Tics, particularly at a young age, may be difficult to distinguish from other abrupt and brief movements, such as myoclonus and chorea. Their suppressibility and association with premonitory urges serve as useful diagnostic clues. However, not all individuals with tics or Gilles de la Tourette syndrome (GTS) are able to exert conscious inhibitory tic control and not all tics are preceded by premonitory sensations. Furthermore, hyperkinesias in other paroxysmal movement disorders, such as in paroxysmal kinesigenic dyskinesia (PKD), a condition associated with several episodic neurologic disorders and for which a causative gene has been recently described,1 are commonly preceded by sensory phenomena, such as numbness of the affected body sites or epigastric area.1, 2, 3 Moreover, the presence of dystonic tics, which differ from clonic tics in phenomenology and duration, might further contribute to diagnostic confusion.4, 5, 6, 7
On the other hand, not all sudden and repetitive movements in GTS are tics and more than one hyperkinesia may co‐occur in a single patient. Their proper recognition bears significant prognostic and therapeutic implications.
Here, we present the case of an adolescent with a classic history of GTS, who was referred to our clinic upon developing longer‐lasting and, on occasion, disabling hyperkinetic episodes at a later age misdiagnosed as complex tics.
Case Presentation
A British 17‐year‐old patient with GTS was referred to our department for treatment of repetitive episodes of cramping and twisting of, predominantly, his right side of the body since the age of 14 considered as being complex tics (see Video 1). These episodes initially affected his feet and, with time, spread to involve his trunk and arms. His jaw would sometimes additionally clench and he could not properly articulate. The episodes lasted up to 40 seconds and occurred with minimal phenomenological variability, but different intensity. Their daily frequency ranged between 5 and 100 times.
Preceding most of these, tension or focal numbness would gradually build up, which was relieved upon their event. None of the episodes could be suppressed. Loud noises or sudden movements would trigger some of the episodes.
The patient, who, between the first 6 to 18 months of his life had had three seizures, was diagnosed with attention‐deficit hyperactivity disorder (ADHD) at the age of 5 and, for that, he had been receiving methylphenidate for 5 years. At age 7, he manifested abrupt, repetitive movements of his eyes and face, such as excessive eye blinking, eye rolling, frowning, tongue rolling, shoulder shrugging, and sounds, such as sniffing and grunting. Although he did not report or exhibit any marked ritualistic behavior, he claimed particular obsessions (e.g., checking door locks or keeping his video games meticulously ordered). At the age of 9, he was also diagnosed with autism spectrum disorder (ASD).
During an otherwise unrevealing neurologic examination, simple motor and phonic tics, as well as complex motor tics and echophenomena, were noted (see Video 2). Most of his tics could be inhibited on demand. No additional movements, in particular, no cramping or twisting were noted during examination. Previous interictal EEG examinations, a cranial MRI, and biochemical examinations (acanthocytes, serum copper, ceruloplasmin, and white cell enzymes) were normal. The patient's mother reported having had two seizures before the age of 2. She and other family members also suffered migrainous headaches (Fig. 1A, pedigree). There was no family history of a tic disorder and no formal diagnosis of obsessive‐compulsive disorder or ADHD. However, the patient's mother had obsessive‐compulsive symptoms related to symmetry and order and reported one of her cousins to be diagnosed with ASD.
Figure 1.
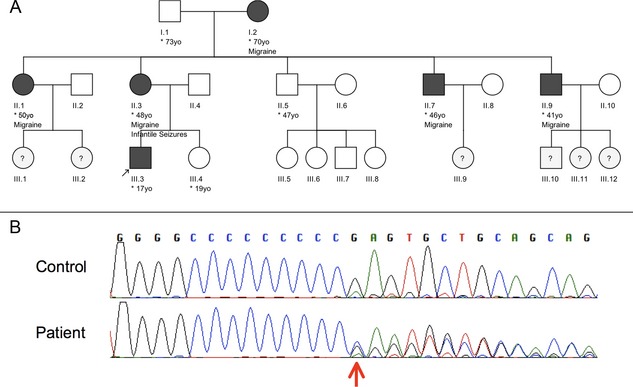
(A) Pedigree of the reported family. Clinically affected members by either migraine and/or infantile seizures are marked with black symbols. The index patient is indicated by an arrow. Individuals where no information was available are marked with a question mark. (B) Chromatograms of the mutation found in the patient (indicated by red arrow, lower row) and a control sequence (upper row). The mutation is the heterozygous insertion of a cytosine at position 649, within a string of nine cytosines leading to a premature stop codon.
The unusual character of the newly described episodes and, in particular, their duration, phenomenological invariability, presence of triggers, involuntariness and lack of suppressibility, absence of waxing and waning, as well as the patient's qualitative distinction of the two phenomena argued against their consideration as complex tics. The reported infantile convulsions of both the patient and his mother, as well as the family history of migrainous headaches, prompted us to screen for mutations in the PRRT2 gene. The patient was found to be a heterozygous carrier of the c.649dupC, p.R217Pfs*8 (Fig. 1B). This frameshift mutation has been recognized as the most common causative mutation for familial PKD.1 Treatment with 100 mg of carbamazepine resulted in complete resolution of PKD attacks.
Discussion
Co‐occurrence of paroxysmal dyskinesias in patients with tics or GTS has been mentioned,2, 8 but rarely highlighted.9 Diagnostic difficulties between, sometimes, akin abrupt and repetitive movements may lead to misdiagnoses as in the presented case.
This is the first report, to our knowledge, of a patient with a classic presentation of full‐blown GTS and PKD resulting from PRRT2 mutation. Given the common nature of GTS in the general population (estimates of up to 1%),10 this might well reflect a coincidental finding. Of interest, however, is the interaction of PRRT2 with the synaptosomal associated protein of 25 kD (SNAP‐25).11, 12 SNAP‐25 has been associated with both ADHD13, 14 and GTS.14 Its expression in peripheral blood has been found to correlate with tic severity of unmedicated GTS children and adolescents.14 Furthermore, in their seminal article on clinical characteristics of PKD, Bruno et al.2 reported on 7 family members of PKD patients with tics. Unfortunately, with the exception of Silveira‐Moriyama et al.,8 who reported on a patient with tics, dysmorphic features, and PKD resulting from PRRT2 mutation, this finding was not followed in recent clinicogenetic studies.1, 15, 16, 17, 18, 19
The discovery of the PRRT2 gene as the cause of PKD has led to the widening of a spectrum of associated episodic neurologic conditions to include hemiplegic migraine, episodic ataxia, and infantile seizures.1, 17, 20 Thus, it currently remains unclear whether this case may lead the way for expanding this phenotypic spectrum of PRRT2‐associated conditions. It, however, highlights the necessity for accurate clinical recognition of new or unusual paroxysmal hyperkinetic phenomena in all movement disorder patients, even in the presence of classic diagnoses such as GTS. Moreover, specialists should feel encouraged to conduct genetic studies in cases where clinical clues point to different than expected directions.
To conclude, here we present, for the first time, an adolescent with GTS, comorbid ADHD, obsessive‐compulsive behavior, and ASD, as well as additional paroxysmal kinesigenic dyskinesia resulting from PRRT2 mutation misdiagnosed as complex tics.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
C.G.: 1A, 1B, 1C, 3A
N.M.: 1C, 3A
A.G.: 1C, 3A
R.E.: 1C, 3A
A.B.: 1C, 3A
H.H.: 1C, 3A
K.P.B.: 1B, 1C, 3A
Financial Disclosures
C.G. has been awarded grants by Actelion, Ipsen, Pharm Allergan, and Merz Pharmaceuticals, as well as academic research support from Deutsche Forschungsgemeinschaft (MU1692/2‐1 and GA 2031/1‐1) and the European Science Foundation. N.M. has been funded by a MRC‐Wellcome Trust grant. A.G. has been funded by the Muscular Dystrophy Campaign. R.E. has been partly supported by COST Action BM1101 (reference no.: ECOST‐STSM‐BM1101‐160913‐035934). H.H. has been funded by the Muscular Dystrophy Campaign. K.B. has received funding for travel from GlaxoSmithKline, Orion Corporation, Ipsen, and Merz Pharmaceuticals, LLC; serves on the editorial boards of Movement Disorders and Therapeutic Advances in Neurological Disorders; receives royalties from Oxford University Press; received speaker honoraria from GlaxoSmithKline (GSK), Ipsen, Merz Pharmaceuticals, LLC, and Sun Pharmaceutical Industries Ltd.; has received personal compensation for serving on the scientific advisory board for GSK and Boehringer Ingelheim; received research support from Ipsen and from the Halley Stewart Trust through the Dystonia Society UK as well as the Wellcome Trust MRC strategic neurodegenerative disease initiative award (reference no.: WT089698); and has been awarded a grant from the Dystonia Coalition and a grant from Parkinson's UK (reference no.: G‐1009).
Supporting information
Video 1. Short lasting attack with classic for paroxysmal kinesigenic dyskinesia generalized dystonic choreic‐ballistic movements.
Video 2. Multiple simple and complex motor tics, as well as simple phonic tics during a video recording of 2.5 minutes, where the patient was alone in the examination room.
References
- 1. Gardiner AR, Bhatia KP, Stamelou M, et al. PRRT2 gene mutations: from paroxysmal dyskinesia to episodic ataxia and hemiplegic migraine. Neurology 2012;79:2115–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bruno MK, Hallett M, Gwinn‐Hardy K, et al. Clinical evaluation of idiopathic paroxysmal kinesigenic dyskinesia: new diagnostic criteria. Neurology 2004;63:2280–2287. [DOI] [PubMed] [Google Scholar]
- 3. Tan LC, Methawasin K, Teng EW, et al. Clinico‐genetic comparisons of paroxysmal kinesigenic dyskinesia patients with and without PRRT2 mutations. Eur J Neurol 2013; doi: 10.1111/ene.12142. [DOI] [PubMed] [Google Scholar]
- 4. Jankovic J. Botulinum toxin in the treatment of dystonic tics. Mov Disord 1994;9:347–349. [DOI] [PubMed] [Google Scholar]
- 5. Jankovic J, Stone L. Dystonic tics in patients with Tourette's syndrome. Mov Disord 1991;6:248–252. [DOI] [PubMed] [Google Scholar]
- 6. Kompoliti K, Goetz CG. Hyperkinetic movement disorders misdiagnosed as tics in Gilles de la Tourette syndrome. Mov Disord 1998;13:477–480. [DOI] [PubMed] [Google Scholar]
- 7. Feinberg TE, Shapiro AK, Shapiro E. Paroxysmal myoclonic dystonia with vocalisations: new entity or variant of preexisting syndromes? J Neurol Neurosurg Psychiatry 1986;49:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silveira‐Moriyama L, Gardiner AR, Meyer E, et al. Clinical features of childhood‐onset paroxysmal kinesigenic dyskinesia with PRRT2 gene mutations. Dev Med Child Neurol 2013;55:327–334. [DOI] [PubMed] [Google Scholar]
- 9. Oyama G, Okun MS, Ashizawa T, Malaty IA. Paroxysmal kinesigenic dyskinesia‐like symptoms in a patient with Tourette syndrome. Tremor Other Hyperkinet Mov (N Y) 2011;1: tre‐01‐31‐157‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Robertson MM. The Gilles De La Tourette syndrome: the current status. Arch Dis Child Educ Pract Ed 2012;97:166–175. [DOI] [PubMed] [Google Scholar]
- 11. Lee HY, Huang Y, Bruneau N, et al. Mutations in the novel protein PRRT2 cause paroxysmal kinesigenic dyskinesia with infantile convulsions. Cell Rep 2012;1:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stelzl U, Worm U, Lalowski M, et al. A human protein‐protein interaction network: a resource for annotating the proteome. Cell 2005;122:957–968. [DOI] [PubMed] [Google Scholar]
- 13. Faraone SV, Mick E. Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am 2010;33:159–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gunther J, Tian Y, Stamova B, et al. Catecholamine‐related gene expression in blood correlates with tic severity in Tourette syndrome. Psychiatry Res 2012;200:593–601. [DOI] [PubMed] [Google Scholar]
- 15. Guerrini R, Mink JW. Paroxysmal disorders associated with PRRT2 mutations shake up expectations on ion channel genes. Neurology 2012;79:2086–2088. [DOI] [PubMed] [Google Scholar]
- 16. Liu Q, Qi Z, Wan XH, et al. Mutations in PRRT2 result in paroxysmal dyskinesias with marked variability in clinical expression. J Med Genet 2012;49:79–82. [DOI] [PubMed] [Google Scholar]
- 17. Marini C, Conti V, Mei D, et al. PRRT2 mutations in familial infantile seizures, paroxysmal dyskinesia, and hemiplegic migraine. Neurology 2012;79:2109–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Meneret A, Grabli D, Depienne C, et al. PRRT2 mutations: a major cause of paroxysmal kinesigenic dyskinesia in the European population. Neurology 2012;79:170–174. [DOI] [PubMed] [Google Scholar]
- 19. van Vliet R, Breedveld G, de Rijk‐van Andel J, et al. PRRT2 phenotypes and penetrance of paroxysmal kinesigenic dyskinesia and infantile convulsions. Neurology 2012;79:777–784. [DOI] [PubMed] [Google Scholar]
- 20. Cloarec R, Bruneau N, Rudolf G, et al. PRRT2 links infantile convulsions and paroxysmal dyskinesia with migraine. Neurology 2012;79:2097–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Short lasting attack with classic for paroxysmal kinesigenic dyskinesia generalized dystonic choreic‐ballistic movements.
Video 2. Multiple simple and complex motor tics, as well as simple phonic tics during a video recording of 2.5 minutes, where the patient was alone in the examination room.


