and Day 30. For 4 μg, 8 μg and 16 μg PS/serotype groups, Day 15 GM fold increases in titer values relative to baseline were as follows, respectively: O1A, 4.2-, 17.0- and 29.1-fold; O2, 23.4-, 38.6- and 57.1-fold; O6A, 2.6-, 2.2- and 5.8-fold; O25B, 4.6-, 2.8- and 2.9-fold. OPK GMT and GM fold increases on Day 15 and Day 30 were similar, indicating the vaccine-mediated immune response was at a maximum by Day 15. Placebo sera exhibited little or no change in functional antibody titer values through Day 30.
On Day 15 compared to baseline, the proportion of participants with ≥2-fold increase in antibody titers across all doses (Figure 2) ranged from 83%-100% (O1A), 100% (O2), 83%-100% (O6A) and 75%-92% (O25B) by ELISA and 67%-100% (O1A), 100% (O2), 50%-75% (O6A) and 50%-67% (O25B) by OPK. The percentage of subjects with ≥2-fold increased antibody titers observed on Day 30 was similar to those on Day 15. None of the participants who received placebo showed ≥2-fold increase in ELISA titers (Fig. 2). Thus, immunogenicity findings described above with ELISA and OPK were generally in agreement with each other. The correlation coefficient between ELISA and OPK for all treatments and age subgroups ranged from 0.5940 to 0.9378 and 0.6249 to 0.9462, respectively.
Figure 2.
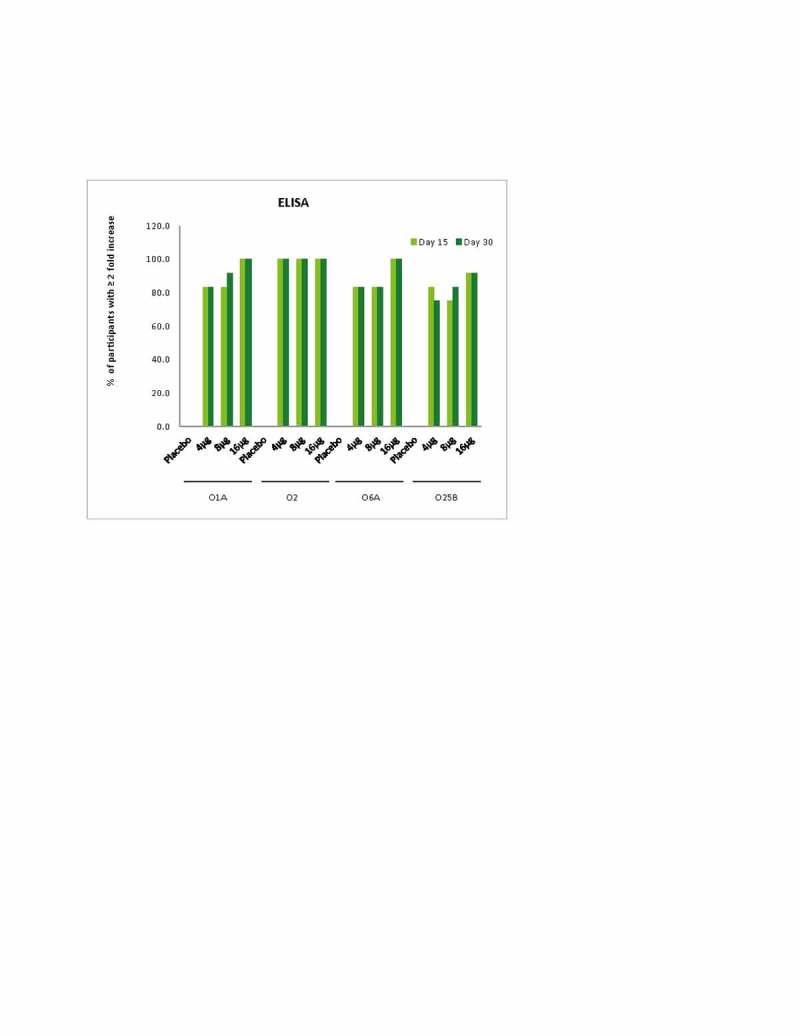
Percentage of participants with ≥2 fold increase at Day 15 and Day 30.
Discussion
The reported Phase 1, placebo-controlled study in healthy Japanese adult subjects evaluated the safety, tolerability, and immunogenicity of an ExPEC4V vaccine at 3 different doses (4, 8, and 16 μg PS/serotype) administered intramuscularly. The vaccine was found to be safe and well tolerated at all dose levels in younger (≥20 to <50 years) and older (≥50 years) adults. Compared to placebo, the vaccine elicited a robust immune response for all serotypes irrespective of the age or dose groups.
Preclinical studies of ExPEC4V vaccine have shown that a dose ranging from 0.4 μg to 20 μg PS/serotype has immunogenicity, and was tolerated.12 In a first-in-human study conducted in women aged 18 to 70 years with a history of recurrent UTI, a single dose of ExPEC4V (4 μg PS/serotype) was found to be safe and immunogenic.2 In the present study, the ExPEC4V vaccine was evaluated progressively from 4 μg PS/serotype to 16 μg PS/serotype since no prior safety data were available in the Japanese population. Similar to the first-in-human study, the present study showed that all ExPEC4V doses were immunogenic and well tolerated. Based on the results from these studies a phase 2 study to evaluate the dose-dependent immunogenicity of ExPEC4V, as measured by ELISA and OPK on Day 15 has been completed and two doses were selected to continue further evaluation (Manuscript under preparation).
In this study, the ExPEC4V vaccine showed no vaccine-related safety signals with a safety profile consistent with the previously studied conjugated vaccines.16–18 As observed in the previous Phase 1 study2 conducted in participants with history of UTI, no vaccine related SAE was observed in the present study conducted in healthy volunteers. Both studies reported a higher incidence of injection site reactions in the vaccine recipients than the placebo group. All solicited local AEs were Grade 1 or 2 (mild or moderate) in severity. The most frequently reported solicited local AE was pain/tenderness at the injection site and systemic solicited AEs were fatigue, headache, and malaise, which were comparable with the previous Phase 1 study.
The ExPEC4V vaccine in the present study demonstrated a vaccine specific immune response for all the 4 serotypes in the Japanese population at Days 15 and 30 indicating a generally stable antibody titer to these E. coli serotypes over this time period. Placebo-treated subjects exhibited little or no change from baseline in antibody titers for the ExPEC4V serotypes for Days 15 and 30 (ELISA and OPK). In contrast, the ExPEC4V vaccine yielded an immunogenic response for all dose groups and for all serotypes, with an increase in antibody titers on Days 15 and 30 compared with baseline, within dose groups and for all serotypes. The wide 95% confidence intervals of GMTs for all serotypes and higher baseline GMT value in the control group versus vaccinated groups of O6A serotype could be due to the small sample size for each study group. There was a positive correlation between ELISA and OPK assay titers for all treatments (3 ExPEC4V groups and placebo group) and age subgroups in all serotypes.
Solicited local and systemic AEs will need to be further evaluated in studies with an increased sample size to exclude unacceptable reactogenicity with increasing doses. Also, further evaluation of different doses of ExPEC4V is needed to investigate the dose immunogenicity response and to analyze dose-dependent cross-reactivity between serotypes.
Conclusion
Overall, the tetravalent ExPEC4V vaccine at 4, 8, and 16 µg PS/serotype was well tolerated and showed no new safety findings in healthy Japanese participants. The ExPEC4V vaccine exhibited strong IgG responses with opsonophagocytic killing activity for all dose groups. Findings of this study will help in the appropriate dose selection for future studies.
Methods
Participants
Healthy Japanese men and women aged ≥20 years, with body mass index ≤30 kg/m2, were considered for inclusion in the study. They had to meet the laboratory criteria defined by the Division of Microbiology and Infectious Diseases (DMID) toxicity table within 7 days preceding vaccination. Participants were of generally stable health, and without acute illness. Women participants of childbearing potential were required to have a negative urine pregnancy test within 24 hours before vaccination to be eligible for participation in the study and agreed to use a highly effective method of birth control until at least 3 months after study vaccine administration.
Participants with acute illness, acute infection, or fever, congenital or acquired immunodeficiency, chronic active hepatitis B or hepatitis C infection, current or history of autoimmune diseases, severe asthma or medically attended wheezing in the 7 days before vaccination were excluded. Participants who had received immunosuppressive therapy within 6 months preceding vaccination, or long-term systemic corticosteroid therapy for more than 2 consecutive weeks within 3 months preceding vaccination were also excluded from the study.
The study protocol was approved by the local Institutional Review Board and the study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, applicable regulatory requirements, and in compliance with the protocol. All participants provided written informed consent to participate in the study.
Study design
This was a Phase 1, double-blind, randomized, placebo-controlled, parallel-group, single center study conducted between 6 May 2016 and 18 August 2016. Participants were stratified according to their age equally with a younger age group (≥20 to <50 years) and older age group (≥50 years). Participants in each age group were then enrolled in a dose ascending approach. The study included a screening phase, vaccination, and a safety and immunogenicity follow-up phase. Following screening, participants were randomized to 1 of the 3 study dose levels of ExPEC4V (4, 8, or 16 µg PS/serotype) or placebo (vaccine buffer) based on a computer-generated randomization schedule (Fig. 3).
Figure 3.
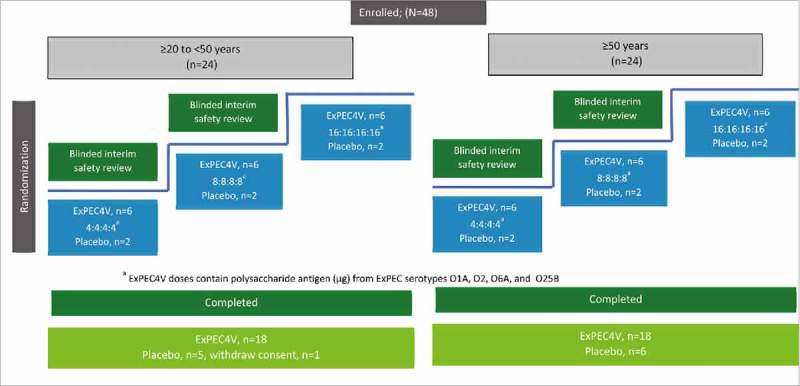
Schematic overview of the study design and participant disposition.
The randomization, vaccination, and post-vaccination procedures were completed on the same day (Day 1). Participants were observed for at least 60 minutes post-vaccination, for adverse events (AEs) or serious AEs, including anaphylactic reactions. At the end of 60-minute observation period, vital signs including body temperature were measured. Solicited local and systemic AEs were collected in a subject diary daily for 8 days post-vaccination. The participants were followed up for 30 days post-vaccination on Days 8 (±1), 15 (±2), and 30 (±3) for safety, and Days 15 (±2) and 30 (±3) for immunogenicity.
Prior to dose escalation, a blinded interim safety review was performed after each dose level and for each age group with a minimum of 6 participants to evaluate safety up to Day 15. The dose escalation decision was based on the available safety data. The dose escalation was done in both age groups separately and in parallel. The study duration for each participant was approximately 38 days (inclusive of screening and post-vaccination follow-up periods).
Concomitant medications
Concomitant therapies including vaccinations, medications, and herbal supplements were recorded throughout the study beginning with vaccine administration to the Day 30 visit. Concomitant therapies were also recorded beyond the Day 30 visit only in conjunction with serious adverse events (SAEs). Concomitant medications for underlying diseases such as hypertension, diabetes, hyperlipoproteinemia, or hypothyroidism were allowed.
Contraindications to vaccination
Acute illness or acute infection (excluding minor illnesses such as mild diarrhea or mild upper respiratory tract infection) and fever (axillary temperature ≥37.5°C) at the time of vaccination were considered contraindications to vaccination.
Study drug
ExPEC4V or placebo was administered as an intramuscular (IM) injection into the deltoid muscle, preferably in the nondominant arm (0.5 mL). Tris buffered saline was used as placebo. Each dose of ExPEC4V contained either 4, 8, or 16 μg PS/serotype.
Assessments
Tolerability and safety
The safety and tolerability endpoints included solicited local and systemic AEs, unsolicited AEs, serious AEs, AEs leading to study discontinuation and AEs leading to death. Safety evaluation included assessment of solicited AEs from the time of vaccination until Day 8 and assessment of unsolicited AEs up to and including Day 30. Also, clinical laboratory tests, vital signs, and physical examinations were performed. Solicited AEs were assessed as either local or systemic. Solicited local AEs included injection site reactions, pain/tenderness, erythema, and induration/swelling, while headache, fatigue, malaise, nausea, myalgia, and body temperature (≥38°C) were analyzed as a solicited systemic AE.
Immunogenicity
The immunogenicity endpoints were to evaluate the serotype-specific immune response in sera of participants, mediated by a single vaccination with different doses of ExPEC4V or placebo, as measured by an Enzyme-linked immunosorbent assay (ELISA) and an opsonophagocytic killing (OPK) assay on Days 1, 15 and 30. The ELISA and OPK assays for ExPEC4V were performed as described previously.2
Statistical methods
Sample size
For this study, 48 participants (24 each in both younger and older age groups) were randomized. The number of participants evaluated in this study was expected to allow preliminary safety and immunogenicity assessment.
Analysis sets
All randomized and vaccinated (different doses of ExPEC4V or placebo) participants were included in the safety analysis set. All randomized and vaccinated (different doses of ExPEC4V or placebo) participants who have antibody titer measures of immunogenicity at baseline, and at least one measures at Day 15 or Day 30 were included in the immunogenicity analysis set.
Statistical methods
Descriptive statistics were used to summarize the safety findings by dose and age groups. The immunogenicity endpoints measured with ELISA and OPK were summarized descriptively, while the correlation between ELISA and OPK was assessed using Pearson correlation coefficient methodology. Immunogenicity endpoints were evaluated on the basis of GMT of antibodies, proportion of subjects with 2-fold, 3-fold, and 4-fold changes in antibody titer, and antibody folder changes.
Funding Statement
This study was funded by Janssen Pharmaceutical K.K.
Disclosure of potential conflicts of interest
TO, HT, YH, YS, PIP, DA, GD, PH and JP are employees of Janssen.
Acknowledgments
Partial data were presented at International Society of Vaccines (ISV) Vaccine Congress 2017 as oral presentation.
Author Contributions
Conception and design: TO, HT, YH, YS. Collection and assembly of data: All authors. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors.
References
- 1.Russo TA, Johnson JR. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J Infect Dis. 2000;181(5):1753–4. doi: 10.1086/315418. PMID:10823778. [DOI] [PubMed] [Google Scholar]
- 2.Huttner A, Hatz C, van den Dobbelsteen G, Abbanat D, Hornacek A, Frölich R, Dreyer AM, Martin P, Davies T, Fae K, et al.. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect Dis. 2017;17(5):528–37. doi: 10.1016/S1473-3099(17)30108-1. PMID:28238601. [DOI] [PubMed] [Google Scholar]
- 3.Pitout JD. Extraintestinal Pathogenic Escherichia coli: A Combination of Virulence with Antibiotic Resistance. Front Microbiol. 2012;3:9. doi: 10.3389/fmicb.2012.00009. PMID:22294983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poolman JT, Wacker M. Extraintestinal Pathogenic Escherichia coli, a Common Human Pathogen: Challenges for Vaccine Development and Progress in the Field. J Infect Dis. 2016;213(1):6–13. doi: 10.1093/infdis/jiv429. PMID:26333944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo TA, Johnson JR. Extraintestinal isolates of Escherichia coli: identification and prospects for vaccine development. Expert Rev Vaccines. 2006;5(1):45–54. doi: 10.1586/14760584.5.1.45. PMID:16451107. [DOI] [PubMed] [Google Scholar]
- 6.2003 Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: Focus on an increasingly important endemic problem. Microbes Infect. ; 5(5):449–56. PMID:12738001. [Google Scholar]
- 7.Roubaud Baudron C, Panhard X, Clermont O, Mentré F, Fantin B, Denamur E, Lefort A. COLIBAFI Group . Escherichia coli bacteraemia in adults: age-related differences in clinical and bacteriological characteristics, and outcome. Epidemiol Infect. 2014;142(12):2672–83. doi: 10.1017/S0950268814000211. PMID:24559489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitout JD, DeVinney R. Escherichia coli ST131: a multidrug-resistant clone primed for global domination. F1000Res. 2017;6. pii: F1000 Faculty Rev-195. doi: 10.12688/f1000research.10609.1. PMID:28344773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mellata M. Human and avian extraintestinal pathogenic Escherichia coli: infections, zoonotic risks, and antibiotic resistance trends. Foodborne Pathog Dis. 2013;10(11):916–32. doi: 10.1089/fpd.2013.1533. PMID:23962019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki S, Shibata N, Yamane K, Wachino J, Ito K, Arakawa Y. Change in the prevalence of extended-spectrum-beta-lactamase-producing Escherichia coli in Japan by clonal spread. J Antimicrob Chemother. 2009;63(1):72–79. doi: 10.1093/jac/dkn463. PMID:19004839. [DOI] [PubMed] [Google Scholar]
- 11.Uchida Y, Mochimaru T, Morokuma Y, Kiyosuke M, Fujise M, Eto F, Eriguchi Y, Nagasaki Y, Shimono N, Kang D. Clonal spread in Eastern Asia of ciprofloxacin-resistant Escherichia coli serogroup O25 strains, and associated virulence factors. Int J Antimicrob Agents. 2010;35(5):444–50. doi: 10.1016/j.ijantimicag.2009.12.012. PMID:20188525. [DOI] [PubMed] [Google Scholar]
- 12.van den Dobbelsteen G, Fae KC, Serroyen J, van den Nieuwenhof IM, Braun M, Haeuptle MA, Sirena D, Schneider J, Alaimo C, Lipowsky G, et al.. Immunogenicity and safety of a tetravalent E. coli O-antigen bioconjugate vaccine in animal models. Vaccine. 2016;34(35):4152–60. doi: 10.1016/j.vaccine.2016.06.067. PMID:27395567. [DOI] [PubMed] [Google Scholar]
- 13.Cryz SJ Jr., Que JO, Cross AS, Furer E. Synthesis and characterization of a polyvalent Escherichia coli O-polysaccharide-toxin A conjugate vaccine. Vaccine. 1995;13(5):449–53. doi: 10.1016/0264-410X(94)00009-C. PMID:7639013. [DOI] [PubMed] [Google Scholar]
- 14. Japan Nosocomial Infections Surveillance. Annual Open Report 2016. Japan: Ministry of Health, Labour and Welfare, Japan; [accessed 2018 Feb 13]. https://janis.mhlw.go.jp/english/index.asp. [Google Scholar]
- 15.Matsumura Y, Noguchi T, Tanaka M, Kanahashi T, Yamamoto M, Nagao M, Takakura S, Ichiyama S.. Population structure of Japanese extraintestinal pathogenic Escherichia coli and its relationship with antimicrobial resistance. J Antimicrob Chemother. 2017;72(4):1040–49. doi: 10.1093/jac/dkw530. [DOI] [PubMed] [Google Scholar]
- 16.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, et al. . Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003;348(18):1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 17.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, et al. . Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. Engl J Med. 2002;346(7):491–6. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 18.Fattom AI, Horwith G, Fuller S, Propst M, Naso R. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: From the lab bench to phase III clinical trials. Vaccine. 2004;22(7):880–7. doi: 10.1016/j.vaccine.2003.11.034. [DOI] [PubMed] [Google Scholar]


