ABSTRACT
Norovirus causes acute and debilitating gastroenteritis, characterized by vomiting and diarrhea. We recently reported a recombinant GII. 4 P domain particle (Pd) vaccine adjuvanted with a flagellin, Vibrio vulnificus FlaB, effectively promoting both humoral and cell-mediated immune responses. In the previous study, we found that sublingual (SL) immunization induced higher fecal secretory IgA (SIgA) responses while intranasal (IN) route provided higher amplitude of humoral and cellular immune responses in the systemic compartment. We hypothesized that the combination of IN and SL routes should induce more potent and sustained SIgA responses in the gut. In this study, we have tried combinatorial prime-boost immunization employing both IN and SL routes. The IN priming and SL boosting with the Pd+FlaB vaccine enhanced highest SIgA responses in feces, accompanying increased Pd-specific memory B cells and plasma cells in spleen and bone marrow, respectively. Notably, the strongest long-lasting SIgA response in feces was induced by combined IN prime and SL boost vaccination, which was sustained for more than 3 months. Significantly enhanced gut-homing B cell and follicular helper T cell responses in mesenteric lymph nodes (mLNs) were observed in the IN prime and SL boost combination. IN priming was a requisite for the robust induction of Pd-specific IFNγ, IL-2, IL-4 and IL-5 cytokine responses in the systemic immune compartment. Collectively, the IN prime and SL boost combination was the best option for inducing balanced long-lasting immune responses against the norovirus antigen in both enteric and systemic compartments. These results suggest that immune responses in specific mucosal compartments may be programmed by employing different prime-boost immunization routes.
KEYWORDS: Immunization route combination, mucosal immunity, norovirus
Introduction
The highly contagious noroviruses (NoVs) are the leading cause of acute epidemic gastroenteritis across all ages, accounting for the loss of 2,496,078 disability-adjusted life years and 34,929 deaths annually worldwide.1 It has also been estimated that NoV infections are responsible for 200,000 child deaths per year in developing countries.2 Several potential candidate vaccines are under development and clinical trials, though there currently are no clinically available vaccines. The absence of culture system and an appropriate animal model for NoVs hampers researches for vaccine development, pathogenesis, and diagnosis of the viruses. NoVs have highly diverse genomes and antigens as a consequence of frequent point mutation and recombination,3 which is also responsible for the lack of effective vaccines and therapeutic agents to combat NoV infections.
NoVs are currently divided into 6 genogroups, with human strains stratified into genogroups I (GI), II (GII), and IV (GIV).4-6 Since NoV infections involve gastrointestinal mucosa, mucosal routes are often employed for vaccination. A monovalent GI.1VLP vaccine administered via the intranasal (IN) route was shown to provide good protection in a clinical trial.7 To overcome the epidemiological differences of GI.1 and GII.4 genotypes, a bivalent VLP vaccine carrying GI.1 and GII.4 genotypes was proposed for IN administration and shown to be immunogenic without the use of adjuvant in a guinea pig model.8 However, intramuscular administration of monovalent GI.1VLPs has been shown to achieve better antigen delivery and antibody responses in adults at a lower dosage than IN immunization.9,10 Intramuscular administration of the bivalent GI.1 and GII.4 VLP vaccine effectively induced genotype-specific antibody titers without adverse events, and the vaccinees were less likely to suffer from gastroenteritis in a challenge study with a natural GII.4 strain.11 Collectively, past studies claim that IN vaccination needs significant improvements in terms of efficacy.
Secretory IgA (SIgA) plays an important role in the protection and homeostatic regulation of mucosal epithelia separating the outer environment from the inside of body. The SIgA production represents the hallmark of immune responses at mucosal sites.12 IgA in mucosal secretions obviously functions in binding to antigens, toxin, foreign proteins, and microorganisms to inhibit penetration of mucosal epithelia.13 Higher quaternary structure SIgA induced by intranasal vaccination displayed increased neutralizing potency against influenza virus.14 Antigen-specific IgA can be induced not only by immunization in the vicinity of corresponding mucosal tissues but also through common mucosal conduits such as oral, sublingual (SL), IN, or rectal routes.15 Mucosa-administered antigens are generally less immunogenic and apt to induce tolerance since the host strives to maintain mucosal homeostasis by responding to mucosal antigens with tolerance. In this regard, the induction of immune responses by mucosal immunization requires the co-administration of appropriate adjuvants that can initiate and support the effective collaboration between innate and adaptive immunity.16 Production of SIgA is finely regulated, and distinct T-independent and T-dependent mechanisms orchestrate class switching. Mucosal or epicutaneous delivery of vaccines helps the activation of effector cells in inductive sites for SIgA responses. Efforts are invested in developing vaccine adjuvants and delivery systems to heighten efficacies of such vaccination efforts.17
FlaB, a major flagellin from Vibrio vulnificus, is a strong mucosal adjuvant for the potentiation of prophylactic and therapeutic vaccines.18,19 In a previous study, we have shown that mucosally administered FlaB-adjuvanted NoV P domain vaccine significantly potentiated balanced humoral and cellular immune responses against the antigen both in the systemic and mucosal compartments.20 In the study, we have used both IN and SL routes for immunization. While overall amplitude of antigen-specific humoral and cellular immune responses was higher in the IN group, fecal SIgA level was significantly higher in the SL group. In this context, we came to hypothesize that the combination of IN and SL routes would provide both higher amplitude of immune responses and profuse SIgA production in the gut. We report here that the IN prime and SL boost combination resulted in a significantly higher fecal IgA secretion accompanying more robust gut-homing and memory B cell responses.
Results
IN prime and SL boost immunization was more effective in inducing IgA responses in both serum and feces
Groups of mice were immunized with a mixture of Pd (0.5 μg) and FlaB (2 μg) via the IN or SL route either alone or in different prime-boost schedules. To make the difference more visible, boosting was done twice (Fig. 1A). NoV Pd-specific antibodies were determined in the serum and feces 2 weeks after the final immunization. Fecal SIgA production was highest in the IN/SL/SL group (Fig. 1B). Employing SL route either in prime or boost schedule induced higher SIgA in the feces than using the IN route for both priming and boosting (IN/IN/IN). On the other hand, both IgG and IgA responses in the serum were highest in the IN/IN/IN and lowest in the SL/SL/SL group (Fig. 1C and D). The IN priming induced higher antibody responses in the serum. Notably, it seemed that the expansion of immune responses in the systemic compartment may a requisite for more robust targeting of enteric compartment through subsequent SL boost immunizations (Fig. 1B). The two IN boosting could not increase serum IgA production in the SL primed groups (SL/IN/IN) to the levels of IN prime-SL boost group (IN/SL/SL). Same tendency was observed with serum IgG levels though the difference between IN/SL/SL and SL/IN/IN groups was statistically insignificant. These results suggested that IN route is better in priming systemic immune responses while SL route is required for specifically targeting the enteric compartment for SIgA production.
Figure 1.
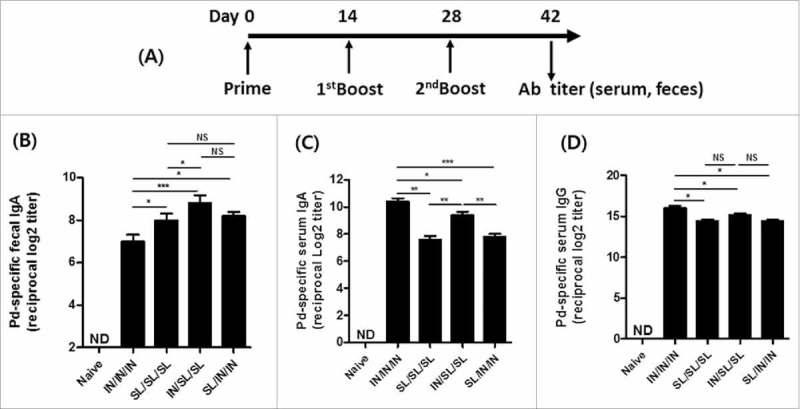
Induction of NoV Pd-specific antibody responses in serum and feces. (A) The immunization schedule: groups of mice were immunized either intranasally (IN) or sublinguallly (SL) or via the combined routes (IN/SL/SL or SL/IN/IN). The fecal IgA (B), serum IgA (C) and serum IgG (D) antibody titers 2 weeks after final immunization. The results are presented as the mean ± SEM and statistical significance was determined using Student's t-test: * p<0.05; ** p<0.01; *** p<0.001; NS, not significant; and ND, not detected.
Generation of α4β7+CCR9+ gut-homing B cells and CXCR5+PD-1+ Tfh cells in mesenteric LNs
Secretory IgA is important for immune exclusion on mucosal surfaces. For the production of SIgA in the mucosal compartment, the mucosal homing of immune effector cells is an important requisite. The class switch recombination (CSR) towards IgA isotypes is modulated by follicular helper T (Tfh) cells. The CCR9 together with α4β7 are best-described factors regulating immune cell homing into the gut.21 To address SIgA responses induced by immunizations, we quantified gut-homing B cells and Tfh cells in the mesenteric lymph nodes (mLNs) (Fig. 2). The CCR9+B220+ cells in mLNs were more abundant in animals received combined route immunizations (IN/SL/SL and SL/IN/IN), suggesting more efficient homing of activated B cells to the mLN germinal centers (Fig. 2A and B). The number of α4β7+B220+ cells in IN/SL/SL mice were higher than that of the SL/SL/SL group. These results indicate that the targeting efficacy of activated lymphocytes could be altered by how prime-boost immunization routes are employed. Since T cell activities also appeared actively modulated by the FlaB mucosal adjuvant in our previous NoV Pd vaccine study,20 we tested whether the different route combinations have also differentially modulated Tfh cell activity in mLNs. The mLN Tfh cell number was significantly higher in combined route groups (IN/SL/SL and SL/IN/IN), suggesting the importance of targeting the gut through the SL immunization (Fig. 2C and D). These results demonstrate that the route combination could affect effector cell population in the gut, which consequently affected intestinal SIgA responses.
Figure 2.
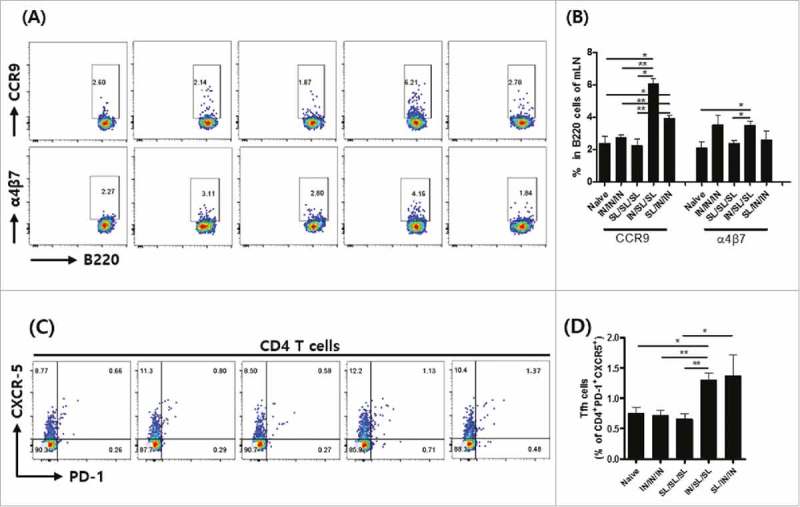
Induction of gut-homing B cell and Tfh cell responses in mesenteric lymph nodes (mLNs). (A) Representative flow cytometry profiles and (B) the statistical analysis of percentage of α4β7 or CCR9 expression in B220+ cells in mLNs. (C) Representative FACS micrograph, and (D) the statistical analysis of percentage of CXCR-5 and PD-1 expressing CD4+ cell population. The results are presented as the mean ± SEM, and statistical significance was determined by Student's t-test: * p < 0.05, ** p<0.01, and *** p < 0.001.
IN priming with the Pd+FlaB vaccine effectively induces significant systemic Th1 and Th2 cytokine responses
We next checked systemic antigen-specific cytokine responses using spleen cells. IL-2, IL-4 and IL-5 are essential for the induction of antigen-specific antibody responses,22 and affect IgM B cells to switch to IgA isotypes.23 Supernatants from spleen cell cultures re-stimulated with the Pd antigen (5 µg/ml) for 5 days were assayed for IFNγ, IL-4, IL-2, and IL-5. In our previous study, IN route appeared superior to SL in inducing IFNγ producing CD4+ and CD8+ cells.20 The IN priming appeared to be a requisite for more robust cytokine production. Dramatically higher antigen-specific production of IFNγ, IL-2, IL-4 and IL-5 was noted only in IN priming (IN/IN/IN and IN/SL/SL) groups (Fig. 3). The SL/SL/SL immunization was far less efficient in stimulating the systemic cytokine responses though the levels were still significantly higher than the naïve control group. In the SL/IN/IN group, two IN boosting increased cytokine production levels, which were still far lower (less than half) than IN priming groups. Notably, the IFNγ response, the hallmark of cell mediated immunity, was also well induced along with Th2 cytokines (Fig. 3D), corroborating our previous results that FlaB enhanced Pd-specific immune responses in the Th1/Th2 balanced fashion.20 Taken together, the combined IN/SL/SL immunization could induce sufficient systemic antigen-specific Th1 and Th2 immune responses comparable to the IN/IN/IN combination as noticed by Pd-specific cytokine productions.
Figure 3.
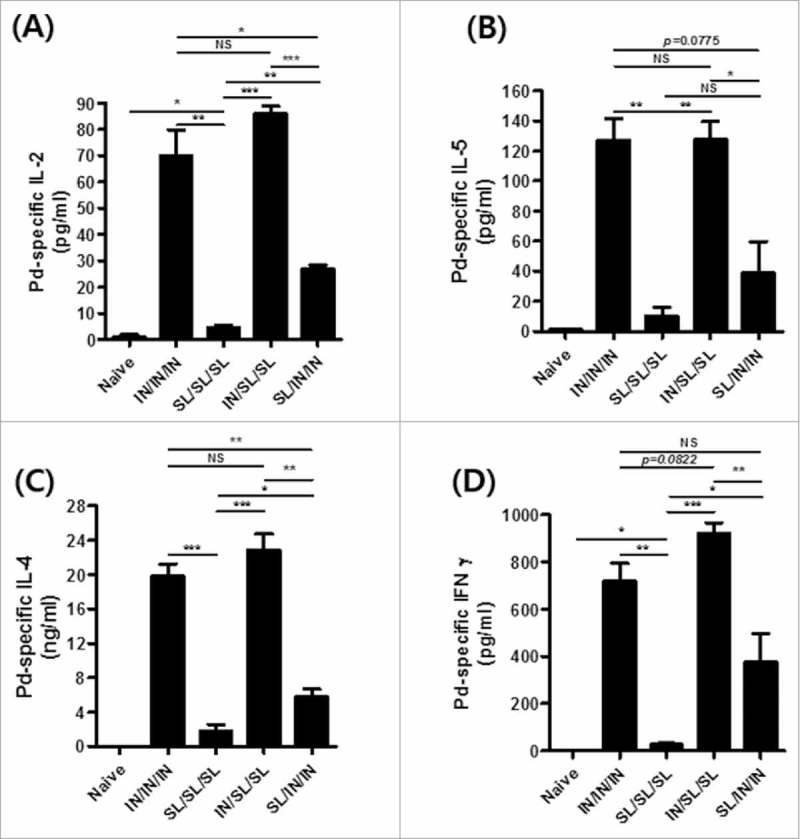
Intranasal priming-induced Pd-specific systemic T cell responses. Levels of Pd-specific IL-2 (A), IL-5 (B), IL-4 (C), and IFNγ (D) in the supernatant of the spleen cell culture re-stimulated with the Pd antigen for 5 days. Results are presented as the mean ± SEM, and statistical significance was determined by Student's t-test: * p<0.05; ** p<0.01; *** p<0.001; and NS, not significant.
Intranasal priming and sublingual boosting maintains significant levels of fecal SIgA and effectively induces long-lasting antigen-specific memory B and plasma cell responses
We next analyzed whether the immunization route combinations could affect immune memories in the mucosal compartment. Antigen-specific IgA and IgG levels in sera did not manifest significant differences among experimental groups 12 weeks after the final immunization (Fig. 4B and C). However, the IN/SL/SL combined immunization resulted in the maintenance SIgA titers in feces at significantly higher levels compared with single route prime-boost (IN/IN/IN or SL/SL/SL) immunizations (Fig. 4A). The SL/IN/IN combination group showed relatively higher SIgA levels in 12 weeks than single route prime-boost schedules though statistical significances were not noted. These results suggest that route combination would be more efficient in maintaining mucosal immune memory regardless of the prime-boost sequences.
Figure 4.
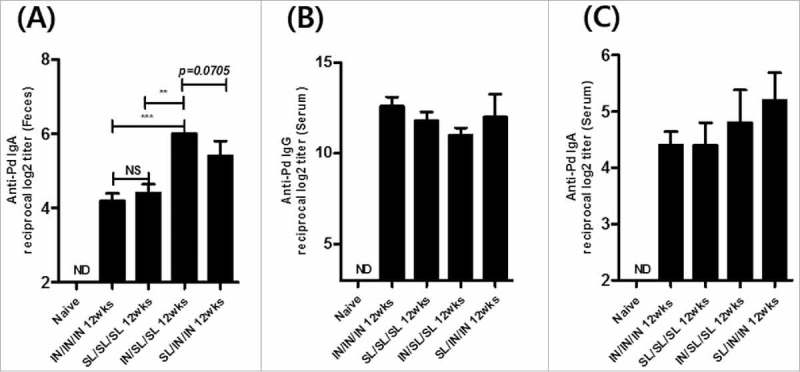
Long-lasting Pd-specific IgA antibody responses in feces. The Pd specific fecal IgA (A), serum IgG (B) or serum IgA (C) titer at 12 weeks after the final boost immunization. The results are presented as the mean ± SEM, and statistical significance was determined by Student's t-test: * p<0.05; ** p<0.01; *** p<0.001; NS, not significant; and ND, not detected.
The induction of long-lasting plasma cells and memory B cells is a major goal of prophylactic vaccination. Antigen-specific germinal center B cells differentiate into Ig-secreting plasma cell and migrate to bone marrow, where they become the long-lasting plasma cells.24 Memory B cells accumulate in the spleen, circulate among the body's chain of lymph nodes and maintain peripheral surveillance for the antigen. We quantified the long-lasting plasma cells in bone marrow (Fig. 5A and B) and memory B cells in spleen (Fig. 5C and D). Consistent with the pattern of the SIgA memory response observed in feces, significantly higher number of Pd-specific IgA-secreting plasma cells were observed in bone marrow of IN/SL/SL mice (Fig. 5A). Higher levels of Pd-specific IgG-secreting plasma cells were observed in bone marrow of mice immunized via combined routes compared with the SL only route (Fig. 5B). On the other hand, both IgA and IgG memory B cells in spleen were more abundant in IN primed groups, like cytokine levels (Fig. 5C and D). Splenic IgG and IgA memory B cells in IN/IN/IN group was significantly higher than that of IN/SL/SL group, suggesting that IN route is better in expanding systemic responses while SL route was superior in targeting mucosa-specific SIgA memory responses. These results also imply that changing prime and boost mucosal immunization routes would differentially affect immune memory generation in mucosal and systemic immune compartments.
Figure 5.
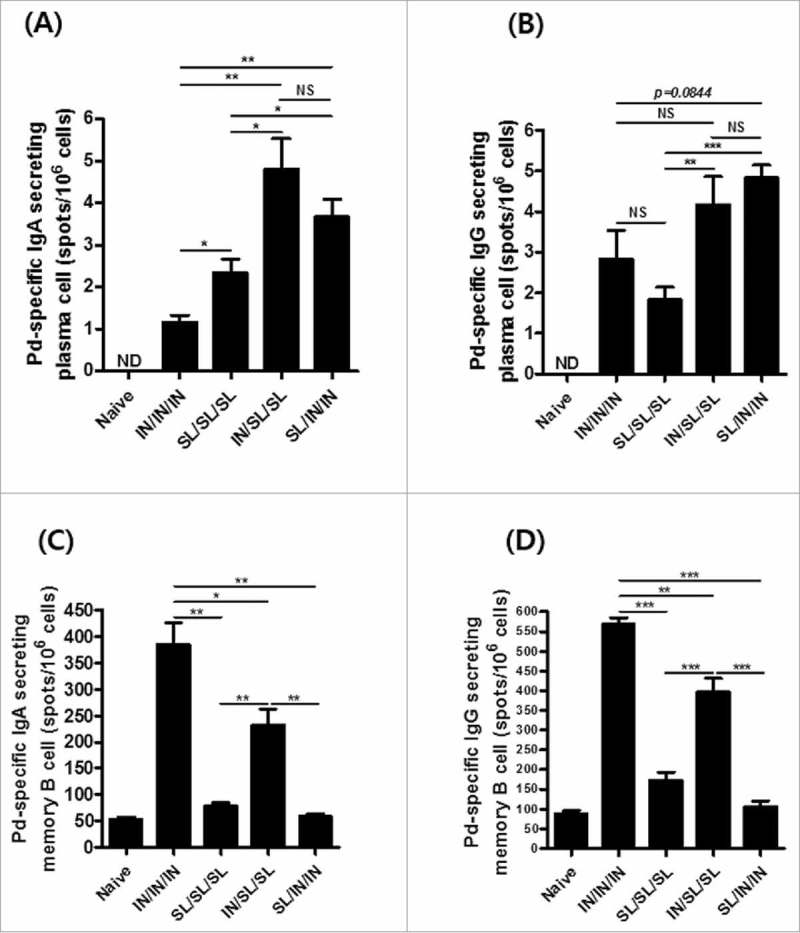
Long-lasting antigen-specific memory B and plasma cell responses. (A and B) Pd-specific IgA- and IgG-secreting plasma cells from bone morrow, respectively, and (C, D) IgA- and IgG-secreting memory B cells from spleen at two weeks after the final vaccination. The results are presented as the mean ± SEM, and statistical significance was determined by Student's t-test: * p<0.05; ** p<0.01; *** p<0.001; NS, not significant, and ND, not detected.
Discussion
It is becoming increasingly clear that mucosal SIgA is a major player in protective mucosal immune responses and serves as an effector in enteric tissue against acute viral infections. In the present study, we propose a route-combination mucosal vaccination strategy for more efficacious SIgA responses in the gut: the combined IN prime and SL boost immunization protocol resulted in a more potent SIgA responses with longer lasting immune memories. The antigen-specific SIgA should effectively exclude NoV infections from the intestinal mucosa. The IN prime and SL boost also induced strong IFNγ responses indicating potent cell mediated immune responses which will play important roles in eliminating virus infected cells. Because of the lack of animal infection models, there is no direct report yet showing protective effects of intestinal SIgA in preventing intestinal NoV infection. On the other hand, there are solid evidences that mucosal rotavirus-specific SIgA strongly correlates with less severe disease and prevention of rotavirus infections in humans.25,26 Given rotavirus and NoV cause similar diarrheal diseases in humans, those reports should rationally be extrapolated to NoV infection.
Most of currently available clinical vaccines are administered via the parenteral route.3 Although parenteral vaccine delivery is more amenable and effective in inducing systemic IgG responses, the protective efficacy of vaccines against mucosal pathogens should be evaluated by the immune responses induced in effector mucosal sites.27 Vaccination via the IN route provides desirable attributes such as easy administration, noninvasiveness, flexibility, and the ability to trigger the common mucosal immune responses in pulmonary, urogenital and gastrointestinal regions.28 Nonetheless, the IN vaccination, which induces α1β4, L-selectin and CCR10, is generally inefficient in inducing SIgA in the gut.17 The sublingual route has been used safely for delivery of chemical medicines and allergen-specific immunotherapy in humans as well as animals. The SL route was recently adopted as a mode of vaccine delivery capable of eliciting both systemic and mucosal immune responses while avoiding many of the adverse effects and formulation difficulties associated with oral or IN vaccination.29 In our previous study for the FlaB-adjuvanted NoV Pd vaccine, we had tried both IN and SL routes for immunization.20 FlaB-mediated TLR5 signaling was responsible for the enhanced immune responses in both system and mucosal compartments. The IN route induced significantly higher serum IgG and IgA titers than SL immunization. Higher number of IFNγ producing CD8+ and CD4+ cells was noted in spleen and mesenteric LNs of IN immunized animals. However, 30-fold or higher fecal IgA titers were observed in the SL immunized mice. Based on these findings, we expected the remarkably augmented SIgA production in the intestinal mucosa by combining IN-mediated immune expansion and SL-mediated gut targeting. The immunization route combination obviously worked evidencing increased SIgA and gut homing B cells (Fig. 1A, 2A and B), higher number of Tfh in mLNs (Fig. 2C and D), enhanced cytokine responses in the systemic compartment (Fig. 3), and longer memory responses (Fig. 4 and 5). More importantly, the sequence of prime-boost schedule played rather a deterministic role. The IN priming seemed to provide more expanded immune responses in the systemic compartment as evidenced by enhanced cytokine production (Fig. 3). The SL boosting appeared to be crucial in inducing more gut-homing B cells and furnishing environments on local LNs for more robust SIgA responses (Fig. 2). On the other hand, the significant differences in specific serum IgA level among groups at 2 weeks after final immunization, where IN priming resulted in significantly higher titers, disappeared at 12 weeks. From 2 weeks to 12 weeks after last immunization, the serum IgA titers decreased by ca. 4–5log2, during which differences among experimental groups. This result shows that IN priming was effective in expanding antibody responses in the systemic compartment, but it was short-lived.
The differential expression of homing receptors on the surface of T and B lymphocytes is dependent on the priming site. For instance, the majority of IgA-secreting cells and T cells activated in GALT express the integrin α4β7.21,30 In contrast, IgA-secreting cells primed at other mucosal inductive sites, such as those elicited by IN immunization in the LNs associated with airways, cannot efficiently occupy plasma cell niches in intestinal sites.31 In addition, α4β7+ plasma and memory B cells can be found after infection or experimental challenge with enteric pathogens as well as oral immunization.32 These differential localization/homing of effector immune cells should be responsible for different immune response patterns in various organs and tissues. Antigens administered through mucosa are transported to specialized mucosal-associated lymphoid tissues. The Peyer's patches, nasopharynx-associaed lymphoid tissue (NALT), and bronchus-associated lymphoid tissue (BALT) are important inductive tissue that generate mucosal immunity upon vaccination via the oral, nasal and pulmonary routes, respectively. Oral/gastrointestinal immunization primarily induce immune responses in the gut from stomach to colon. The IN immunization induces robust immune responses in the respiratory and genitourinary tracts. On the other hand, the SL immunization induces good immune responses in respiratory, gastrointestinal, and genitourinary tract.33 Immune responses in distant effector sites after mucosal immunization is explained by the existence of common mucosal immune system.34 The SIgA is locally produced in effector tissues. The presence of Ag-specific SIgAs at mucosal effector sites distant from the inductive sites where initial Ag sampling occurred is definitive evidence for the common mucosal immune system (CMIS). Activated lymphocytes at inductive secondary lymphoid tissues are imprinted with specific addressins and chemokine receptors to be homed into specific effector sites.35 IN and SL routes should share same draining LNs, such as cervical LNs while antigen presenting cell (APC) population in nasal and sublingual mucosae are different.33,34 Imprinting and activation of B and T lymphocytes in the same LNs by different APCs from nasal or sublingual mucosae might have directed different SIgA responses in the gut. This should be proven by more defined cellular immunological studies of draining LNs of immunized mice in the future.
Systemically administered vaccines can often elicit mucosal responses, as observed by the detection of primed T cells in the spleen, resulting in a promiscuous homing program that can drive cells to the intestinal mucosa.36 The, IL-2, IL-4, and IL-5 cytokines were reported to play important roles in inducing class switching and promote IgA production by enhancing post-switch maturation.23 In the present study, IN priming strongly induced Pd-specific IL-2, IL-4 and IL-5 cytokine-secreting T cells in the spleen. Moreover, systemic T cell priming effect via the IN immunization was well maintained even after two SL boost immunizations (Fig. 3). However, relatively poor T cell activation by SL priming could not be escalated to the IN/SL/SL level by additional two IN boost immunizations, suggesting the importance of initial imprinting of effector cells by the priming immunization. These findings highlight the importance of the priming route in the antigen-specific systemic T cell response and indicate that the priming immunization route plays a povotal role in modulating CMIS responses.
Materials and methods
Protein production and vaccination schedule
The norovirus (NoV)-specific P domain (Pd) antigen and V. vulnificus flagellin (FlaB) were prepared as previously described.20 Eight-week-old female BALB/c mice were immunized with a mixture of Pd (0.5 μg) plus FlaB (2 μg) three times at 2-week intervals through the IN route (IN/IN/IN), SL route (SL/SL/SL) or one of two combined routes, employing IN prime and SL boost (IN/SL/SL) or SL prime and IN boost (SL/IN/IN) schedules. The immunization protocol is illustrated in Figure 1. All animal experimental procedures were conducted in accordance with the guidelines of the Animal Care and Use Committee of Chonnam National University.
Determination of antigen-specific antibodies and cytokines by ELISA
Two weeks after the final immunization, serum and feces samples were collected for the determination of NoV Pd-specific antibody titers by ELISA as previously reported.20 Briefly, 96-well plates were coated with 1 μg/ml of Pd recombinant protein overnight at 4°C and then blocked with 1 mM EDTA (BIONEER, C-9007) in PBST containing 0.5% BSA (Sigma, cat. A2153-50G) at 37°C for one hour. After washing with PBST (0.05% Tween-20 in 1x PBS), 2-fold serially diluted sera were added, followed by incubation at room temperature for 2 hours. Antibody responses were detected using HRP-conjugated goat anti-mouse IgG and IgA secondary antibodies, then developed with 3,3′,5,5′-tetramethylbenzidine (TMB, BD OptEIA, 555214) and stopped with 1 N H2SO4. The OD was measured at 450 nm using a microplate reader (Molecular Devices Corp., Menlo Park, CA). The titers were expressed as the reciprocal log2 values of the dilution. We regarded IgA detected in feces as functional SigA following previous reports.37,38
NoV Pd-specific interferon gamma (IFNγ), interleukin-4 (IL-4), IL-2, and IL-5 produced by splenocytes were determined by ELISA.20 Splenocytes (106) were incubated with 5 μg/ml of Pd recombinant protein for 5 days. The supernatant from the re-stimulated cells was then collected and kept at −80°C until use for cytokine measurement. The cytokines were measured using ELISA kits (eBioscience, San Diego, CA) according to the manufacturer's instructions.
Determination of Ab-secreting cells by ELISPOT assays
Splenocytes and bone marrow cells from mice were used for ELISPOT analysis. To assess Pd-specific antibody-secreting cells (ASCs), multi-screen 96-well plates (BD Biosciences) were coated with recombinant Pd protein (5 µg/ml) overnight at 4°C. After blocking, 106 splenocytes and bone marrow cells were added to the Pd-coated plates, followed by incubation for 1 day for bone marrow cells, or 5 days for spleen cells. The plates were subsequently developed with HRP-conjugated anti-mouse IgG or IgA, and the spots were visualized with the AEC substrate (Sigma) and counted with a CTL-Immunospot Analyzer (Cellular Technology, Shaker Heights, OH, USA).
Flow cytometry
Immune cells isolated from spleen or mLNs were used for determination of gut-homing B cells and follicular helper T cells (Tfh cells). Gut-homing cells were assayed by anti-B220-FITC, anti-α4β7-APC (Thermo fisher) and anti-CCR9-PE (Biolegend). Tfh cells were stained with anti-CD4-FITC (Biolegend), anti-PD-1-PE (Biolegend) and anti-CXCR5-APC (Biolegend). After staining, cells were analyzed with the Accuri C6 Flow Cytometer System (BD Bioscience).
Statistical analysis
The results are expressed as the mean ± SEM unless otherwise noted. Student's t-test was used to compare two groups. All experiments were repeated more than three times, and the results from representative experiments are shown.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2017K000360, NRF-2016R1A2B4009611, 1720120(HA17C0038), NRF-2017M3A9E2056372).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake RJ, Praet N, Bellinger DC, de Silva NR, Gargouri N, et al.. World health organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12:e1001923. doi: 10.1371/journal.pmed.1001923. PMID:26633896.9382757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel MM, Hall AJ, Vinje J, Parashar UD. Noroviruses: a comprehensive review. Journal of clinical virology: the official publication of the Pan American Society for Clinical Virology. 2009;44:1–8. doi: 10.1016/j.jcv.2008.10.009. PMID:19084472.9382757 [DOI] [PubMed] [Google Scholar]
- 3.Lucero Y, Vidal R, O'Ryan GM. Norovirus vaccines under development. Vaccine. 2017. doi: 10.1016/j.vaccine.2017.06.043 [DOI] [PubMed] [Google Scholar]
- 4.Vidal R, Roessler P, Solari V, Vollaire J, Jiang X, Matson DO, Mamani N, Prado V, O'Ryan ML. Novel recombinant norovirus causing outbreaks of gastroenteritis in Santiago, Chile. J Clin Microbiol. 2006;44:2271–5. doi: 10.1128/JCM.01890-05. PMID:16757638.9382757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansman GS, Natori K, Shirato-Horikoshi H, Ogawa S, Oka T, Katayama K, Tanaka T, Miyoshi T, Sakae K, Kobayashi S, et al.. Genetic and antigenic diversity among noroviruses. J Gen Virol. 2006;87:909–19. doi: 10.1099/vir.0.81532-0. PMID:16528040.9382757 [DOI] [PubMed] [Google Scholar]
- 6.Phumpholsup T, Chieochansin T, Vongpunsawad S, Vuthitanachot V, Payungporn S, Poovorawan Y. Human norovirus genogroup II recombinants in Thailand, 2009–2014. Arch Virol. 2015;160:2603–9. doi: 10.1007/s00705-015-2545-5. PMID:26215446.9382757 [DOI] [PubMed] [Google Scholar]
- 7.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, et al.. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med. 2011;365:2178–87. doi: 10.1056/NEJMoa1101245. PMID:22150036.9382757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Springer MJ, Ni Y, Finger-Baker I, Ball JP, Hahn J, DiMarco AV, Kobs D, Horne B, Talton JD, Cobb RR. Preclinical dose-ranging studies of a novel dry powder norovirus vaccine formulation. Vaccine. 2016;34:1452–8. doi: 10.1016/j.vaccine.2016.01.064. PMID:26873053.9382757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, Ferreira J, Chen WH, Sublett R, Richardson C, et al.. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010;202:1649–58. doi: 10.1086/657087. PMID:20979455.9382757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Treanor JJ, Atmar RL, Frey SE, Gormley R, Chen WH, Ferreira J, Goodwin R, Borkowski A, Clemens R, Mendelman PM. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate–reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J Infect Dis. 2014;210:1763–71. doi: 10.1093/infdis/jiu337. PMID:24951828.9382757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernstein DI, Atmar RL, Lyon GM, Treanor JJ, Chen WH, Jiang X, Vinjé J, Gregoricus N, Jr Frenck RW, Moe CL, et al.. Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J Infect Dis. 2015;211:870–8. doi: 10.1093/infdis/jiu497. PMID:25210140.9382757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corthesy B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front Immunol. 2013;4:185. doi: 10.3389/fimmu.2013.00185. PMID:23874333.9382757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blutt SE, Conner ME. The gastrointestinal frontier: IgA and viruses. Front Immunol. 2013;4:402. doi: 10.3389/fimmu.2013.00402. PMID:24348474.9382757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki T, Kawaguchi A, Ainai A, Tamura S, Ito R, Multihartina P, Setiawaty V, Pangesti KN, Odagiri T, Tashiro M, et al.. Relationship of the quaternary structure of human secretory IgA to neutralization of influenza virus. Proc Natl Acad Sci U S A. 2015;112:7809–14. doi: 10.1073/pnas.1503885112. PMID:26056267.9382757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mestecky J, Michalek SM, Moldoveanu Z, Russell MW. Routes of immunization and antigen delivery systems for optimal mucosal immune responses in humans. Behring Inst Mitt. 1997;98:33–43. PMID:9382757. [PubMed] [Google Scholar]
- 16.Rhee JH, Lee SE, Kim SY. Mucosal vaccine adjuvants update. Clin Exp Vaccine Res. 2012;1:50–63. doi: 10.7774/cevr.2012.1.1.50. PMID:23596577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyaka PN. Inducing mucosal iga: a challenge for vaccine adjuvants and delivery systems. J Immunol. 2017;199:9–16. doi: 10.4049/jimmunol.1601775. PMID:28630108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong SH, Byun YH, Nguyen CT, Kim SY, Seong BL, Park S, Woo GJ, Yoon Y, Koh JT, Fujihashi K, et al.. Intranasal administration of a flagellin-adjuvanted inactivated influenza vaccine enhances mucosal immune responses to protect mice against lethal infection. Vaccine. 2012;30:466–74. doi: 10.1016/j.vaccine.2011.10.058. PMID:22051136. [DOI] [PubMed] [Google Scholar]
- 19.Lee SE, Kim SY, Jeong BC, Kim YR, Bae SJ, Ahn OS, Lee JJ, Song HC, Kim JM, Choy HE, et al.. A bacterial flagellin, Vibrio vulnificus FlaB, has a strong mucosal adjuvant activity to induce protective immunity. Infect Immun. 2006;74:694–702. doi: 10.1128/IAI.74.1.694-702.2006. PMID:16369026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verma V, Tan W, Puth S, Cho KO, Lee SE, Rhee JH. Norovirus (NoV) specific protective immune responses induced by recombinant P dimer vaccine are enhanced by the mucosal adjuvant FlaB. J Transl Med. 2016;14:135. doi: 10.1186/s12967-016-0899-4. PMID:27184355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mora JR, von Andrian UH. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008;1:96–109. doi: 10.1038/mi.2007.14. PMID:19079167. [DOI] [PubMed] [Google Scholar]
- 22.Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21:1303–9. doi: 10.1093/intimm/dxp102. PMID:19819937. [DOI] [PubMed] [Google Scholar]
- 23.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–34. doi: 10.1038/nri2322. PMID:18483500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellyard JI, Avery DT, Phan TG, Hare NJ, Hodgkin PD, Tangye SG. Antigen-selected, immunoglobulin-secreting cells persist in human spleen and bone marrow. Blood. 2004;103:3805–12. doi: 10.1182/blood-2003-09-3109. PMID:14701691. [DOI] [PubMed] [Google Scholar]
- 25.Coulson BS, Grimwood K, Hudson IL, Barnes GL, Bishop RF. Role of coproantibody in clinical protection of children during reinfection with rotavirus. J Clin Microbiol. 1992;30:1678–84. PMID:1321167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Offit PA. Correlates of protection against rotavirus infection and disease. Novartis Found Symp. 2001;238:106–13; discussion 14–24. PMID:11444023. [DOI] [PubMed] [Google Scholar]
- 27.Yuki Y, Kiyono H. New generation of mucosal adjuvants for the induction of protective immunity. Rev Med Virol. 2003;13:293–310. doi: 10.1002/rmv.398. PMID:12931340. [DOI] [PubMed] [Google Scholar]
- 28.Ogra PL, Faden H, Welliver RC. Vaccination strategies for mucosal immune responses. Clin Microbiol Rev. 2001;14:430–45. doi: 10.1128/CMR.14.2.430-445.2001. PMID:11292646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, Hofman P, Holmgren J, Anjuère F, Czerkinsky C. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007;25:8598–610. doi: 10.1016/j.vaccine.2007.09.073. PMID:17996991. [DOI] [PubMed] [Google Scholar]
- 30.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–9. doi: 10.1038/nri1203. PMID:14523388. [DOI] [PubMed] [Google Scholar]
- 31.Agnello D, Denimal D, Lavaux A, Blondeau-Germe L, Lu B, Gerard NP, Gerard C, Pothier P. Intrarectal immunization and IgA antibody-secreting cell homing to the small intestine. J Immunol. 2013;190:4836–47. PMID:23547118. [DOI] [PubMed] [Google Scholar]
- 32.Kuklin NA, Rott L, Feng N, Conner ME, Wagner N, Muller W, Greenberg HB. Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J Immunol. 2001;166:1894–902. doi: 10.4049/jimmunol.166.3.1894. PMID:11160237. [DOI] [PubMed] [Google Scholar]
- 33.Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij JP. Buccal and sublingual vaccine delivery. Journal of controlled release: official journal of the Controlled Release Society. 2014;190:580–92. doi: 10.1016/j.jconrel.2014.05.060. PMID:24911355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujkuyama Y, Tokuhara D, Kataoka K, Gilbert RS, McGhee JR, Yuki Y, Kiyono H, Fujihashi K. Novel vaccine development strategies for inducing mucosal immunity. Expert Rev Vaccines. 2012;11:367–79. doi: 10.1586/erv.11.196. PMID:22380827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J exp med. 2002;195:135–41. doi: 10.1084/jem.20011502. PMID:11781372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al.. Dynamic T cell migration program provides resident memory within intestinal epithelium. J exp med. 2010;207:553–64. doi: 10.1084/jem.20090858. PMID:20156972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim KH, Lee YT, Hwang HS, Kwon YM, Kim MC, Ko EJ, Lee JS, Lee Y, Kang SM. Virus-Like Particle Vaccine Containing the F Protein of Respiratory Syncytial Virus Confers Protection without Pulmonary Disease by Modulating Specific Subsets of Dendritic Cells and Effector T Cells. J virol. 2015;89:11692–705. doi: 10.1128/JVI.02018-15. PMID:26355098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung Y, Wen T, Mingler MK, Caldwell JM, Wang YH, Chaplin DD, Lee EH, Jang MH, Woo SY, Seoh JY, et al.. IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production. Mucosal Immunol. 2015;8:930–42. doi: 10.1038/mi.2014.123. PMID:25563499. [DOI] [PMC free article] [PubMed] [Google Scholar]


