The subthalamic nucleus (STN) is the most commonly targeted structure for deep brain stimulation (DBS) in Idiopathic Parkinson's disease (IPD).1 Microelectrode recording (MER) and stimulation can be useful in localization of the physiological target during STN DBS.2 Intraoperative imaging can supplement physiological techniques in order to achieve optimal lead placement.3
We report here on a case in which intraoperative macrostimulation produced a false localizing sign, which has not been previously reported. A 65‐year‐old male with IPD was admitted for bilateral STN DBS. For the right STN, the initial planned stereotactic coordinates based on direct visualization by MRI were X = 11.97, Y = −2.41, Z = −3.76, Ring = 118, and Arc = 112.4. MER documented typical STN cells beginning at 0.03 mm above target and ending at 2.9 mm below target. No kinesthetic cells were found. At target depth (0.0 mm), we performed macrostimulation through the flexible sideport.4 At a current of 1.0 mA, the patient felt mild left‐leg contraction and thigh tightness, which was not clearly visible to the examiners. When stimulation was stopped, this tightness immediately subsided according to the patient. With increased stimulation at 2.0 mA, the left‐leg tightness returned and was more pronounced along with left‐arm tightness. Again, this tightness subsided after stimulation ceased. Stimulation was further increased to 4.0 mA and now involved visible contraction of the left leg and arm, without facial contraction or speech involvement. No oculomotor abnormalities or autonomic symptoms were observed. An intraoperative computed tomography (CT) scan was performed, which showed that the track was 2 mm medial to the intended target with the stereotactic coordinates X = 10.22, Y = −2.99, and Z = −5.07 (Fig. 1A). The microelectrode was moved 2 mm lateral, and a second track was performed. STN was encountered at 2.4 mm above target and ended at 3.7 mm below target. Five of seventeen cells tested had kinesthetic responses to the lower extremity. The lead was placed in this position (stereotactic coordinates: X = 11.97, Y = 2.41, and Z = 3.76) with good benefit and no side effects (Fig. 1B).
Figure 1.
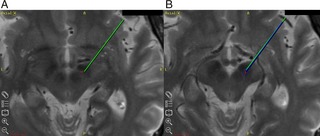
A: Green line points to intended target; red dot indicates the tip of the microelectrode after track 1. B: Green line indicates intended target (different axial slice from A). Dark blue line indicates microelectrode placement after track 2, with red dot indicating the tip of the microelectrode. In both images, the red dot is based on intraoperative CT visualization of the microelectrode tip. The CT image has been blended out to reveal the relationship with the brain anatomy on the merged preoperative MRI.
Using this case, we wish to highlight several points. First, we encountered STN near target depth, which was later than expected, suggesting (given the anatomical orientation of the nucleus) that our first track was medial or posterior, not anterior. The initial track was unlikely to be too anterior because the STN was encountered relatively late (+0.03 mm above target), whereas STN is usually encountered higher than anticipated if the track is too anterior, based on the shape of the STN.5 Furthermore, our intraoperative CT scan confirmed that our track was not too anterior (Y = −2.99). The patient did not experience any paresthesias during test stimulation to suggest a posterior track, leading us to believe that the track was medial to the intended target. To our surprise, during test stimulation, the patient developed limb contractions, suggesting that the track was possibly too lateral, resulting in corticospinal tract activation. However, corticospinal tract activation and corticobulbar fibers are in close promixity, and therefore activation of these fibers would be expected to result in facial contraction or changes in speech in addition to limb contractions6, but this pattern was not observed in our case. Thus, our suspicion remained that our track was too medial to the intended target, though this was not completely in line with our neurophysiological data. Also, we wish to point out that though it is theoretically possible that the limb contracture observed was dystonia, the pattern of contraction observed was not typical of dystonia in PD. Typical PD dystonia usually involves unilateral equinovarus foot, upper arm/forearm, or forearm/hand flexion,7 none of which was observed in our case. In fact, STN stimulation typically results in improvement of dystonia,8 rather than worsening. Thus, it is most probable that our patient had “capsular‐like” limb contraction, rather than dystonia, which was arising from a medial trajectory, and intraoperative imaging was used to verify this.
The use of intraoperative imaging can be used to provide accurate three‐dimensional confirmation of electrode tip locations relative to preoperative images and surgical plans.9 Intraoperative neurophysiology is used to increase the accuracy of DBS, but there are times when the physiological data are conflicting or confusing,10 as noted in our case. This can result in additional penetrations of the brain, thus increasing surgical risk.9 To avoid increasing surgical risk and to clarify the target, intraoperative CT was obtained. The intraoperative CT scan confirmed our initial suspicion that our track was, in fact, medial to the intended target. This imaging was helpful because it allowed us to confidently move the electrode 2 mm lateral during MER of the second track. As a result, we obtained clear leg kinesthetic cells, suggesting that this track was still in the medial sensorimotor part of the STN and confirming that our initial track was clearly too medial. This is in line with a reported finding that if a MER track shows greater than three movement‐related cells involving the leg, there is a 90% likelihood that the MER track maps to the medial part of the motor STN.11
This medial MER trajectory resulted in capsular‐like side effects in an unusual pattern (face sparing) without clear autonomic or oculomotor side effects, other signs that are typically observed with a medial trajectory.12 This leads us to conclude that muscle contraction during stimulation does not always suggest a lateral trajectory, especially if the pattern of muscle contraction is atypical as described in our case. Though fusion of MRI and CT can produce merging error,13 this is typically in the dorsoventral direction and so is not pertinent to our discussion. Our case deals with accuracy of the lead in the mediolateral direction, and we have reported a unique finding that is supported with our imaging and neurophysiological data. This case is an example where intraoperative imaging can be particularly valuable during DBS surgery.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
G.D.P.: 1A, 1B, 1C, 2A, 2B, 2C, 3A, 3B
S.S.: 2A, 2B, 2C, 3B
L.V.: 1B, 1C, 2A, 2B, 2C, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for previous 12 months: The authors declare that there are no disclosures to report.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Sanghera MK, Desaloms JM, Stewart RM. High‐frequency stimulation of the subthalamic nucleus for the treatment of Parkinson's disease–a team perspective. J Neurosci Nurs 2004;36:301–311. [DOI] [PubMed] [Google Scholar]
- 2. Romanelli P, Heit G, Hill BC, Kraus A, Hastie T, Bronte‐Stewart HM. Microelectrode recording revealing a somatotopic body map in the subthalamic nucleus in humans with Parkinson disease. J Neurosurg 2004;100:611–618. [DOI] [PubMed] [Google Scholar]
- 3. Smith AP, Bakay RA. Frameless deep brain stimulation using intraoperative O‐arm technology. Clinical article. J Neurosurg 2011;115:301–309. [DOI] [PubMed] [Google Scholar]
- 4. Broggi G. Chronic deep brain stimulation: clinical results In: Germano IM, ed. Neurosurgical Treatment of Movement Disorders. Park Ridge, Illinois: AANS Publications Committee; 1998:169. [Google Scholar]
- 5. Baker K, Boulis N, Rezai A, Montgomery E, Jr . Target selection using microelectrode recording In: Zvi Israel, Burchiel Kim J., ed. Microelectrode Recording in Movement Disorder Surgery. New York, NY: Thieme Medical Publishers, Inc.; 2004:140. [Google Scholar]
- 6. Gorgulho AA, Shields DC, Malkasian D, Behnke E, Desalles AA. Stereotactic coordinates associated with facial musculature contraction during high‐frequency stimulation of the subthalamic nucleus. J Neurosurg 2009;110:1317–1321. [DOI] [PubMed] [Google Scholar]
- 7. Tolosa E, Compta Y. Dystonia in Parkinson's disease. J Neurol 2006;253(suppl 7):VII7–VII13. [DOI] [PubMed] [Google Scholar]
- 8. Ostrem JL, Racine CA, Glass GA, et al. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology 2011;76:870–878. [DOI] [PubMed] [Google Scholar]
- 9. Shahlaie K, Larson PS, Starr PA. Intraoperative computed tomography for deep brain stimulation surgery: technique and accuracy assessment. Neurosurgery 2011;68(1 suppl Operative):114–124; discussion 124. [DOI] [PubMed] [Google Scholar]
- 10. Holloway K, Docef A. A quantitative assessment of the accuracy and reliability of O‐arm images for deep brain stimulation surgery. Neurosurgery 2013;72(1 suppl Operative):47–57. [DOI] [PubMed] [Google Scholar]
- 11. Theodosopoulos PV, Marks WJ, Jr , Christine C, Starr PA. Locations of movement‐related cells in the human subthalamic nucleus in Parkinson's disease. Mov Disord 2003;18:791–798. [DOI] [PubMed] [Google Scholar]
- 12. Rodriguez‐Oroz MC, Rodriguez M, Leiva C, et al. Neuronal activity of the red nucleus in Parkinson's disease. Mov Disord 2008;23:908–911. [DOI] [PubMed] [Google Scholar]
- 13. Bakay RA, Falowski S. Perspectives on “fusion image‐based programming after subthalamic nucleus deep brain stimulation”. World Neurosurg 2011;75:436–437. [DOI] [PubMed] [Google Scholar]


