SCA12 is an autosomal dominant cerebellar ataxia (ADCA) associated with an expanded cytosine‐adenine‐guanine (CAG) repeat in the 5′‐untranslated region of PPP2R2B, a gene encoding a regulatory subunit of protein phosphatase PP2A.1 SCA12 is characterized by a slowly progressive cerebellar syndrome that is frequently preceded by action tremor. Although considered rare, SCA12 is estimated to account for up to 16% of ADCA cases in northern India, likely resulting from a founder effect.2 Thus, SCA12 is an important diagnostic consideration for patients of Indian origin presenting with familial tremor. Though it is well recognized that SCA12 can present with tremor, videos of SCA12 patients have not been previously published to our knowledge. Here, we present the first SCA12 case report with video to illustrate the phenomenology of the associated tremor.
Case Report
A right‐handed man from India noted “shaky” handwriting and subtle voice tremor at age 59. At age 63, he developed mild dysarthria and gait difficulties. He initially reported that his tremor improved with alcohol, but his gait worsened. His only medications were warfarin and sotalol for atrial fibrillation as well as tamulosin for benign prostatic hypertrophy. Family history was significant for gait impairment with or without tremor or dementia affecting his mother, maternal aunt, and maternal grandmother (Fig. 1). His mother and maternal grandmother also had anxiety. He denied anxiety, depression, or delusions. Brain MRI revealed mild cerebral and cerebellar atrophy (Fig. 2). He was diagnosed with essential tremor (ET). Symptoms slowly progressed and he was examined at age 69 (see Video). Montreal Cognitive Assessment score was 25/30 with language and memory deficits. There was minimal saccadic pursuit, no nystagmus, and mildly dysmetric saccades. He had scanning dysarthria with superimposed voice tremor. Tone was normal in the upper extremities and there was spasticity in the lower extremities. There was no bradykinesia or rest tremor. He had a very asymmetric, low‐amplitude, 4‐Hz postural tremor primarily involving his distal right‐upper extremity. Tremor maintained its frequency, but increased in amplitude during target‐directed movements and tasks. There was generalized hyper‐reflexia and plantar responses were flexor. There was mild dysmetria in all four limbs. He had a normal‐based gait, but displayed difficulties with tandem walking. Genetic testing was abnormal only for PPP2R2B with ≥67 CAG repeats in one allele (normal range: 4–32), consistent with a diagnosis of SCA12. Genetic testing of affected family members could not be pursued because they were deceased. At‐risk family members declined genetic testing and neurological assessment. None of the following medications provided any significant symptomatic benefit: propranolol, primidone, clonazepam, gabapentin, topiramate, or acetazolamide.
Figure 1.
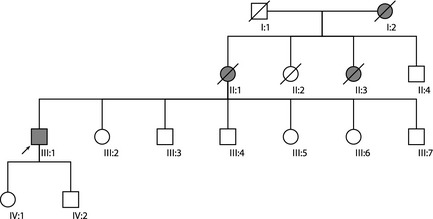
Pedigree of patient with SCA12. Arrow indicates our patient (III:1). Shaded symbols represent affected individuals. Based on history, II:1 had gait impairment, hand tremor, anxiety, dementia, and died in her eighties, II:3 had gait impairment, dementia, and died in her seventies, and I:2 had gait impairment, anxiety, possible hand tremor, and died in her seventies. Unshaded symbols represent unaffected individuals.
Figure 2.
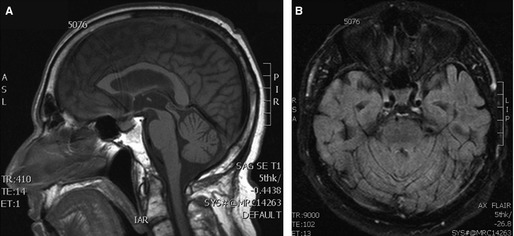
MRI brain of patient with SCA12. Sagittal T1 weighted (A) and axial FLAIR (B) images show only mild generalized atrophy.
Discussion
Tremors as part of a cerebellar syndrome are common in SCAs and include postural and intention tremor of limbs, titubation of head and trunk, stance tremor, and voice tremor.3 These tremors result from cerebellar dysfunction and thus are typically associated with ataxic signs of comparable severity. Because these tremors are nonspecific features of cerebellar impairment, they are not indicative of specific SCA subtypes. In contrast, rest tremor as part of coexisting parkinsonism can be characteristic of SCA2 or 3; palatal tremor can be a distinguishing feature of SCA20 and, less commonly, SCA7. Similarly, action tremor of the head and/or hands, which precedes the onset of ataxia, is suggestive of SCA12, as illustrated by this case, or the rarer SCA27.4 Fragile X‐associated tremor ataxia syndrome is an X‐linked disorder that can have prominent tremor with varying phenomenology: cerebellar; essential‐like; and parkinsonian.5 No autosomal recessive ataxia syndromes have a characteristic noncerebellar tremor.
There are no previously published videos of patients with SCA12. Based on previous case descriptions,6 this patient demonstrates the characteristic tremor associated with SCA12, which is a postural and kinetic, lower‐frequency tremor involving the hands. Previous reports have not commented on tremor asymmetry, so it is unclear whether the striking asymmetry observed in this patient is typical. Our patient also had a voice tremor and this has not been mentioned in previous reports. Earlier in his disease course, the patient was diagnosed with ET, as has been described for other SCA12 patients.6 There are several features common to ET and SCA12, including tremor characteristics, frequent onset in later adulthood, and positive family history. Mild gait ataxia in early SCA12 may not be a differentiating feature because a proportion of ET patients, especially those with intention tremor, have a gait disorder indistinguishable from that of mild cerebellar disease.7 Thus, it may be difficult to make an early diagnosis of SCA12 when cerebellar features are subtle. However, the patient's Indian ethnicity is a notable clinical clue to the diagnosis. The implications of a correct diagnosis are important for prognosis, because some SCA12 patients have ataxia progressing to wheelchair dependence or develop late‐onset dementia.6 Furthermore, counseling other family members regarding their risk of inheriting this ADCA may be necessary.
There are limited reports on treatment response of tremor in SCA12. One earlier report described SCA12 patients with tremor who had no significant improvement on propranolol.6 Our patient had no appreciable benefit from drugs typically used for ET. Whether tremor associated with SCA12 may be responsive to DBS remains to be determined. A previously reported case of a patient with SCA2 and severe action tremor documented significant improvement with combined DBS of the thalamus (ventral intermediate nucleus [VIM] and ventral oralis posterior nucleus) and a subthalamic region.8 Our patient is currently being considered for VIM DBS.9
Funding Agencies
L.V.K. is supported by a Canadian Health Institutes of Research Clinician‐Scientist Award.
Acknowledgment
The authors are thankful to the patient for contributing his time to this case study.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
L.V.K.: 1A, 1B, 1C, 3A, 3B
C.R.‐G.: 1C, 3B
A.B.: 1C, 3B
A.E.L.: 1B, 1C, 3B
Financial Disclosures
L.V.K. is funded by a Clinician‐Scientist Phase I Award from the Canadian Institutes of Health Research (CIHR). A.B. has served as an advisor for Abbott and UCB Canada Inc. A.E.L. has served as an advisor for Abbott, Abbvie, Allon Therapeutics, Avanir Pharmaceuticals, Biogen Idec, Boerhinger‐Ingelheim, Ceregene, Medtronic, Merck, Novartis, NeuroPhage Pharmaceuticals, Teva, and UCB; received grants from Brain Canada, CIHR, Edmond J. Safra Philanthropic Foundation, Michael J. Fox Foundation, National Parkinson Foundation, Parkinson Society Canada, Tourette Syndrome Association, and W. Garfield Weston Foundation; received publishing royalties from Saunders, Wiley‐Blackwell, Johns Hopkins Press, and Cambridge University Press; and served as an expert witness in cases related to the welding industry.
Supporting information
Video. A 69‐year‐old man with SCA12. The video demonstrates his ataxic speech with superimposed voice tremor, asymmetric postural and kinetic tremor involving the upper extremities, mild dysmetria in all four limbs, and difficulties with tandem gait.
Relevant conflicts of interest/financial disclosures: Nothing to report.
References
- 1. Holmes SE, O'Hearn EE, McInnis MG, et al. Expansion of a novel CAG trinucleotide repeat in the 5′ region of PPP2R2B is associated with SCA12. Nat Genet 1999;23:391–392. [DOI] [PubMed] [Google Scholar]
- 2. Bahl S, Virdi K, Mittal U, et al. Evidence of a common founder for SCA12 in the Indian population. Ann Hum Genet 2005;69:528–534. [DOI] [PubMed] [Google Scholar]
- 3. Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Mov Disord 1998;13(suppl 3):2–23. [DOI] [PubMed] [Google Scholar]
- 4. van Gaalen J, Giunti P, van de Warrenburg BP. Movement disorders in spinocerebellar ataxias. Mov Disord 2011;26:792–800. [DOI] [PubMed] [Google Scholar]
- 5. Apartis E, Blancher A, Meissner WG, et al. FXTAS: new insights and the need for revised diagnostic criteria. Neurology 2012;79:1898–1907. [DOI] [PubMed] [Google Scholar]
- 6. O'Hearn E, Holmes SE, Calvert PC, Ross CA, Margolis RL. SCA‐12: tremor with cerebellar and cortical atrophy is associated with a CAG repeat expansion. Neurology 2001;56:299–303. [DOI] [PubMed] [Google Scholar]
- 7. Stolze H, Petersen G, Raethjen J, Wenzelburger R, Deuschl G. The gait disorder of advanced essential tremor. Brain 2001;124:2278–2286. [DOI] [PubMed] [Google Scholar]
- 8. Freund HJ, Barnikol UB, Nolte D, et al. Subthalamic‐thalamic DBS in a case with spinocerebellar ataxia type 2 and severe tremor‐A unusual clinical benefit. Mov Disord 2007;22:732–735. [DOI] [PubMed] [Google Scholar]
- 9. Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. N Engl J Med 2000;342:461–468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video. A 69‐year‐old man with SCA12. The video demonstrates his ataxic speech with superimposed voice tremor, asymmetric postural and kinetic tremor involving the upper extremities, mild dysmetria in all four limbs, and difficulties with tandem gait.


