ABSTRACT
Background: Chickenpox is a contagious airborne disease. Immunization by varicella vaccine is an effective preventive measure. The objective of this study is to evaluate the impact and effectiveness of a single-dose vaccination against chickenpox at 15 months of age.
Methods: Observational study based on data from the Epidemiological Surveillance System of the Autonomous Community of Madrid from 2001 to 2015. The years were grouped into 4 periods according to epidemic cycles and vaccination schedule: 2001–06, 2007–10, 2011–13 and 2014–15. The impact was calculated as Relative Risk (RR) between the incidence of chickenpox in children between 15 months and 13 years of age between 2011–13 and 2001–06 through Poisson regression using notifications made to the Diseases of Compulsory Declaration (DCD) system, the Sentinel Physicians Network (SPN) and hospital discharge records noted as Minimum Basic Data Set (MBDS). The vaccine effectiveness (VE) was calculated using the screening method and a 1:2 case-control study paired by age and paediatrician in population from 15 months to 13 years and between 2007 and 2015 using SPN source data.
Results: The RR2011–13/2001–06 using data from the DCD was 0.14 (95% CI: 0.14 to 0.15), 0.07 (95% CI: 0.06 to 0.08) from SPN and 0.17 (95% CI: 0.15 to 0.20) from MBDS. A total of 338 cases were included in the VE screening obtaining an overall of 76.7% (IC 95%: 71.9 to 80.7%). For a case-control study, 120 cases and 247 controls were recruited obtaining a VE of 92.4% (IC 95%: 80.8 to 97.0%).
Conclusions: The single-dose vaccination against chickenpox at 15 months of age has high impact and effectiveness.
KEYWORDS: Chickenpox, Epidemiological Monitoring, Sentinel Surveillance, Varicella vaccine
Introduction
The varicella-zoster virus (VZV) is a DNA virus of the herpesvirus group that produces two types of disease (chickenpox and shingles).1 Chickenpox is an acute disease that mostly affects children and usually consists of a moderate fever, mild general symptoms and a vesicular rash that develops scabs in a few days. In some cases, this disease can be serious, with complications such as pneumonia or encephalitis, especially in children under 1 year of age, adults and immunocompromised population.
Chickenpox is one of the most contagious airborne diseases. The period of transmissibility ranges from 2 days before the onset of the rash until all lesions are scabbed over. The incubation period is 2 to 3 weeks, although it may be longer in immunocompromised individuals since it takes longer to solve the disease. Susceptibility is universal. In temperate zones, 90% of the population has contact with the virus before the age of 15 years. Data from the IV Community of Madrid Sero-Surveillance Survey show that the prevalence of antibodies against chickenpox after the age of 11 years exceeds 90%.2
Vaccination against chickenpox is effective in protecting against VZV infection and in reducing the severity of the disease if contracted. The vaccine is a live attenuated virus vaccine that is safe and well tolerated.3,4 Adverse reactions are generally mild.5 The therapeutic indication is two doses for people aged 9 months to 12 years.6
In Spain, the first chickenpox vaccine was approved for hospital use with at-risk groups in 1998. In 2004, the vaccine was given extra-hospital indications, which initiated its commercialization in pharmacies. In March 2005, the Interterritorial Council of the National Health System recommended the inclusion of the vaccine in the schedule of systematic childhood vaccinations in the susceptible population between 10 and 14 years.
In the Community of Madrid, unlike the rest of the country, publicly founded chickenpox vaccine was included in the routine childhood immunization schedule at 15 months of age with single dose of Varivax©, on November 1, 2006,.7 In January 2014 varicella vaccination was withdrawn from the official childhood vaccination schedule. The dose administered at 11 years remained under the same indication as described in 2005. For other schedules, the vaccine was available only through the private market.
The objective of this study is to evaluate the impact in the general population and effectiveness in the child population born after 2006, of a single-dose of varicella vaccine in the Community of Madrid.
Results
Impact of vaccination
A total of 318,697 cases were reported using the DCD system. Additionally, 8,189 cases were obtained through the SPN, and 3,067 admissions were obtained through the MBDS during the period from 2001 to 2015 (Fig. 1). The highest incidence was reported in the period prior to the introduction of the vaccine to the schedule at 15 months (2001–06) for the three sources and in all age groups (Table 1). From that year forward, the incidence showed a downward trend in all three information sources. This reduction was maintained until period 2011–13, when the rates reached the lowest value. From that year forward, the incidence showed an upward trend (Fig. 2).
Figure 1.
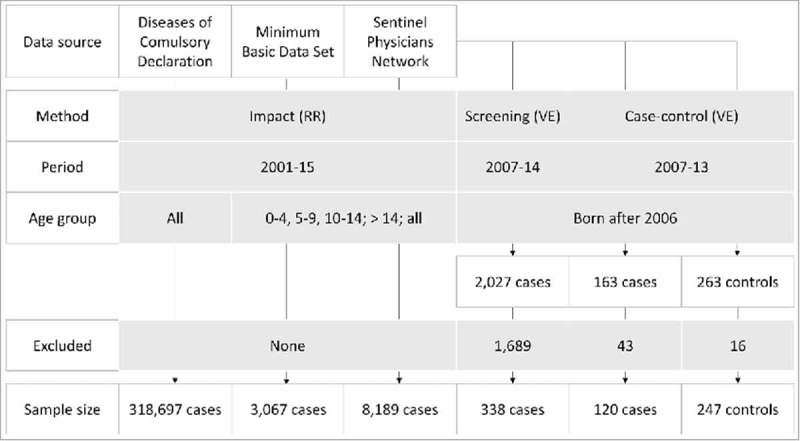
Chickenpox Epidemiological Surveillance System. 2001 to 2015. Community of Madrid.
Table 1.
Incidence and Risk Ratio (RR) of chickenpox according to the source of information and age group. Epidemiological Surveillance System. 2001 to 2015. Community of Madrid.
| 2001–06 | 2007–10 | 2011–13 | 2014–15 | ||||
|---|---|---|---|---|---|---|---|
| Cases (incidence) | Cases (incidence) | 2007–10 / 2001–06 RR (95% IC) | Cases (incidence) | 2011–13 / 2001–06 RR (95% IC) | Cases (incidence) | 2014–15 / 2001–06 RR (95% IC) | |
| Rates | |||||||
| 0–4 | 3806 (8439.61) | 689 (2219.76) | 0.26 (0.24 to 0.28) | 96 (358.15) | 0.04 (0.03 to 0.05) | 140 (975.75) | 0.12 (0.10 to 0.14) |
| 5–9 | 1597 (2906.96) | 443 (1381.07) | 0.48 (0.43 to 0.53) | 171 (622.95) | 0.21 (0.18 to 0.25) | 138 (804.37) | 0.28 (0.23 to 0.33) |
| 10–14 | 386 (672.79) | 51 (165.3) | 0.25 (0.18 to 0.33) | 30 (134.21) | 0.20 (0.14 to 0.29) | 44 (293.15) | 0.44 (0.32 to 0.59) |
| > 14 | 347 (114.53) | 145 (52.96) | 0.46 (0.38 to 0.56) | 71 (22.47) | 0.20 (0.15 to 0.25) | 35 (20.21) | 0.18 (0.12 to 0.25) |
| Addmission rates | |||||||
| 0–4 | 1082 (60.91) | 314 (22.43) | 0.37 (0.32 to 0.42) | 42 (3.82) | 0.06 (0.05 to 0.09) | 52 (7.59) | 0.12 (0.09 to 0.16) |
| 5–9 | 162 (10.36) | 76 (6.17) | 0.60 (0.45 to 0.78) | 25 (2.44) | 0.24 (0.15 to 0.36) | 16 (2.27) | 0.22 (0.13 to 0.37) |
| 10–14 | 42 (2.59) | 15 (1.33) | 0.52 (0.29 to 0.93) | 4 (0.44) | 0.17 (0.06 to 0.48) | 5 (0.79) | 0.30 (0.12 to 0.77) |
| > 14 | 756 (2.57) | 256 (1.19) | 0.46 (0.40 to 0.54) | 128 (0.78) | 0.30 (0.25 to 0.37) | 92 (0.85) | 0.33 (0.27 to 0.41) |
| All ages | |||||||
| DCD | 227165 (660.45) | 58423 (231.85) | 0.35 (0.35 to 0.35) | 18552 (95.22) | 0.14 (0.14 to 0.15) | 14557 (112.92) | 0.17 (0.17 to 0.17) |
| SNP | 6136 (1324.69) | 1328 (361.08) | 0.27 (0.26 to 0.29) | 368 (93.74) | 0.07 (0.06 to 0.08) | 357 (145.19) | 0.11 (0.10 to 0.12) |
| MBDS | 2042 (5.94) | 661 (2.62) | 0.44 (0.40 to 0.48) | 199 (1.02) | 0.17 (0.15 to 0.20) | 165 (1.28) | 0.22 (0.18 to 0.25) |
DCD: Diseases of Compulsory Declaration; SPN: Sentinel Physician Network; MBDS: Minimum Basic Data Set.
Figure 2.
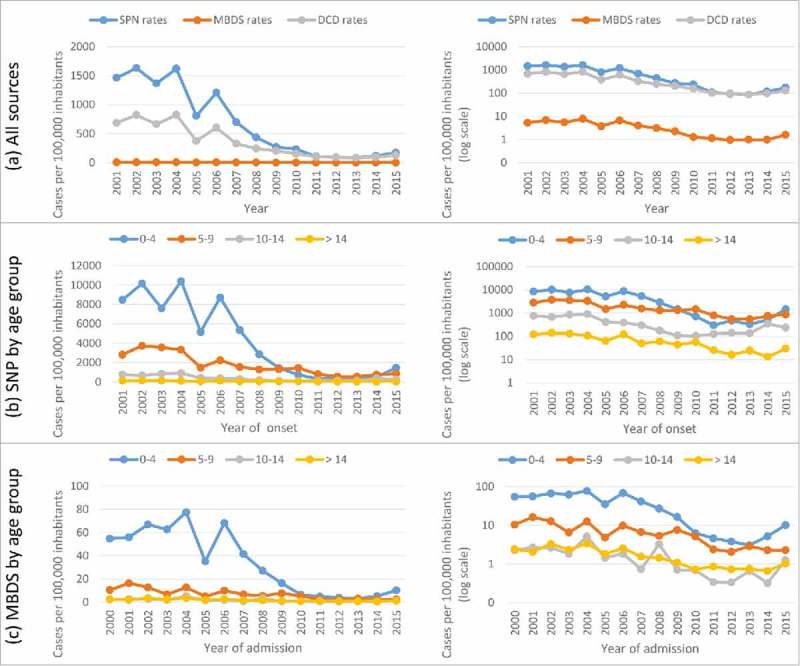
Chickenpox rates. Epidemiological Surveillance System. 2001 to 2015. Community of Madrid.
From the reference period (2001–06) to the last period with the vaccine in the schedule at 15 months (2011–13), RR of chickenpox was 0.14 (95% CI: 0.14 to 0.15) according to the DCD system, 0.07 (95% CI: 0.06 to 0.08) according to the SPN and 0.17 (95% CI: 0.15 to 0.20) according to the MBDS (Table 1).
Vaccine effectiveness
Screening method
A total of 2,027 cases of chickenpox were notified to the SPN from 2007 to 2014. The following cases were excluded: 1,424 cases born before 2006, 202 cases less than 15 months old at symptom development, 33 cases without information on the vaccination status and 30 cases with an incomplete birth date. A total of 338 cases were included in the study (Fig. 1).
The year of the withdrawal (after 2013) of the vaccine from the schedule showed the lowest coverage in both the cases and the general population (Fig. 3). The highest proportion of vaccinated cases occurred in those born in 2011 after a sustained period of high coverage. During the period in which the vaccine was on the schedule, population coverage exceeded 90% in the target cohorts of the vaccination programme.
Figure 3.
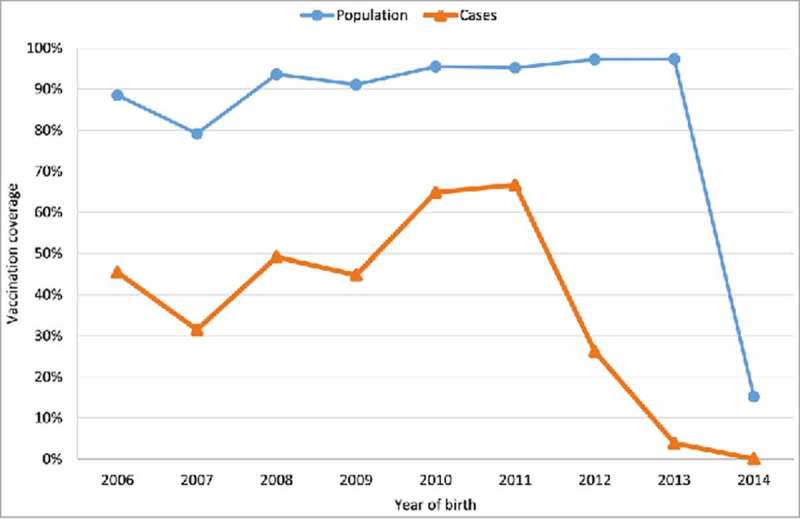
Vaccination against chickenpox coverage by year of birth. Community of Madrid.
The overall VE calculated by the screening method was 93.1% (IC95%: 90.9 to 94.8). The VE decreased with time from vaccination from 98.2% (IC95%: 96.5 to 99.9) in the first year to 93.1% (IC95%: 90.9 to 94.8%) after 9 years of follow-up. This decrease can be considered as statistically significant since the confidence intervals are not overlapped. Most vaccinated cases occur in the first 2 years of follow-up (97/129) (Fig. 4).
Figure 4.
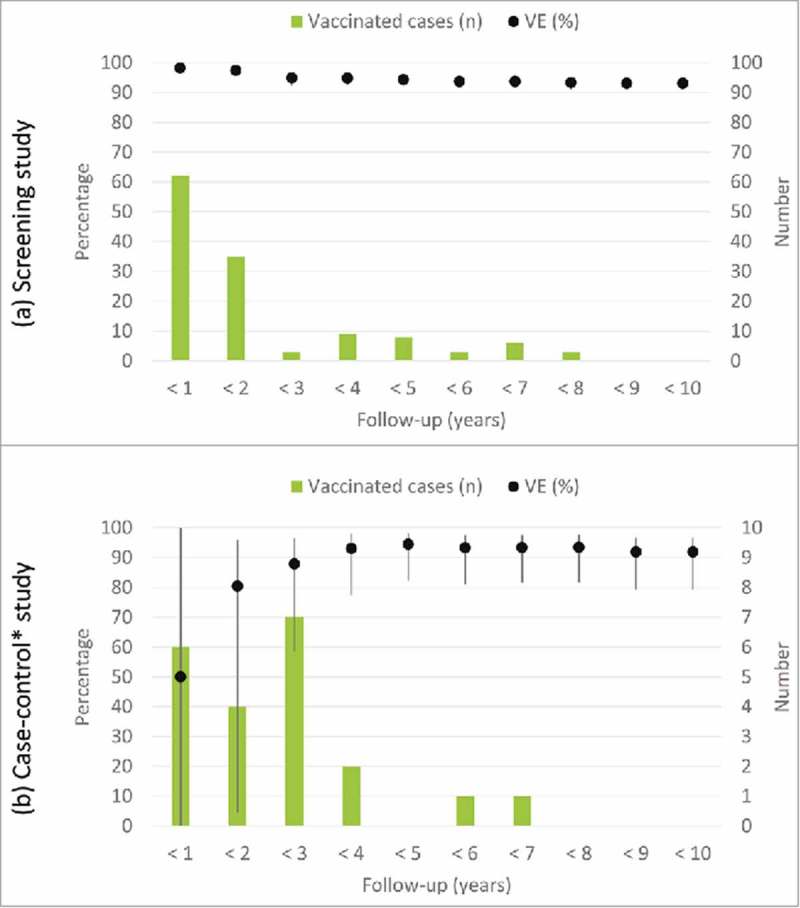
Evolution of vaccine effectiveness and vaccinated cases over time. Community of Madrid.
Case-control method
Of the 163 cases detected by the Sentinel Physicians Network between 2007 and 2013, 43 cases met the exclusion criteria so 120 were included in the case-control study. The characteristics of these 120 cases and their corresponding controls are shown in Table 2. The distributions of demographic factors, including sex, age and social factors, such as the country of origin, were similar in both groups. Other possible factors that might influence the development of the disease, such as prematurity and MMR vaccination, were also similar.
Table 2.
Characteristics of study population. Case-control study based on the Sentinel Physicians Network. 2007 to 2015. Community of Madrid.
| |
Cases |
Controls |
|---|---|---|
| Characteristics | (n = 120) | (n = 247) |
| Age (years) | ||
| Median | 4 | 4 |
| Range | 1 to 12 | 1 to 13 |
| Sex: female | 55 (46%) | 112 (45%) |
| Prematurity: yes | 10 (8%) | 22 (9%) |
| Origin: Spain | 112 (93%) | 232 (94%) |
| MMR vaccination: yes | 117 (98%) | 238 (96%) |
| Chickenpox vaccination: yes | 19 (16%) | 124 (50%) |
| Age at vaccination: ≥ 15 months | 15 (79%) | 95 (77%) |
| Vaccination follow up (months) | ||
| Median | 19 | 30 |
| Range | 1 to 80 | 0 to 75 |
| Case type: confirmed/susceptible | 44 (37%) | — |
| Chickenpox: moderate/severe | 31 (26%) | — |
In nearly 47% (n = 76) of the cases, we could not extract a biological sample, but the VE was similar for all cases and laboratory-confirmed cases (Table 3). None of the severe cases were vaccinated, and thus, the VE was 100%. The 7 confirmed vaccinated cases exhibited mild symptoms.
Table 3.
VE against chickenpox. 2003 to 2015. Community of Madrid.
| Vaccinated cases |
||||
|---|---|---|---|---|
| Method | VE (95% CI) | Cases (N) | (N) | (%) |
| Screening | ||||
| All cases | 93.1 (90.9 to 94.8) | 338 | 129 | 38.2 |
| Cases and controls | ||||
| All cases | 92.4 (80.8 to 97.0) | 120 | 19 | 15.8 |
| Confirmed cases | 91.8 (64.3 to 98.1) | 44 | 4 | 2.7 |
| Moderate/severe cases | 100 | 31 | 0 | 0.0 |
There are no moderate/severe cases among the vaccinated population.
Estimates of the VE during the first three years were unstable. After the third year of follow-up, the VE was approximately 90%. Most vaccinated cases occur in the 3 five years of follow-up (17/23) (Fig. 4).
Discussion
The community of Madrid is the only region in Spain that has implemented a population-based one-dose publicly funded chickenpox vaccination at 15 months between November 2006 and January 2014. The vaccination strategy used in our region has significantly reduced the burden of disease, as seen in other countries.13-18 However, there was a slight increase in the incidence of all ages after the exclusion of the dose at 15 months from the public funded vaccination schedule. This modification was made in the context of laws to reduce health expenditure and seeking cohesion with the vaccine schedules of other Autonomous Communities. Subsequent consensus at the national level has reintroduced the vaccine into the schedule in 2016 (Revisión del calendario de vacunación 2016).
The VE calculated by the screening method was higher than that calculated by the case-control method, but both methods yielded results compatible with one another. The cases and controls were recruited consecutively so the epidemic environment was similar at the time of its inclusion, reducing the bias that seasonality could induce. In addition, the screening method is overestimated if the population vaccine coverage is biased upwards. Therefore, the results of the case-control study could be more reliable, despite its limitation due to the sample size.19
A study conducted in Spain found a reduction of 83.5% in hospitalizations among Autonomous Communities with the vaccine on the schedule.20 The findings of these studies are very similar to the impact described in the present study.
A paired case-control study conducted in Bavaria showed an 86% VE (95% CI: 77–92) after more than 5 years of follow-up.21 Another study conducted in Connecticut, USA, with the same method showed a VE of 87% (95% CI: 81 to 91) for a follow-up of 4 years.22 In Italy, the VE determined using the screening method.23 was 91% (95% CI: 90–92).
In Spain, the VE against the disease confirmed by PCR for a single dose in a case-control study conducted in Navarra was 93% (95% CI: 34 to 100) in the first year of follow-up,24 results very similar to those found in case-control study against confirmed cases (VE: 92%; 95% CI: 64 to 98). For a retrospective cohort study conducted in Murcia in a population under 13 years of age, the VE against clinical disease was 89% (95% CI: 75 to 95), although this study is framed in the context of reported epidemic outbreaks in the school setting.25
The immunity conferred by a single dose of vaccine followed up for at least 10 years suggests a possible decrease in the VE over time. The VE follows patterns similar to those described in other studies.21,26 The highest VE was found in the first year of continuous follow-up, followed by a decline in the following years, a subsequent stabilization and, finally, a gradual decrease. The most likely explanation for this stabilization is an immune response secondary to exposure to free VZV occurring at the time of schooling among the child population.21,27
Relevant strength is the information systems on which this study is based. The vaccination record collects information on the vaccination status of the population of Madrid regardless of where the vaccine is administered (primary or specialized care or a public or private clinic). Moreover, the EHR of specialized care is interoperable with primary care, which allows the users' conditions or vaccines to be reviewed. Thus, the classification of exposure to the vaccine was very comprehensive.
Our study has some limitations to take into account. Due to the high vaccination coverage during the vaccine publicly founded period, practically all cases with a follow-up of less than one year were vaccinated. This increase the difficulties of finding the required number of controls for each case and calculate long-term VE using a case-control study.
Although information on the severity of symptoms was collected in the case-control study, no cases were vaccinated among the severe cases, which resulted in insufficient power to calculate the VE for this group. However, this lack of severe cases among those receiving 1 dose of vaccine is a positive result.
There is a possible misclassification bias and a loss of power in the study of confirmed cases because in many cases a clinical sample could not be extracted. However, despite the low incidence of childhood chickenpox in our setting, the professionals involved in the SPN performed identical protocols to define monitored diseases and had a high level of alertness in their diagnoses. Therefore, very few cases should be misclassified.
The WHO makes clear recommendations for the chickenpox vaccination policy as follows: one dose is sufficient if the objective of the programme is to reduce morbidity and mortality, whereas the 2 dose regimen is more effective and is recommended if the objective is to reduce the number and size of outbreaks and limit virus circulation.28
In summary, the dose regimen administered in the Community of Madrid is highly effective in the prevention of cases of chickenpox and, with less certainty, is effective in the prevention of severe cases. Additional studies are needed that include more patients with diagnostic confirmation information to better describe the changes in immunity produced by vaccination.
Material and methods
Data sources and variables
The Epidemiological Surveillance System obtains information from several sources. To perform this study, the following sources were used:
The Diseases of Compulsory Declaration (DCD) system. This system collects information from the entire population. Chickenpox is viral infection included in the DCD system with weekly numerical data. Variables were used by referring to the year and week of notification and the number of cases declared. The notification is made by automatically downloading of basic information from the Electronic Health Records (EHR) of primary care. The EHR is encoded following the International Classification of Primary Care (ICPC) developed by the WONCA.8 The episodes with the A72 ICPC code were selected. Through the EHR of primary care, health care providers can access the EHR of hospital care, which allows completing the information on the evolution of cases. In addition to this system, all healthcare workers are required to notify any possible, probable or confirmed cases. Clinical case definition was: acute generalized onset disease with mild fever and rapidly evolving rash from superficial papules to vesicles and eventually scabs.
Minimum Basic Data Set (MBDS). This system collects information on the population covered by the national health system. It is based on hospital discharges; each discharged patient generates a record in this information system. Records with the following ICD9-MC codes as the main diagnosis or first secondary diagnosis were selected: 052.0 (encephalitis caused by VZV), 052.1 (pneumonitis caused by VZV), 052.2 (myelitis caused by VZV), 052.7 (chickenpox with other specified complications), 052.8 (chickenpox with other unspecified complications) and 052.9 (chickenpox without complications). All hospitals, public and private, in the region where included for the whole period of the study. Variables referring to the primary and secondary diagnosis, age, sex, year of notification and place of residence were used.
Sentinel Physicians Network (SPN). The SPN is formed by 136 physicians who monitors a representative population of the Community of Madrid (199,044 inhabitants, 3% of the population).9 and reports diseases with homogenous criteria using a standardized questionnaire with the following variables: year and week of notification, age, sex and vaccination status. This system uses the same case definition as DCD, but the physicians receive specific training on case detection and gather clinical and epidemiological information. For the case-control study, the information also included the date of symptom onset, disease severity, microbiological results and the vaccination status of the cases and controls. A valid vaccine dose was considered if more than 15 days elapsed between the date of the dose and the onset of symptoms for both calculations of vaccine effectiveness. The disease was measured using a modified version of the scale used in clinical trials on the chickenpox vaccine (Appendix 1). A score ≤ 7 was considered mild, 8 to 15 moderate and ≥ 16 severe.10 Serum samples were collected from the cases for the determination of chickenpox-specific IgM antibodies within 5 days after vesicles appeared. Vesicle swab were also collected for identification of viral DNA by PCR. These samples were sent to the Regional Public Health Laboratory of the Community of Madrid.
Vaccine registration. This registry began in 2006, it contains a nominal record of vaccination and it automatically incorporates vaccines registered in Primary Healthcare Centres of the Regional Health Service, vaccination centres of the Madrid City Council, as well as a manual registry from other vaccination centres, mainly from the private sector. Annual coverage of the chickenpox vaccine was estimated, and the vaccination status (number of doses and date of administration) of the cases notified to the SPN was completed.
Rates denominators. The annual resident population data included in the Continuous registry of the Community of Madrid were used for the calculation the MBDS and DCD rates. However, for the SPN, the denominator incorporates the weekly population assigned to each physician adjusted for their attendance. The population of sentinel physicians without assistance is not counted. Sentinel physicians with assistance who did not complete any protocol receive an automatic email that includes a submit type field to notify zero cases. The population of sentinel physicians confirming zero cases is incorporated and those who do not confirm are excluded as long as they do not.
Study design
Study of the impact of vaccination
Population-based follow-up study was conducted using data obtained from the Epidemiological Surveillance System of the Community of Madrid from 2001 to 2015.
Cases of all ages of chickenpox reported to the Surveillance System during the study period were selected. There also was analysed the evolution of the incidence of hospitalization. All admissions presenting ICD-9-MC codes compatibles with chickenpox attending to public or private hospitals during the study period were selected.
Study of the vaccine effectiveness
Two different methods were used to calculate the vaccine effectiveness:
-
Screening method.
To this end, were selected those cases reported between 2007 and 2014 by the Sentinel Physicians Network (SPN) of individuals older than 15 months of age at symptom onset and born after 2006, who were eligible for varicella vaccine. Vaccination coverage was calculated for the population that was ≥15 months of age from 2006 to 2014.
-
Paired case-control study.
The case-control study was paired by physician, age of the case and consultation date. Two controls were selected for each case. The inclusion criteria for cases were as follows: 1) individuals between 15 months and 13 years of age and 2) individuals who presented with a clinical profile compatible with chickenpox according to the case definition used in the SPN. The first two patients who consecutively attended the same clinic after the case were selected as controls if they met the following criteria: 1) an age similar to the case (± 5 months), 2) treated by the same paediatrician and 3) not suffering from the disease at the time of selection. The following exclusion criteria were applied to both the cases and controls: 1) vaccination against chickenpox not recommended (immunosuppression due to underlying disease or medication), 2) personal history of chickenpox recorded in the medical history or reported by the parents prior to the vaccination date and 3) unknown vaccination status or date of birth. Inclusion in the study was established prior to the delivery of the information sheet and signing of the informed consent form by the parents. The selection of establishments was conducted between 2007 and 2013.
Statistical analysis
Analysis of the impact of vaccination
The annual incidence rates (cases per 100,000 inhabitants) and annual hospital admissions (admissions per 100,000 inhabitants) for chickenpox were calculated for specific age groups (0 to 4, 5 to 9, 10 to 14 and > 14 years old) and for all age groups.
The years were grouped into 4 periods according to changes in the incidence and in the vaccination schedule: 2001–06, 2007–10, 2011–13 and 2014–15. The impact of vaccination was calculated by comparing the rates of the last period with the vaccine in the children's vaccine schedule (2011–13) with the rates of the reference period (2001–06) by calculating relative risks (RR) through Poisson regression models.
Analysis of the vaccine effectiveness
The follow-up time was defined as years elapsed between the date of vaccination and the date of symptom onset, if the cases were not vaccinated the date of recommended vaccination at 15 months of age was consider as time of vaccination. The number of vaccinated cases was defined as the occurrence of suspected case in a vaccinated patient with a valid dose.
Vaccine effectiveness was estimated using the screening method.11 based on a comparison of the proportion of vaccinated cases with the proportion of the vaccinated population. The approach described by Farrington.12 was adopted; this approach allows adjustment of the VE with confounding factors using logistic regression models. To control the effect of variation in cohort coverage, the proportion of vaccinated population under 15 months corresponding to each year of symptoms onset was considered for each case and incorporated into the logistic regression models. Also the results are presented globally and disaggregated by the years of follow-up.
Subsequently, the effectiveness of the vaccine was calculated using the case-control method. Only paired groups that had at least one control were included: 11 groups 1:1, 345 groups 1:2, 8 groups 1:3 and 4 groups 1:4. A conditional logistic regression model was used to calculate the relationship between the probability of having chickenpox and the probability of being vaccinated. These regression models allow flexibility in the number of controls recruited for each case and adjustment by possible confounding variables. The VE was calculated according to the types of cases (total, confirmed and severe). The case was confirmed if the DNA of the virus was identified by PCR in the clinical samples. The case was severe if the severity scale of the disease was equal to or greater than 16.
In both cases, effectiveness was calculated using the formula (1-OR) × 100 for the entire study period and disaggregated by the follow-up time from vaccination.
Statistical analyses were performed with calculated by Stata software, version 12.0 (Stata Corp., College Station, TX) with a confidence level of 95%.
Appendix 1. Criteria for the severity of chickenpox
| Clinical signs | Presentation | Score |
|---|---|---|
| Number of lesions | 1 to 50 | 1 |
| 51 to 100 | 2 | |
| 101 to 500 | 4 | |
| ≥ 501 | 6 | |
| Type of injuries | Macular or papular | 2 |
| Vesicular | 4 | |
| Haemorrhagic | 4 | |
| Fever | < 38°C | 0 |
| 38.8 to 39.9°C | 1 | |
| ≥ 40°C | 3 | |
| Systemic Signs | No signs | 0 |
| Pain in the back or abdomen | 4 | |
| Pneumonia | 5 | |
| Encephalitis | 5 | |
| Subjective assessment | Does not appear sick | 0 |
| Appears moderately ill | 2 | |
| Appears seriously ill | 5 |
Score: ≤ 7 mild; 8 to 15 moderate; ≥ 16 severe.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure of potential conflicts of interest
AGM have received travel and research grants from Sanofi Pasteur MSD and GSK.
Bibliography
- 1.Bennett JE, Dolin R, Blaser MJ. Mandell, Douglas and Bennett's Infectious Diseases Essentials. Philadelphia, PA: Elsevier Inc; 2016. [Google Scholar]
- 2.García-Comas L, Ordobás Gavín M, Sanz Moreno JC, Ramos Blázquez B, Gutiérrez Rodríguez MA, Barranco Ordóñez D. [Seroprevalence of varicella-zoster virus antibodies after the recent introduction of the universal childhood immunisation schedule in the Community of Madrid]. Enferm Infecc Microbiol Clin. 2016. December;34(10):633–8. doi: 10.1016/j.eimc.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Galea SA, Sweet A, Beninger P, Steinberg SP, Larussa PS, Gershon AA, Sharrar RG. The safety profile of varicella vaccine: a 10-year review. J Infect Dis. 2008. March 1;197(Suppl 2):S165–169. doi: 10.1086/522125. [DOI] [PubMed] [Google Scholar]
- 4.Goulleret N, Mauvisseau E, Essevaz-Roulet M, Quinlivan M, Breuer J. Safety profile of live varicella virus vaccine (Oka/Merck): five-year results of the European Varicella Zoster Virus Identification Program (EU VZVIP). Vaccine. 2010. August 16;28(36):5878–82. doi: 10.1016/j.vaccine.2010.06.056. [DOI] [PubMed] [Google Scholar]
- 5.Black S, Shinefield H, Ray P, Lewis E, Hansen J, Schwalbe J, Coplan P, Sharrar R, Guess H. Postmarketing evaluation of the safety and effectiveness of varicella vaccine. Pediatr Infect Dis J. 1999. December;18(12):1041–6. doi: 10.1097/00006454-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Agencia española de medicamentos y productos sanitarios, Ministerio de Sanidad, Servicios Sociales e Igualdad. Ficha técnica Varivax polvo y disolvente para suspensión inyectable [Internet]. Available from: http://www.aemps.gob.es/cima/pdfs//ft/65709/FT_65709.pdf.
- 7.World Organization of National Colleges, Academies, and Academic Associations of General Practitioners/Family Physicians. ICPC-2: International classification of primary care. Oxford: Oxford University Press. 1998 [accessed 2017 Dec 29]. Available from: http://www.who.int/classifications/icd/adaptations/icpc2/en/. [Google Scholar]
- 8.WHO | International Classification of Primary Care Second edition (ICPC-2) [Internet]. WHO. [cited 2017December29]. Available from: http://www.who.int/classifications/icd/adaptations/icpc2/en/. [Google Scholar]
- 9.Pérez-Farinós N, Galán I, Ordobás M, Zorrilla B, Cantero JL, Ramírez R. Diseño de selección de muestra para una red de medicos centinela. Gac Sanit. 2009. June;23(3):186–91. doi: 10.1016/j.gaceta.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Vázquez M, LaRussa PS, Gershon AA, Steinberg SP, Freudigman K, Shapiro ED. The effectiveness of the varicella vaccine in clinical practice. N Engl J Med. 2001. March 29;344(13):955–60. doi: 10.1056/NEJM200103293441302. [DOI] [PubMed] [Google Scholar]
- 11.Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, Bart KJ, Sirotkin B. Field evaluation of vaccine efficacy. Bull World Health Organ. 1985;63(6):1055–68. PMID:3879673. [PMC free article] [PubMed] [Google Scholar]
- 12.Farrington CP. Estimation of vaccine effectiveness using the screening method. Int J Epidemiol. 1993. August;22(4):742–6. doi: 10.1093/ije/22.4.742. [DOI] [PubMed] [Google Scholar]
- 13.Wutzler P, Bonanni P, Burgess M, Gershon A, Sáfadi MA, Casabona G. Varicella vaccination - the global experience. Expert Rev Vaccines. 2017. Aug;16(8):833–43. doi: 10.1080/14760584.2017.1343669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mirinaviciute G, Kristensen E, Nakstad B, Flem E. Varicella-Related Primary Healthcare Visits, Hospitalizations and Mortality in Norway, 2008–2014. Pediatr Infect Dis J. 2017. Nov;36(11):1032–38. doi: 10.1097/INF.0000000000001656. [DOI] [PubMed] [Google Scholar]
- 15.Sheridan SL, Quinn HE, Hull BP, Ware RS, Grimwood K, Lambert SB. Impact and effectiveness of childhood varicella vaccine program in Queensland, Australia. Vaccine. 2017. June 14;35(27):3490–7. doi: 10.1016/j.vaccine.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Fu J, Jiang C, Wang J, Zhao F, Ma T, Shi R, Zhao Y, Zhang X. Epidemiology of varicella in Haidian district, Beijing, China-2007–2015. Vaccine. 2017. April 25;35(18):2365–71. doi: 10.1016/j.vaccine.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Avila-Aguero ML, Ulloa-Gutierrez R, Camacho-Badilla K, Soriano-Fallas A, Arroba-Tijerino R, Morice-Trejos A. Varicella prevention in Costa Rica: impact of a one-dose schedule universal vaccination. Expert Rev Vaccines. 2017. March;16(3):229–34. doi: 10.1080/14760584.2017.1247700. [DOI] [PubMed] [Google Scholar]
- 18.Boccalini S, Bonanni P, Bechini A. Preparing to introduce the varicella vaccine into the Italian immunisation programme: varicella-related hospitalisations in Tuscany, 2004–2012. Euro Surveill. 2016. June 16;21(24). [DOI] [PubMed] [Google Scholar]
- 19.Torvaldsen S, McIntyre PB. Observational methods in epidemiologic assessment of vaccine effectiveness. Commun Dis Intell Q Rep. 2002;26(3):451–7. PMID:12416713. [PubMed] [Google Scholar]
- 20.Gil-Prieto R, Garcia-Garcia L, San-Martin M, Gil-de-Miguel A. Varicella vaccination coverage inverse correlation with varicella hospitalizations in Spain. Vaccine. 2014. December 12;32(52):7043–6. doi: 10.1016/j.vaccine.2014.10.076. [DOI] [PubMed] [Google Scholar]
- 21.Liese JG, Cohen C, Rack A, Pirzer K, Eber S, Blum M, Greenberg M, Streng A. The effectiveness of varicella vaccination in children in Germany: a case-control study. Pediatr Infect Dis J. 2013. September;32(9):998–1004. doi: 10.1097/INF.0b013e31829ae263. [DOI] [PubMed] [Google Scholar]
- 22.Vázquez M, LaRussa PS, Gershon AA, Niccolai LM, Muehlenbein CE, Steinberg SP, Shapiro ED. Effectiveness over time of varicella vaccine. JAMA. 2004. February 18;291(7):851–5. doi: 10.1001/jama.291.7.851. [DOI] [PubMed] [Google Scholar]
- 23.Pieri L, Porchia BR, Pieralli F, Varone O, Niccolai G, Roselli A, Boccalini S, Bonanni P, Bechini A; Working Group of Tuscan LHU's, et al.. Assessment of the effectiveness of the universal varicella vaccination program in Toscana (Italy), in the period 2010–2013. Epidemiol Prev. 2015. August;39(4 Suppl 1):119–23. [PubMed] [Google Scholar]
- 24.Cenoz MG, Martínez-Artola V, Guevara M, Ezpeleta C, Barricarte A, Castilla J. Effectiveness of one and two doses of varicella vaccine in preventing laboratory-confirmed cases in children in Navarre, Spain. Hum Vaccines Immunother. 2013. May;9(5):1172–6. doi: 10.4161/hv.23451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romera-Guirado FJ, Molina-Salas Y, Pérez-Martín JJ, Ruzafa-Martínez M. Varicella vaccine effectiveness in schoolchildren in outbreaks in a semi-urban area. An Pediatr (Barc). 2016. Jan;84(1):30–8. doi: 10.1016/j.anpedi.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 26.Fu C, Wang M, Liang J, Xu J, Wang C, Bialek S. The effectiveness of varicella vaccine in China. Pediatr Infect Dis J. 2010. August;29(8):690–3. doi: 10.1097/INF.0b013e3181d7380e. [DOI] [PubMed] [Google Scholar]
- 27.Chaves SS, Gargiullo P, Zhang JX, Civen R, Guris D, Mascola L, Seward JF. Loss of Vaccine-Induced Immunity to Varicella over Time. N Engl J Med. 2007. March 15;356(11):1121–9. doi: 10.1056/NEJMoa064040. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization Varicella and herpes zoster vaccines: WHO position paper, June 2014. Wkly Epidemiol Rec. 2014. June 20;89(25):265–87. [PubMed] [Google Scholar]


