Abstract
Pantothenate‐kinase–associated neurodegeneration (PKAN) is an autosomal recessive disorder characterized by iron deposits in basal ganglia. The aim of this study was to quantify iron concentrations of deep gray matter structures in heterozygous PANK2 mutation carriers and in PKAN patients using quantitative susceptibility mapping MRI. By determining iron concentration, we intended to find mutation‐specific brain parenchymal stigmata in heterozygous PANK2 mutation carriers in comparison to age‐matched healthy volunteers. We studied 11 heterozygous PANK2 gene mutation carriers (mean age: 43.4 years; standard deviation [SD]: 10.5), who were found to be clinically asymptomatic by neurological examination. These carriers were compared to 2 clinically affected PKAN patients 21 and 32 years of age and to 13 age‐matched, healthy controls (mean age: 39.7; SD, 13.6). Scanning was performed on a 7.0‐Tesla whole‐body scanner applying three‐dimensional susceptibility‐weighted gradient echo acquisitions. Susceptibility maps were calculated by threshold‐based k‐space division with single‐orientation acquisition. Magnetic susceptibility values, relative to the occipital white matter, were determined for the following regions of interest (ROI): globus pallidus (GP), thalamus, putamen, internal capsule (IC), caudate nucleus, substantia nigra (SN), and red nucleus. Heterozygous PANK2 mutation carriers did not show increased brain iron concentrations, compared to healthy controls (P > 0.05), in any of the examined ROIs. In PKAN patients, more than 3 times higher concentrations of iron were found in the GP, SN, and IC. Our results suggest that heterozygous mutations in PANK2 gene do not cause brain iron accumulation nor do they cause movement disorders.
Keywords: pantothenate kinase associated neurodegeneration (PKAN), neurodegeneration with brain iron accumulation (NBIA), 7‐Tesla MRI, quantitative susceptibility mapping, iron
The syndromes of neurodegeneration with brain iron accumulation (NBIA) are defined by a progressive hypo‐ and/or hyperkinetic movement disorder and pathological excess of iron deposition in the brain, particularly affecting the basal ganglia, mainly the globus pallidus (GP). Of these, pantothenate‐kinase–associated neurodegeneration (PKAN) is the most common form caused by mutations in the PANK2 gene. However, PKAN is an orphan disease with an estimated world‐wide prevalence of approximately 1 in 1 million.1
In NBIA syndromes, iron accumulation observed on gross pathological assessment as brown discoloration can be detected noninvasively as a prominent hypointensity in T2‐ and T2*‐weighted MRI.2
In PKAN, a specific MRI pattern of iron deposition, the “eye of the tiger sign” consistent with a central hyperintensity within a surrounding area of hypointensity in the anterior‐medial part of the GP, is thought to be virtually pathognomonic. However, emergence of this MRI pattern appears to be a dynamic process.3, 4 It has been shown that alterations in GP detected by MRI may precede the development of clinical signs5 (i.e., in asymptomatic carriers of homozygous mutations), but may also be absent in early disease stages.3, 6, 7 Additionally, the bright spot within the surrounding area of hypointensity may vanish over time.3
Recently, a 7‐Tesla (T) MRI study in 3 PKAN patients was performed using the method of field‐dependent R2 relaxivity increase.8 Iron concentration in GP of PKAN patients was more than doubled, compared to controls. Moreover, based on the differences in the field dependency of the relaxation rates, the researchers suggested that, in PKAN patients, iron deposited in forms other than ferritin also contributes to the MRI signal.
On the other hand, little is known about brain iron content in heterozygotes. Results of recent 3‐T MRI studies suggested no signs of increased brain iron content in the 13 examined heterozygous PANK2 mutation carriers.3 However, the latter study showed subtle differences in mean water diffusivity (MD) and fractional water diffusion anisotropy (FA) in GP, cerebral peduncles, and internal capsule (IC) between heterozygous carriers and controls, which may be indirect effects reflecting altered iron content. Indeed, correlations between MD, FA, and ferritin‐iron content were observed in an in vitro phantom study.9
Taking these results into account, the question remains of whether iron accumulation occurs in heterozygous PANK2 mutation carriers. To answer this question, iron quantification techniques using ultra‐high‐field MRI can be very helpful. Changes in magnetic susceptibility of tissue resulting from iron accumulation cause magnetic field shifts on the microscopic scale. These field shifts are more closely related to iron concentration than spin‐spin relaxation processes and can be detected in phase‐sensitized MRIs.2 Quantitative susceptibility mapping (QSM) techniques were developed to determine tissue susceptibility.10, 11, 12, 13 Ultra‐high‐field imaging using magnetic fields of B0 ≥7 T is particularly sensitive to very small susceptibility changes because of the linear relationship between magnetic field strength and the field shift.14, 15, 16, 17 We used this advantage of ultra‐high‐field MRI to study iron concentration in PANK2 mutation carriers.
The aims of our study were to (1) quantify iron concentration in basal ganglia, IC, and thalami (THAs) of PKAN patients and heterozygous PANK2 mutation carriers by employing QSM with ultra‐high‐field MRI and (2) seek for differences between heterozygous PANK2 mutation carriers and healthy volunteers.
Methods
Participants
The study was approved by the relevant local ethic committees, and participants gave written informed consent before the study.
Two PKAN patients (both female, 21 and 32 years of age) and 11 clinically unaffected relatives of these and other PKAN patients (4 female and 7 male; mean age: 43.4 years; strandard deviation [SD]: 10.5) were recruited. Patients were examined in order to determine reference susceptibility values of pronounced brain iron deposits at ultra‐high field. Both patients had the atypical form of PKAN with disease onset at 12 to 14 years and duration of 7 to 20 years. Genetic testing with full sequencing of the PANK2 gene revealed homozygous and compound heterozygous mutations, respectively, in the 2 patients and heterozygous mutations in all nonaffected family members. Thirteen age‐ and sex‐matched healthy individuals (5 female and 8 male; mean age: 39.7 years; SD, 13.6) served as controls. All participants were neurologically examined with a focus on presence (patients) or absence (nonaffected mutation carriers and controls) of movement disorder abnormalities. Demographic and clinical characteristics are shown in Table 1.
Table 1.
Demographic and clinical characteristics of patients and heterozygous mutation carriers
| Proband | Nationality | Age | Clinical Status (Age at Onset) | Disease Severity (BFM Score) | Genetic Status |
|---|---|---|---|---|---|
| PKAN‐1 | Czech | 21 | Generalized dystonia, spasticity (R>L), micrographia, dysarthria, dysphagia, drooling, personality changes (14 years) | 27 | c.1253C>T (homozygous) |
| PKAN‐2 | Czech | 32 | Generalized dystonia, Babinski sign positive (R>L), gait freezing, dysarthria, dysphagia, drooling, personality changes (12 years) | 62 | c.1369G>T and c.1561G>A |
| Non‐Manif‐1 | Polish | 43 | Unaffected | n/a | c.573delC |
| Non‐Manif‐2 | Polish | 41 | Unaffected | n/a | c.1583C>T |
| Non‐Manif‐3 | Czech | 29 | Unaffected | n/a | c.1253C>T |
| Non‐Manif‐4 | Czech | 26 | Unaffected | n/a | c.1253C>T |
| Non‐Manif‐5 | Czech | 56 | Unaffected | n/a | c.1253C>T |
| Non‐Manif‐6 | Czech | 47 | Unaffected | n/a | c.1253C>T |
| Non‐Manif‐7 | Argentinian | 47 | Unaffected | n/a | c.821‐822del |
| Non‐Manif‐8 | Czech | 54 | Unaffected | n/a | c.1369G>T |
| Non‐Manif‐9 | Czech | 56 | Unaffected | n/a | c.1561G>A |
| Non‐Manif‐10 | German | 40 | Unaffected | n/a | Deletion exon 5 |
| Non‐Manif‐11 | German | 39 | Unaffected | n/a | c.1663‐1G>C |
BFM, Burke Fahn Marsden Dystonia Rating Scale; Non‐Manif, nonmanifesting heterozygous gene mutation carrier; n/a, not applicable.
MRI Data Acquisition
MRIs were acquired on a Magnetom 7‐T whole‐body MR scanner (Siemens Healthcare, Erlangen, Germany), using a 24‐channel receive head coil (Nova Medical, Wilmington, MA). The subject's head was carefully padded with foam cushions during the entire measurement to avoid head movements. A three‐dimensional (3D) flow‐compensated susceptibility‐weighted imaging (SWI) gradient echo technique was employed (echo time [TE] = 15 ms; repetition time [TR] = 25 ms; flip angle = 12 degrees; 72 slices; matrix = 448 × 352; spatial resolution: 0.5 × 0.5 × 1.0 mm3). In PKAN patients, turbo inversion recovery magnitude (TIRM; TE = 90 ms; TR = 10,070 ms; inversion time [TI] = 2,614 ms; flip angle = 128 degrees; 35 slices; matrix = 512 × 448; spatial resolution: 0.5 × 0.5 × 3.0 mm3) and 3D magnetization‐prepared rapid acquisition with gradient echo (MP‐RAGE; TE = 2.98 ms; TR = 2,300 ms; TI = 900 ms; flip angle = 5 degrees; matrix = 256 × 240; spatial resolution: 1.0 × 1.0 × 1.0 mm3) techniques were also employed.
Data Processing and Susceptibility Calculation
For the calculation of the susceptibility maps, the phase images derived from the high‐resolution SWI data sets were high‐pass filtered and divided into the original complex image to remove effects of large‐scale background magnetic fields (e.g., air‐tissue boundaries at the sinuses). This processing yields a homodyne high‐pass‐filtered phase image, with most low spatial frequency phases removed.18
To keep the field of view with an aspect ratio of 1:1:4, k‐space was interpolated by zero filling the phase images to a 512 × 512 × 128 three‐dimensional matrix. Susceptibility calculations using a regularized inverse filter were performed in the selected 3D regions of the Fourier transform of the high‐pass‐filtered phase image (Fig. 1).19
Figure 1.
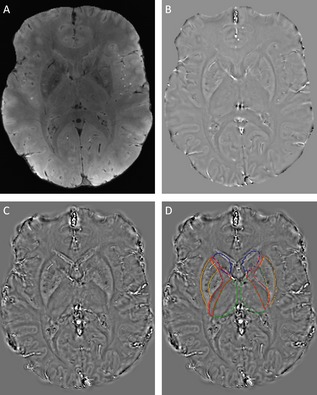
Output of image‐processing steps. (A) Magnitude image, (B) unwrapped filtered phase image, (C) susceptibility map calculated by thresholded k‐space division with single‐orientation acquisition, and (D) susceptibility map with ROIs highlighted in color: blue, caudate nucleus; orange, putamen; dark green, globus pallidus; red, internal capsule; green, thalamus.
Image and Region of Interest Analysis
Seven regions of interest (ROIs) were placed into the deep gray matter structures bilaterally to evaluate the dependence of the calculated susceptibility values in the iron‐rich deep gray matter structures on the iron content, namely, the GP, THA, putamen (PUT), caudate nucleus (CN), substantia nigra (SN), and red nucleus (RN). An additional ROI was placed into the IC. ROIs were manually plotted on the susceptibility maps.
We calculated the difference in susceptibility values of each structure relative to the occipital white matter; values of both hemispheres were averaged. The occipital white matter was used as a reference, considering that this region exhibits the lowest intersubject variance in iron concentration and shows no iron deposits in pathological states.20
Statistical Analysis
Statistical analyses were conducted using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). A normality test was performed using Kolmogorov‐Smirnov's test, and the phase and susceptibility data showed a normal distribution. Two‐tailed t tests with false discovery rate correction were performed to compare ROI susceptibility values of relatives and healthy controls. To calculate iron content, the following steps were performed: In healthy volunteers, the mean susceptibility values in every ROI were plotted against the iron content of each corresponding region, as reported by Hallgren and Sourander, in a postmortem analysis.21 Pearson's correlation coefficient and linear regression fit were calculated. Using the linear regression fit, iron content was then calculated based on measured susceptibility values for all study participants.
Results
In healthy volunteers, a strong positive linear correlation between regional bulk magnetic susceptibility and reported postmortem iron concentration21 was found (Fig. 2A). Including all ROIs, the linear regression fit yielded: χ = 0.57 ppb × [Fe] + 43.625, where [Fe] is the iron concentration in μg/g wet tissue mass and the susceptibility value χ is referenced to the mean susceptibility of occipital white matter (r² = 0.9321). The results of the regional QSM analysis to calculate iron content using the linear regression fit are summarized in Table 2.
Figure 2.

(A) Average susceptibility values in healthy controls plotted against estimated nonheme iron content based on the work of Hallgren and Sourander21 for the following brain regions (iron concentration values presented as mean ± SD μg/g): TH (47.6 ± 1); CN (92.8 ± 2); PUT (133.2 ± 3); SN (184.6 ± 7); RN (194.8 ± 7); and GP (213.0 ± 3). The calculated slope of the linear regression fit 0.57 ± 0.1 ppb per μg iron per g of wet tissue, which is in good agreement with the values of 0.56 and 0.6 previously reported by Shmueli et al.31, and Wharton and Bowtell32, respectively. (B) Differences in the magnetic susceptibility of the ROIs, relative to the occipital white matter, for all subjects in both study groups. Heterozygotes showed no significant increased magnetic susceptibility versus the healthy controls (P > 0.05) in any of the ROIs. Y‐axis scale is equal for part (A) and (B).
Table 2.
Mean bulk tissue magnetic susceptibilities (in ppb) and the corresponding iron concentrations (μg/g wet tissue) in healthy volunteers, heterozygous relatives, and patients
| ROI | Controls | Relatives | Patient 1 | Patient 2 | ||||
|---|---|---|---|---|---|---|---|---|
| Bulk Susceptibility (ppb) | Iron Concentration (μg/g wet tissue) | Bulk Susceptibility (ppb) | Iron Concentration (μg/g wet tissue) | Bulk Susceptibility (ppb) | Iron Concentration (μg/g wet tissue) | Bulk Susceptibility (ppb) | Iron Concentration (μg/g wet tissue) | |
| GP | 173 ± 45 | 228 | 169 ± 47 | 220 | 605 | 986 | 465 | 740 |
| RN | 137 ± 39 | 164 | 136 ± 40 | 162 | 156 | 198 | 142 | 173 |
| SN | 153 ± 46 | 193 | 145 ± 45 | 178 | 628 | 1026 | 593 | 965 |
| PUT | 122 ± 42 | 138 | 144 ± 32 | 176 | 135 | 161 | 138 | 166 |
| CN | 104 ± 51 | 107 | 98 ± 55 | 96 | 110 | 117 | 106 | 110 |
| THA | 65 ± 27 | 37 | 75 ± 26 | 55 | 56 | 21 | 73 | 52 |
| IC | 4 ± 2 | N.D. | 4 ± 2 | N.D. | 19 | N.D. | 22 | N.D. |
Susceptibility values are given relative to the occipital white matter region. Iron concentration was not calculated for IC because the relationship between susceptibility and iron concentration is not linear in white matter and cannot be reliably derived from the linear regression equation calibrated on deep gray matter structures. N.D., not done.
Figure 2B shows the mean susceptibility values in each ROI for healthy controls and PANK2 mutation carriers. PANK2 mutation heterozygotes did not exhibit significantly increased concentration of iron, compared to healthy controls (P > 0.05), in any ROI nor did they exhibit any signs of movement disorders in neurological examination.
We found the typical eye of the tiger sign in patients showing iron‐deposits–related hypointensity in T2‐weighted (T2w) MRIs with a central T2w hyperintensity, presumably representing gliotic changes and edema, as indicated by the magnitude images derived from TIRM and SWI imaging (Fig. 3). In PKAN patients, considerably higher susceptibility values were found in the GP, SN, and IC (Table 2). In the GP of the 2 PKAN patients, susceptibility values were 465 to 605 parts per billion (ppb), in comparison to the mean value 173 ± (SD) 45 ppb in healthy controls. In the SN, the 2 patients had 4 times higher susceptibility values (593–628 ppb), compared to healthy controls (153 ± 46 ppb); in the IC, susceptibility values were 5 times increased in patients (19–22 ppb; healthy controls: 4 ± 2 ppb). No differences in iron concentrations were detected in other ROIs (THA, PUT, CN, and RN). When the calculated iron concentrations in GP for PANK2 mutation heterozygotes and healthy controls were plotted against age, we did not reveal differences in the slope of age‐related iron content changes between these groups (data not shown).
Figure 3.

PKAN patient: the eye of the tiger sign, that is, hypointense signal in the GP with a central region of hyperintensity clearly visible in the TIRM image (A) that is barely apparent on the SWI image (B). MP‐RAGE image shows only hypointense signal in the anterio‐medial part of the GP (C).
Discussion
We employed QSM using 7‐T MRI to characterize and quantify the iron distribution in the basal ganglia of PKAN patients and asymptomatic heterozygous PANK2 gene mutation carriers and compared these to age‐matched healthy controls. QSM was used to make sure that the susceptibility weighting would be dictated by microscopic B0 susceptibility gradients, rather than by macroscopic B0 field inhomogeneities. For this purpose, a threshold k‐space division with single‐orientation acquisition was employed. Our iron concentration estimates derived from susceptibility values are highly correlated with postmortem iron concentration values,21 confirming the validity of this approach.
The major aim of this study was to ascertain whether asymptomatic relatives with heterozygous gene mutations show subtle signs of iron accumulation that could be used as an endophenotypic marker, similar to mild abnormalities observed in heterozygous gene mutation carriers of other recessive movement disorders, such as Parkin‐related parkinsonism.22, 23 However, we did not detect signs of abnormal iron deposits in healthy heterozygotes with high spatial resolution 7‐T MRI. This finding argues against abnormalities in brain iron metabolism in subjects carrying a single abnormal PANK2 allele. Consequently, our results do not support the idea that subtle changes in FA and MD in heterozygous gene carriers detected by Delgado et al. are reflections of iron deposits.
The group of examined heterozygotes covers the age spectrum of the third to sixth decade, and the slope of age‐related iron accumulation in GP in this age group was not significantly different, compared to healthy controls. However, because we did not examine heterozygous subjects in the seventh decade or older, we cannot confidently exclude that abnormal iron accumulation occurs in this age group. It is also important to note that the PANK2 enzyme is not involved in iron metabolism, but in coenzyme A synthesis. Thus, iron accumulation may be only a secondary factor in the pathophysiology of PKAN and may not be best suited as an endophenotypic marker of heterozygous PANK2 mutation. Instead, heterozygotes may manifest other subtle metabolic abnormalities (e.g., in lipid or energy metabolism).24 Absence of movement disorders in examined heterozygotes suggests, however, that any abnormality, if potentially present, would not be clinically significant.
It was suggested that, besides ferritin, part of the iron deposits in PKAN patients is composed of a ferromagnetic compound with similar properties as superparamagnetic iron oxide nanoparticles.8 Our approach does not distinguish between these iron forms. Nevertheless, the QSM method is capable of detecting iron in any form with magnetic properties. Thus, even if superparamagnetic iron accumulation occurred in heterozygous gene carriers, it would have been detected by our approach.
As expected, we detected a significant increase of iron in the GP of our patients of more than 3 times, compared to controls. The iron concentration in GP measured by QSM (mean, 863 μg/g) was higher than previous results obtained by R2 relaxivity measurements (mean, 443 and 480 μg/g, respectively).8, 25 Because we scanned only 2 PKAN patients, our sample is not representative, and no solid conclusion can be drawn regarding over‐ or underestimation of the iron concentration while using the QSM or R2 relaxivity approach, respectively. Notably, no MRI method is sufficiently validated for measurement of brain iron concentration in pathological states with profound iron deposits, and calculated values should be thus treated as rough estimates.
The eye of the tiger sign was best appreciated using the TIRM sequence, whereas in the SWI sequence, the sign was not as clearly visible as a result of the strong T2w signal extinction resulting from the deposited iron (Fig. 3). The presence of the eye of the tiger sign thus strongly depends on the MRI parameters used.
In line with recent literature,3, 26, 27 iron deposition extended to adjacent areas in our patients, namely, to the SN and IC, which was reflected in clinical symptoms of mild parkinsonism and pyramidal signs in addition to generalized dystonia. Indeed, dopamine transporter single‐photon emission CT, as a marker of dopamine loss in Parkinson's disease, may be abnormal in PKAN.28 Similarly, GP and SN signal abnormalities in PKAN patients were reported for diffusion tensor imaging,3, 29 presenting with pseudoincreased FA and abnormal MD. These abnormalities are, however, likely to be consequences of iron deposits disturbing the local magnetic field.9 Transcranial ultrasound investigations30 also support the involvement of GP and SN, showing hyperechogenicity of the SN and nucleus lentiformis hypothesized to represent the correlate of iron deposits.
In conclusion, we did neither observe signs of movement disorders nor increased iron accumulation in heterozygous carriers of PANK2 gene mutation. This implies that a single functional PANK2 allele is sufficient for normal function.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
P.D.: 1A, 1B, 1C, 3A, 3B
E.M.T.M.: 1A, 1B, 1C, 2B, 3B
V.I.M.: 1A, 1B, 1C, 3B
R.J.: 1A, 1B, 1C, 3B
J.S.: 1A, 1B, 1C, 3B
F.P.: 1A, 1B, 1C, 3B
T.N.: 1A, 1B, 1C, 3B
J.W.: 1A, 1B, 1C, 3B
S.A.S.: 1A, 1B, 1C, 3A, 3B
Disclosures
Funding Sources and Conflicts of Interest: Petr Dusek received funding from the Charles University in Prague with the grant PRVOUK‐P26/LF1/4. Vince Madai and Jan Sobesky received funding from the German Federal Ministry of Education and Research with the grant “Center for Stroke Research Berlin” (01 EO 0801; http://www.bmbf.de). Robert Jech received funding from the Czech Ministry of Health with the grant IGA MZ ČR NT12282‐5/2011 and from the Charles University in Prague with the grant PRVOUK‐P26/LF1/4. Friedemann Paul is supported by the German Research Foundation (Exc 257). Thoralf Niendorf has nothing to disclose related to the project. Susanne A. Schneider received funding from Else Kroener‐Fresenius‐Stiftung, Eva Luise und Horst Köhler‐Stiftung, and the Novartis Pharma GmbH.
Financial Disclosures for previous 12 months: The study was supported by the Else Kröner‐Fresenius‐Stiftung, the Eva Luise und Horst Köhler Stiftung, the Novartis Pharma GmbH, the German Federal Ministry of Education and Research, and
by the Czech Ministry of Education (research project PRVOUKP26/LF1/4). None of the funding bodies had any input into the study design or analysis.
Acknowledgments
The authors thank the patients and their relatives, as well as the healthy controls, for participating in our study and Hoffnungsbaum e.V. for their support.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Kruer MC, Boddaert N, Schneider SA, et al. Neuroimaging features of neurodegeneration with brain iron accumulation. AJNR Am J Neuroradiol 2012;33:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haacke EM, Cheng NY, House MJ, et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 2005;23:1–25. [DOI] [PubMed] [Google Scholar]
- 3. Delgado RF, Sanchez PR, Speckter H, et al. Missense PANK2 mutation without “eye of the tiger” sign: MR findings in a large group of patients with pantothenate kinase‐associated neurodegeneration (PKAN). J Magn Reson Imaging 2012;35:788–794. [DOI] [PubMed] [Google Scholar]
- 4. Hayflick SJ, Hartman M, Coryell J, Gitschier J, Rowley H. Brain MRI in neurodegeneration with brain iron accumulation with and without PANK2 mutations. AJNR Am J Neuroradiol 2006;27:1230–1233. [PMC free article] [PubMed] [Google Scholar]
- 5. Hayflick SJ, Penzien JM, Michl W, Sharif UM, Rosman NP, Wheeler PG. Cranial MRI changes may precede symptoms in Hallervorden‐Spatz syndrome. Pediatr Neurol 2001;25:166–169. [DOI] [PubMed] [Google Scholar]
- 6. Chiapparini L, Savoiardo M, D'Arrigo S, et al. The “eye‐of‐the‐tiger” sign may be absent in the early stages of classic pantothenate kinase associated neurodegeneration. Neuropediatrics 2011;42:159–162. [DOI] [PubMed] [Google Scholar]
- 7. Aggarwal A, Schneider SA, Houlden H, et al. Indian‐subcontinent NBIA: unusual phenotypes, novel PANK2 mutations, and undetermined genetic forms. Mov Disord 2010;25:1424–1431. [DOI] [PubMed] [Google Scholar]
- 8. Dezortova M, Herynek V, Krssak M, Kronerwetter C, Trattnig S, Hajek M. Two forms of iron as an intrinsic contrast agent in the basal ganglia of PKAN patients. Contrast Media Mol Imaging 2012;7:509–515. [DOI] [PubMed] [Google Scholar]
- 9. Rulseh AM, Keller J, Tintera J, Kozisek M, Vymazal J. Chasing shadows: what determines DTI metrics in gray matter regions? An in vitro and in vivo study. J Magn Reson Imaging 2013;38:1103–1110. [DOI] [PubMed] [Google Scholar]
- 10. Liu J, Liu T, de Rochefort L, et al. Morphology enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage 2012;59:2560–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marques JP, Bowtell R. Application of a Fourier‐based method for rapid calculation of field inhomogeneity due to spatial variation of magnetic susceptibility. Concepts Magn Reson Part B Magn Reson Eng 2005;25B:65–78. [Google Scholar]
- 12. Salomir R, de Senneville BD, Moonen CTW. A fast calculation method for magnetic field inhomogeneity due to an arbitrary distribution of bulk susceptibility. Concepts Magn Reson 2003;19B:26–34. [Google Scholar]
- 13. Schweser F, Deistung A, Lehr BW, Reichenbach JR. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? Neuroimage 2011;54:2789–2807. [DOI] [PubMed] [Google Scholar]
- 14. de Rochefort L, Liu T, Kressler B, et al. Quantitative susceptibility map reconstruction from MR phase data using Bayesian regularization: validation and application to brain imaging. Magn Reson Med 2010;63:194–206. [DOI] [PubMed] [Google Scholar]
- 15. Wharton S, Schafer A, Bowtell R. Susceptibility mapping in the human brain using threshold‐based k‐space division. Magn Reson Med 2010;63:1292–1304. [DOI] [PubMed] [Google Scholar]
- 16. Turner R, Jezzard P, Wen H, et al. Functional mapping of the human visual cortex at 4 and 1.5 Tesla using deoxygenation contrast EPI. Magn Reson Med 1993;29:277–279. [DOI] [PubMed] [Google Scholar]
- 17. Meloni A, Hezel F, Positano V, et al. Detailing magnetic field strength dependence and segmental artifact distribution of myocardial effective transverse relaxation rate at 1.5, 3.0, and 7.0 T. Magn Reson Med 2014;71:2224–2230. [DOI] [PubMed] [Google Scholar]
- 18. Haacke EM, Xu Y, Cheng YC, Reichenbach JR. Susceptibility weighted imaging (SWI). Magn Reson Med 2004;52:612–618. [DOI] [PubMed] [Google Scholar]
- 19. Haacke EM, Tang J, Neelavalli J, Cheng YC. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. J Magn Reson Imaging 2010;32:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Langkammer C, Schweser F, Krebs N, et al. Quantitative susceptibility mapping (QSM) as a means to measure brain iron? A post mortem validation study Neuroimage 2012;62:1593–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hallgren B, Sourander P. The effect of age on the non‐haemin iron in the human brain. J Neurochem 1958;3:41–51. [DOI] [PubMed] [Google Scholar]
- 22. Binkofski F, Reetz K, Gaser C, et al. Morphometric fingerprint of asymptomatic Parkin and PINK1 mutation carriers in the basal ganglia. Neurology 2007;69:842–850. [DOI] [PubMed] [Google Scholar]
- 23. Schneider SA, Talelli P, Cheeran BJ, et al. Motor cortical physiology in patients and asymptomatic carriers of parkin gene mutations. Mov Disord 2008;23:1812–1819. [DOI] [PubMed] [Google Scholar]
- 24. Leoni V, Strittmatter L, Zorzi G, et al. Metabolic consequences of mitochondrial coenzyme A deficiency in patients with PANK2 mutations. Mol Genet Metab 2012;105:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hajek M, Adamovicova M, Herynek V, et al. MR relaxometry and 1H MR spectroscopy for the determination of iron and metabolite concentrations in PKAN patients. Eur Radiol 2005;15:1060–1068. [DOI] [PubMed] [Google Scholar]
- 26. Fermin‐Delgado R, Roa‐Sanchez P, Speckter H, et al. Involvement of globus pallidus and midbrain nuclei in pantothenate kinase‐associated neurodegeneration: measurement of T2 and T2* time. Clin Neuroradiol 2013;23:11–15. [DOI] [PubMed] [Google Scholar]
- 27. Kruer MC, Hiken M, Gregory A, et al. Novel histopathologic findings in molecularly‐confirmed pantothenate kinase‐associated neurodegeneration. Brain 2011;134(Pt 4):947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Antonini A, Goldwurm S, Benti R, et al. Genetic, clinical, and imaging characterization of one patient with late‐onset, slowly progressive, pantothenate kinase‐associated neurodegeneration. Mov Disord 2006;21:417–418. [DOI] [PubMed] [Google Scholar]
- 29. Awasthi R, Gupta RK, Trivedi R, Singh JK, Paliwal VK, Rathore RK. Diffusion tensor MR imaging in children with pantothenate kinase‐associated neurodegeneration with brain iron accumulation and their siblings. AJNR Am J Neuroradiol 2010;31:442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kostic VS, Svetel M, Mijajlovic M, Pavlovic A, Jecmenica‐Lukic M, Kozic D. Transcranial sonography in pantothenate kinase‐associated neurodegeneration. J Neurol 2012;259:959–964. [DOI] [PubMed] [Google Scholar]
- 31. Shmueli K, de Zwart JA, van Gelderen P, Li TQ, Dodd SJ, Duyn JH. Magnetic susceptibility mapping of brain tissue in vivo using MRI phase data. Magn Reson Med 2009;62:1510–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wharton S, Bowtell R. Whole‐brain susceptibility mapping at high field: a comparison of multiple‐ and single‐orientation methods. Neuroimage 2010;53:515–525. [DOI] [PubMed] [Google Scholar]


