Abstract
Hypersexuality (HS) was one of the earliest examples of an impulse control disorder (ICD) or behavior to be associated with treatment for Parkinson's disease (PD), with an estimated prevalence of approximately 3.5%. Here, we report on a systematic review of the published literature of HS in PD with a view to uncovering evidence as to whether it is distinct from other ICDs. In addition, we report on 7 new cases that had broad neuropsychological testing, including a gambling test, which taps into reward and inhibitory mechanisms. The review uncovered a number of case series and cohorts that comment on the prevalence of HS, but very few made systematic comparisons with other ICDs, although younger onset and male sex are usually noted. A few studies have begun to map out a neuropsychological profile for HS, and our own cases show particular deficits in learning from negative outcomes, but, overall, there are insufficient data to draw firm conclusions. Functional imaging has shown patterns of increased content‐specific activation in response to sexual material and this might relate to increased dopamine release. We conclude with a brief survey of the neurobiology of sexuality, which suggests possible avenues for further research and treatment of HS.
Keywords: Impulse control, Parkinson's, sexuality, gambling task, systematic review
Hypersexuality (HS) is usually considered to constitute a marked increase in sexual interest, arousal, and behavior, which has adverse consequences for the patient and their partner or carers, and is out of keeping with premorbid personality. It is often characterized by a preoccupation with sexual thoughts, frequent demands, and desire for sexual practice that might be quite different from those previously engaged in, and currently, habitual use of sex lines and Internet pornography or contact with sex workers. HS is classified as an impulse control disorder (ICD), along with problem gambling (PG), compulsive shopping (CS), compulsive eating (CE), and compulsive hobbyism and punding (repetitive goal‐less tasks), complicating the treatment of Parkinson's disease (PD), and has been operationally defined.1 ICD in PD may be seen as an example of disinhibitory psychopathology2—a failure of executive function to inhibit reinforcing responses—although alternative theories regard it as an increase in incentive salience akin to that observed in substance misuse disorders.3
HS, as a complication of treated PD, is rare in absolute terms, with estimates of prevalence from postal questionnaires and clinical surveys of approximately 2% to 4% (see below1, 4, 5), with prevalence among users of dopamine agonists (DAs) reported as 7.2%1 and 3.5%4 in two of these articles (Cooper et al., not drawing the distinction). A recent review offered an overall rate of 3.5% based on 29 epidemiological studies.6 Variations in rates may be explained by differential rates of reporting, which may underestimate prevalence in certain populations for whom these symptoms might be seen as shameful, decreasing disclosure. Hypersexuality in PD includes not only quantitative changes in behavior, but also encompasses a range of qualitative alterations in sexuality,7, 8 such as transvestism9 or paraphilias,10, 11 and even gender identity disorders.12 Case descriptions of HS in PD often note that patients deny having such impulses before treatment for PD.13 Cases have been described with levodopa13, 14, 15 and DBS,16, 17 although the majority are observed with use of DAs.18, 19, 20 This correlation with DA use is more established in pathological gambling.21
Hypersexuality in the General Population
Prevalence estimates of compulsive sexual behavior range from 3% to 6% in the general U.S. population22, 23 and was first described in 1886,24 although operational criteria are a relatively new phenomenon.25 The most established view of HS in the general population is that of a behavioral addiction, with significant comorbidity with substance misuse disorders, and higher scores than population average on impulsivity, as well as the similarities (appetitive cravings and persistently engaging in the activity despite adverse social consequences) between the disorders.26 Nevertheless, in proposing Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5), criteria, Kafka27 preferred the term hypersexual disorder, rather than “sexual addiction,” reflecting ongoing uncertainty. Alternative theories are that HS represents an impulsive/compulsive phenomenon28 or that it represents an obsessive‐compulsive pathology. This is born from the high levels of comorbidity with anxiety disorders and a theory that compulsive fantasizing may be a means of decreasing anxiety.26
ICDs in PD
Behavioral symptoms in PD have attracted much attention since the landmark publication in 2000 by Giovannoni et al.,29 and coinciding with the increasing use of potent30 DA medications. Although ICDs are present in the population, patients treated with DAs have been shown to be at increased risk of ICDs above that of the general population, as well as of untreated PD.31 Neurobiological mechanisms for ICDs have been postulated with localized brain abnormalities centering on the STN,32 ventral striatum,33 and frontal cortex.34, 35 Cognitive testing and functional imaging data have implicated such domains as general impulsivity35, 36, 37 and frontal lobe dysfunction,38 poor negative reinforcement sensitivity,39, 40 increased positive reinforcement sensitivity,33, 34, 41, 42 poor recall, lack of ability to estimate risk,43 and increased temporal discounting,44 although Siri et al.45 found preserved executive function despite increased aggressiveness and impulsivity.
HS can be distinguished from other ICDs having been identified in an earlier era,7, 8, 13, 14, 46, 47, 48 but as with other ICDs, reports indicate that these impulses generally recede on withdrawal of the dopamine replacement or agonist drug,49, 50 although HS may persist.51 Early literature on dopamine replacement in PD also describes hypomanic symptoms.47
Understanding HS
Potential mechanisms by which HS might be mediated can be drawn from comparisons with fields such as addictions,3 anxiety disorders, and ICDs,52 as well as through research into psychological aspects of sexuality.53, 54 Dopamine in the mesolimbic area is recognized as a mediator of reinforcement and is implicated in drug addiction55 and is also recognized to play an important role in the regulation of sexuality.54 However, hypersexual behavior has also been described as part of a general loss of impulse control associated with lesions in the prefrontal cortex.55, 56
There is, however, a growing evidence base for a regulatory mechanism specific for sexuality. Bancroft et al.53 provide a compelling argument that sexual behavior is mediated by separate systems of inhibitory and excitatory controls. For example, individuals with erectile dysfunction score highly on measures of inhibition proneness, whereas males with hypersexual behavior show higher sexual excitation scores.57 Given data from animal studies in which environmentally induced sexual inhibition could be overcome using amphetamine in rats,58 this offers an intriguing possible alternative explanation for the link between dopamine and hypersexual behavior. Studies in humans have shown some effect of amphetamine59 and yohimbine60—both of which are known to be dopaminergic, in the treatment of erectile dysfunction. Finally, it has been noted that HS can occur independently of any improvement in sexual function that dopamine replacement might incur.15, 61, 62
Aims of the Review
Given the differing characteristics of HS, its distinctive neurobiology, and the recent explanatory models put forward by Bancroft et al., we decided to search the published literature for evidence that HS fits into the current ICD paradigm, as well as whether a more complex explanation is required for this variety of behaviors. A systematic literature review was conducted using terms related to PD and HS, looking for studies that considered HS in relation to other ICDs. We also report on 7 new cases of our own from a clinical case register that had additional neuropsychological testing, including a gambling test that taps into neuropsychological mechanisms governing response to risk and reward.
Methods
An initial search was run using MEDLINE, EMBASE, and PsycINFO 1984 to the present (May 2013) in order to identify previous meta‐analyses or systematic reviews on HS and PD. Next, in order to identify original contributions to this topic, the same three databases were searched by OVID using Medical Subject Headings (MeSH) terms Parkinson's disease AND hypersexuality OR paraphilia OR impulse control disorder OR ICD OR QUIP OR dopamine dysregulation syndrome OR impulsive‐compulsive OR impulsivity. The title and abstract of the articles were then analyzed to look for relevance. All articles that made any reference to ICDs in general or HS (or another term denoting this, e.g., compulsive sexual behavior, or a subset thererof, e.g., paraphilias) were considered. Those found to be relevant were vetted against the inclusion criteria. Bibliographies from relevant articles found in the primary search were reviewed and additional references obtained. Inclusion criteria were:
HS assessed using standardized criteria. Given that there is not currently any single recognized set of criteria, those using any validated criteria set (e.g., the MIDI or QUIP criteria) were included.
Cases should consist entirely of idiopathic PD (iPD) and relate to pharmacological treatment.
Article distinguishes HS from other ICDs.
An attempt was made to distinguish some etiologically relevant criterion.
Study was not a case report or case series of n < 5.
Results
The initial search revealed 1,219 articles. After being scanned for relevance, several7, 8, 9, 10, 11, 12, 49, 50, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78 were rejected for being case reports, five46, 79, 80, 81, 82 were review articles, and 1,181 were irrelevant to the study. Twenty‐three articles were studied further, with the majority being rejected for not examining etiological or pathological factors, followed by not using recognized or any specific criteria for HS, with the rest failing to draw conclusions specifically related to HS. These articles that explored the area in question, but did not fulfill criteria for inclusion, are tabulated in Supporting Appendix 1.
The references of the accepted articles were scanned and a further two relevant articles were found. Table 1 summarizes the characteristics of eight studies that looked at prevalence and etiological associations of HS comparative to other ICDs that met our criteria. Two categories of articles were recognized: cross‐sectional studies, in which etiological factors relating to the different ICDs were explored, and case‐control studies, which attempted to explore the pathology underlying the process.
Table 1.
Studies of hypersexuality in PD meeting criteria for systematic review
| Reference | Study Type | Case Selection | Location | Evaluation | Participants | Comparisons |
|---|---|---|---|---|---|---|
| Fan et al.83 | Case control | iPD on medication using patient database | China | Initial screening and Voon criteria | 400 | Demographics, LEDD, smoking, and alcohol status |
| Solla et al.84 | Cohort | Fulfilling Brain Bank criteria for PD, presenting to PD clinic | Italy | Voon criteria | 349 | Demographics, medication, neuropsychiatric conditions, MMSE |
| Voon et al.1 | Cohort | iPD, presenting to movement disorder clinic | Canada | Voon criteria | 297 | Psychiatric disorders, demographics, and medications |
| Cooper et al.5 | Cohort study | All iPD registered at an outpatient clinic | USA | Voon criteria | 141 | Demographics, health history, NPI, and BDI |
| Lee et al.87 | Cross‐sectional | iPD, presenting to movement disorder clinic | Korea | MIDI | 1,167 | Length of PD, age of onset, motor symptoms, and LEDD |
| Weintraub et al.4 | Cross‐sectional | Patients with PD receiving follow‐up at 46 centers | USA/Canada | MIDI | 3,090 | Demographics including education, FH of mental health problems, alcohol, and smoking |
| Vitale et al.89 | Case–control | iPD attending PD OP clinic | Italy | MIDI | 63 | UPDRS, LEDD, LEDD‐DA, frontal lobe: WCST, TMT, Stroop |
| Politis et al.93 | Case–control | iPD with and without hypersexuality | UK | Voon criteria | 24 | fMRI of patients viewing neutral, reward, and erotic stimuli |
MMSE: Mini‐mental status exam; VIQ: verbal IQ; PIQ: performance IQ; NART: National Adult Reading Test; FH: family history; UPDRS: Unified Parkinson's disease rating scale; OP: out‐patient.
Fan et al.83 studied the demographics and treatment regimens of patients with ICDs, as well as smoking and alcohol status, in a Chinese population. A screening questionnaire for ICDs was sent to 400 patients with iPD, with those positive for ICDs compared to those without. Criteria for HS proposed by Voon et al.1 were used. The study found that HS had the highest prevalence of any ICD (1.92%) and showed an increased prevalence, compared to controls, in contrast to other ICDs, which did not.
Solla et al.84 took 349 subjects presenting consecutively to a PD clinic who fulfilled criteria for iPD. They then screened these patients for ICDs, using the Voon et al.1 criteria for HS. They were concurrently assessed for motor complications, neuropsychiatric disorders, dementia, and PD severity. Of the ICDs, HS was the only one significantly associated with motor complications, with all ICDs associated with longer disease duration and higher l‐dopa equivalent daily dose (LEDD), calculated according to previous criteria.85
Voon et al.1 used a tripartite method of assessment of HS based around DSM criteria86 and enquiry surrounding degree of functional impairment caused by the symptoms with concurrent mania/hypomania as an exclusion criterion. They studied 297 patients attending a PD clinic, who filled in a survey based around ICD criteria. They found a lifetime prevalence of 2.4%, but 7.2% in DA‐treated PD, which was found to be associated with increased LEDD, independent of whether it was a consequence of increased DA use. Six of seven patients were also found to have comorbid depression. HS had a statistically significant association with male sex and earlier‐onset PD.
In a cross‐sectional study of 1,167 patients presenting to a PD clinic in Korea, which used the Minnesota Impulsive Disorders Interview (MIDI) as a screening tool, Lee et al.87 found 33 HS patients (2.8%). HS and PG were both associated with male gender (75.8% for HS). Total LEDD was calculated, as well as LEDD accounted for by agonists. All ICDs had nonsignificantly higher agonist usage than controls.
Weintraub et al.4 performed a cross‐sectional study of 3,090 patients in 46 different movement disorder centers in North America. HS was identified using the MIDI as well as assessment by a specialist consultant using the patient notes. Demographic data were taken and LEDD was calculated; 3.5% had “compulsive sexual behavior.” HS was associated with male sex. HS was the only one of the ICDs measured not to be related to gambling problems in a first‐degree relative. A selection of 282 ICD cases identified in this study and 282 PD controls went on to have further tests.88 As a group, ICD patients scored more highly on measures of impulsivity and general psychopathology, although the subgroup with HS (n = 47) differed from PD controls only on state anxiety, except that all but 1 was male.
Vitale et al.89 took 49 patients with ICDs—PG, HS, CE, and multiple ICDs—all diagnosed using the MIDI, as well as 14 parkinsonian controls without ICDs for neuropsychological profiling. This consisted of memory testing and tests of frontal/executive function: Wisconsin Card Sorting Test (WCST)90; Trail Making Test (TMT)91; Rey‐Osterrieth Complex Figure Test; matrices; and Stroop Color Word Test. Whereas there was no significant difference in demographics or disease burden associated with PD between groups, there were differences in neuropsychological profile. All ICD cases scored worse on the Rey figure test and TMT, suggesting difficulties with spatial planning and set shifting. Patients with HS, however, scored significantly worse on memory function and on the Stroop test. The researchers suggest that this indicates that HS, more than other ICDs, is primarily a disorder of impulse control (see a previous work92).
A study specifically into HS was performed by Cooper et al.,5 who mailed 400 patients with questions regarding demographics, the Neuropsychiatric Inventory (NPI), Beck's Depression Inventory (BDI), as well as a screening questionnaire for HS, which included a list of recognized behaviors. Those who were positive and accepted were then invited for interview against the Voon criteria. Overall, 15 of the 141 returners were screened positive for HS, although only 6 of these were positive on interview. Of these, correlation was found with earlier onset of disease, but there was no correlation found with marital status or psychiatric illness, as measured on the BDI and NPI. Significantly, there was no correlation found with gender or use of DAs.
Politis et al.93 used functional MRI (fMRI) in 12 PD patients with and 12 without HS to examine functional activation when they watched neutral and then erotic stimuli. Those patients with HS were found to have greater activation of limbic, paralimbic, temporal, occipital, somatosensory, and prefrontal cortices, which the researchers suggest correspond to emotional, cognitive, autonomic, visual, and motivational processes. Enhanced activation in cingulate and orbitofrontal cortices strongly correlated with increased sexual desire. The study had relatively few patients and found diffuse changes in activation, making the drawing of straightforward conclusions difficult. It is also difficult to determine whether these patients are merely more “reward sensitive” in general or whether this is specific to erotic cues, owing to the fact that only erotic cues were given.
Mamikonyan et al.94 followed up patients diagnosed with ICDs, contacting them by telephone on average 29.3 months after diagnosis with an ICD. Fifteen subjects were successfully contacted and were administered a MIDI as well as being asked about subjective severity of ICD symptoms at follow‐up. Seven patients were identified with HS, 6 of whom had their DA decreased or discontinued at follow‐up with a lower contribution of DA to total LEDD. Of these, 6 had complete remission of symptoms and no longer met MIDI criteria for compulsive HS, and 1 described a subjective partial remission. Similar results were found in CG and CS. For ICDs as a whole, LEDD was not statistically different, although DA LEDD was decreased and LEDD increased with statistical significance. The study suggests that DA LEDD is implicated in the genesis and continuation of HS symptoms as well as those of other ICDs (see also a previous work51).
New Cases
A database of 592 patients with iPD attending the regional Neurosciences Clinic at King's College Hospital in London was screened to identify patients whose notes explicitly mentioned symptoms suggestive of HS or associated behaviors. The patients identified were then interviewed by a psychiatrist using the Structured Clinical Interview for DSM‐IV supplemented by a detailed psychosexual history. Seven patients met the criteria for HS and their demographic, clinical, and neuropsychological details are given in Table 2.
Table 2.
Clinical and neuropsychological features of PD cases with hypersexuality
| Variable | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
|---|---|---|---|---|---|---|---|
| Gender | M | M | M | M | M | M | M |
| Age (yr) | |||||||
| Onset of PD | 50 | 61 | 42 | 54 | 32 | 43 | 20 |
| Onset of HS | 53 | 65 | 60 | 58 | 38 | 55 | 30 |
| At assessment | 54 | 70 | 63 | 60 | 41 | 58 | 51 |
| H & Y stage | 2 | 3 | 3 | 2 | 2.5 | 3 | 4 |
| Medication at onset of symptoms |
Sinemet Cabergoline Entacopone |
Sinemet Entacapone |
Madopar Benzhexol |
Madopar Entacapone Ropinorol |
Madopar Pramiprexole |
Madopar Pergolide |
Madopar Cabergoline |
| Associated symptoms | |||||||
| Sexual behavior and other impulse control problems |
Fetishism Pathological gambling |
Phone lines Change in orientation Prostitutes Exhibitionism Possible pedophilic tendencies |
Phone lines Incessant demands for sex Prostitutes Benzhexol misuse |
Pornography Prostitutes Incessant demands May have assaulted daughter |
Pornography Phone lines Including gambling; misuse of DA meds |
Excessive masturbation Frequent demands Kleptomania |
Excessive masturbation; frequent demands Misuse of DAergic meds |
| Mood disturbance | Depression | Hypomania | — | — | Hypomania | — | — |
| Other | Pathological jealousy toward wife | — | — | — | — | Hypochondrical delusions and second person AHs | – |
| IQ | |||||||
| FSIQ (VIQ/PIQ) | 98 (98/98) | 114 (127/93) | 103 (105/101) | 98 (106/95) | 84 (88/80) | 58 (62/55) | 80 (84/77) |
| Premorbid (NART) | 102 | 113 | 109 | 120 | 92 | 78 | 94 |
| Memory | |||||||
| Logical (immediate/delayed) | 9/9 | 7/6 | 8/4 | 6/4 | 10/9 | 3/2 | 4/4 |
| Executive function | |||||||
| Hayling | Low average | Low average | Low average | Low average | Poor | Poor | — |
| Brixton | Impaired | Low average | Poor | Poor | Abnormal | Impaired | — |
| Stroop (centile) | 25th | 25th–50th | 10th–25th | 10th–25th | <2nd | <2nd | — |
| Gambling task | Impaired | Impaired | Impaired | Intact | Impaired | Impaired | N/A |
FSIQ, VIQ, NART and PIQ.
All patients reported an intense preoccupation with sex, with mounting levels of tension until they found a sexual outlet, which resulted in a transient sense of relief. The most frequent sexual activities were masturbation (most patients trying to masturbate at least twice a day) and use of telephone and Internet sex lines. Five patients described sex with prostitutes (which only 1 admitted to premorbidly) and 4 were noted to proposition female friends and clinical staff. Several patients developed paraphilias; 1 described a completely new interest in masochistic sex; another admitted to indecently exposing himself on several occasions; and 1 patient drilled holes in the bathroom walls of his house so he could watch his unsuspecting partner undress. Another was accused of sexually assaulting his daughter by inappropriate physical contact. The behaviors led to relationships with partners ending in 4 cases.
In 5 of these patients, there was no clear temporal relationship between adjustments in medication and the onset of hypersexual symptoms, nor in fluctuations of the levels of medication and symptom intensity. All patients had their dopaminergic medication reduced, but in 4 this merely changed the ability to act upon sexual desire, not the intense sexual preoccupations themselves. Misuse of medication was present in 3 cases and 1 of these patients linked his escalating libido to intentional overuse of anticholinergic, but not dopaminergic, medication.
Neuropsychological Testing
We tested 6 of our hypersexual patients' performance on a short battery of neuropsychological tests, including one designed to examine decision making under ambiguous circumstances. We used the Iowa Gambling Task95 (IGT) in order to examine patient tendency to balance large rewards/losses over smaller rewards/losses. Other neuropsychological tests, including an estimate of IQ using the shortened version of the Wechsler Adult Intelligence Scale—Second Edition (WAIS‐II), the Wechsler Memory Scale (logical memory immediate and delayed), and a short executive battery that included the Hayling Sentence Completion test (which requires subjects to inhibit an obvious missing word at the end of a sentence), the Brixton Spatial Anticipation test96 (which examines the ability to detect rules in sequences non‐verbal symbols—somewhat analogous to the WCST)—and the Stroop colour‐word interference test (an index of selective attention92). The standard clinical scoring of the Stroop was used, namely, number of correct responses in 45s converted into a centile score.
These patients were compared to a control group of 11 subjects with PD with no sexual dysfunction (non‐HS PD) who were matched as a group for demographic (age, 57 years, and gender), clinical stage of PD (median H & Y stage = 3; range, 2–4), and medication. Seven of the eleven controls were on agonist medication in addition to optimized doses of l‐dopa; 3 were on entacapone and 1 on benzhexol and 1 on amantadine. Regarding neuropsychological variables, WAIS IQ showed an HS mean of 90.7 (range, 58–114) versus a non‐HS PD mean of 94.0 (range, 68–112; t test, P > 0.1). The groups had a similar spread of low average to definitely impaired scores on the Hayling and Brixton Tests (although this was not subjected to statistical comparison).
Results
In over 100 trials of the IGT, the hypersexual patients tended to pick more disadvantageous cards than the PD control group (t = 2.02; df = 15; P = 0.06). The group difference reached significance in the latter stages of the task, with the PD control group behaving in a normative manner and choosing progressively more from the advantageous cards, in contrast to the hypersexual subjects who persisted in making disadvantageous choices (over the last 60 trials, hypersexual mean number of disadvantageous choices = 34; standard deviation [SD] = 3; PD controls mean = 26; SD = 8; t = 2.3; df = 15; P = 0.03; see Fig. 1; scoring system explained in legend). This behavior cannot be attributed simply to the impairments in response inhibition or perseveration among the hypersexual patients given that the comparison group had similar levels of executive dysfunction.
Figure 1.
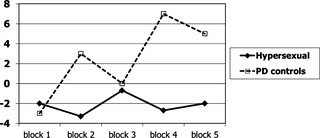
Performance on the IGT. “Hypersexual” PD patients with hypersexual behavior (n = 6; solid line) and PD controls (n = 11; broken line). Y‐axis shows mean net score from advantageous decks (C and D) minus disadvantageous decks (A and B) over the course of five blocks (each block represents 20 consecutive card selections). HS patients show significantly poorer learning.
Discussion: Gambling Task
The study adds HS to the list of impulse control and substances misuse disorders in which deficits in the IGT have been reported, implicating ventromedial prefrontal cortex (VMPFC) dysfunction in their pathogenesis.95, 97 The study is small, so the results must be interpreted with caution, although, as our review shows, studies exploring cognitive underpinnings of specific ICDs are rare. As well as studies in PD per se,98 there are two previous studies that have examined decision making under varying conditions of risk and ambiguity in PD patients with and without ICDs. Rossi et al.99 compared 7 PD patients with PG (2 of whom were also said to have HS) and 13 without ICDs. In general, the differences between the groups were minor, although the PG group showed a significant tendency to select disadvantageous decks, compared to the PD controls. Performance on other decision tasks, including those where risk and advantages were explicit, did not differ between groups. Bentivoglio et al.100 recruited 17 PD patients with ICDs (8 had “compulsive sexual behavior”) and contrasted them with 17 matched PD controls. They found no statistically significant neuropsychological differences, although there were trends for the ICD group to do somewhat worse on frontal‐executive tests and to show some increases in risky decision making. Voon et al.33 examined decision making using a gambling task in 14 PD patients with and 14 without ICD (though none had HS), of whom 11 from each group underwent a series of fMRI studies. Performance was studied ON and OFF DA. The results were complex, with the ICD patients prone to make more risky choices when starting at 0 dollars relative to when starting at a loss and showing reduced ventral striatal activity. ICD patients showed increased sensitivity to risk with agonists, unlike PD controls. The same PD patients with ICD who took part in a reinforcement learning paradigm34 showed faster rates of learning from gain outcomes with DAs, compared to PD controls, and when OFF agonists. Agonists appeared to increase striatal prediction error activity, perhaps signifying a “better than expected” outcome and, by implication, encouraging further “bets.”
Neuropathologically, it might imply that the hypersexual PD patients, assuming they have most in common with other ICD patients, may have either a relatively heavy local loss of dopaminergic neurons in the VMPFC or in the striatal projections to this area, both of which could increase sensitivity to DAs. Therapeutically, it suggests that reduction of dopaminergic medication is only one component of treatment, and future therapies based on an appreciation of the cognitive substrate of the problem may be more effective.
General Discussion
We carried out a systematic literature review in order to see whether there was emerging evidence that HS is a distinct entity from other ICDs with more subtle deficits than the category nomenclature implies. However, this review has shown that there is as yet insufficient evidence to draw this conclusion. Review of the literature showed that there were few articles that actively sought to distinguish HS from its umbrella term. Over 30 studies were identified initially, which did this with most looking solely at prevalence and obtaining very different figures (see Supporting Appendix 1) and most (except for eight; Table 1) failing to meet our entry criteria for the review. There is a lack of a defined standard for the diagnosis of HS, with the QUIP, MIDI, and Voon et al.1 criteria all being used as part of the definition.
Demographically, there seems to be a consensus that HS is associated with younger onset of disease,1 with most suggesting a preponderance in male patients1, 4, 19, 87, 100 and increased LEDD,1, 84 whereas Solla et al.84 also noted that it was related to increased motor complications, suggesting a possible direct effect of dopaminergic stimulation. Interestingly, it was the only ICD that displayed this in what was a numerically small study. The gender bias in the study seems less easy to explain, but would seem to indicate that there are potential physiological predisposing factors to HS. Hence, the main demographic risk factors previously uncovered for ICDs as a whole (earlier age of onset and male gender) seem to be especially relevant for HS.
Cognitive Mechanisms
The current data on the pathological mechanisms of HS are quite sparse in the general population. However, from our systematic review of the literature, there were eight studies in PD (Table 1). Those that have looked at ICDs in general have concluded that they show increased impulsivity, decreased ability to learn from negative outcomes, and decreased ventral striatal activation, suggesting loss of inhibitory control. Vitale et al.89 suggest primarily a deficit in impulse control with poor performance on the Stroop test and also poorer performance in memory testing, although evidence that resistance to attentional interference is a good measure of behavioral impulsivity is weak.92 The generalizability of this study is limited by the small number of patients. The researchers' conclusion that development of ICDs is likely owing to pre‐existing cognitive deficits, based on the lack of difference in LEDD between groups with and without ICDs, would be stronger if individuals underwent neuropsychological testing before developing ICDs. Voon et al.88 found a consistent pattern in a larger group of patients with ICD, although neuropsychological variables did not point to profiles for each ICD subtype. There were some differences in terms of other psychiatric measures.
Recent efforts to understand the cognitive basis of behavioral addictions—such as HS—have emphasized the tendency to engage in immediately gratifying behavior, despite full awareness of adverse long‐term consequences. Bechara et al.95 have developed a paradigm to assess this tendency in which participants choose between packs of cards that bring high immediate monetary reward, but even higher intermittent associated losses (the “disadvantageous” packs) and packs that bring smaller immediate monetary gain, but even smaller losses (the “advantageous” packs). Whereas findings on the IGT and related gambling tasks have been mixed, both in detailed studies of PD patients per se98 and those contrasting patients with and without ICDs,33, 34, 45, 99, 100 our own data suggest that there are particular difficulties in this domain in HS patients that cannot be explained by general cognitive or executive dysfunction. However, in order to show that such deficits are specific to HS, carefully matched patients with other ICDs would need to be examined on this task as well.
Neuroimaging data from a smaller pool of patients, albeit one consisting entirely of HS patients, suggest that there may be some increased element of sexual desire, with patients showing increased activation of reward pathways in response to erotic cues.93 Finally, in a PET study of 18 PD patients with a range of ICDs (5 of whom had HS), O'Sullivan et al.102 used11 C‐raclopride PET to infer D2 receptor availability. ICD patients evinced greater reduction of raclopride‐binding potential in the ventral striatum after reward‐related cue exposure (which included some erotic images), relative to neutral cues, after l‐dopa challenge. This was interpreted as a heightened response of striatal reward circuitry to reward‐related visual cues consistent with a global sensitization to appetitive behaviors with dopaminergic therapy in ICDs.
The Neurobiology of HS
As with ICDs in general, there is currently a debate in the literature as to whether hypersexual disorder per se can be categorized as a behavioral addiction, an impulse control problem, or is more akin to an obsessive‐compulsive disorder (OCD).51, 103 Current understanding of the neurobiology of sexuality may help move this debate forward (for review, see a previous work54).
An established model of human sexual behavior uses the cycle of excitement, plateau, orgasm, and refraction and is largely based around reward. Animal studies have shown large increases in DA in the nucleus accumbens (NA) of rats around the time of orgasm, which rapidly decreases during the refractory phase.104 This can be compared to the rush of euphoria produced by administration of opiates followed by a long period of relaxation. This is often referred to as the “pharmacological orgasm,” and endogenous opioids have been found to be released during ejaculation in male rats. This rather unique signature may be of value in differentiating HS from other ICDs. Alongside human data showing that individuals with high synaptic DA and strongest NA response to reward showed a lower age of first sexual intercourse and increased number of sexual partners,105, 106 this gives a good theoretical underpinning for an addictive model of hypersexual behavior, which suggests that increased reinforcement may lead to an increase in sexual “wanting.”107 It also hints at a possible role for opiate agonists in the treatment of HS—although this would require an extremely careful risk‐benefit analysis.
It is also the case that hypersexual behavior shares many of the characteristics of an addiction with: continued engagement despite adverse consequences; appetitive urge or craving; and diminished self‐control over engagement and compulsive engagement.103 Brain‐imaging studies indicate that sexual arousal and orgasm affect the mesolimbic reward systems, including the striatum and VMPFC,54 which have been implicated in both substance misuse disorders and problem gambling, and are of interest in ICDs owing to the proposed increase ratio of D3 to other receptor subtypes in mesolimbic regions.102, 108 Indeed, one of the distinguishing features of DA drugs versus l‐dopa is affinity to D3, and hence the increased risk of ICD conferred by agonist treatment4 has led to the inference that this receptor subtype is especially relevant to ICDs.109 Nevertheless, whereas PG has been shown to correspond to high sensation seeking,41, 88 this may be less true of hypersexual disorder.
As noted earlier, an alternative model of hypersexual behavior involves loss of inhibition. Bancroft et al.53 propose a theory of sexuality involving dual control that consists of both inhibition and excitation, which are dissociable from each other. It is argued that inhibition may occur at times in which alternative challenges or threats are requiring of attention. Fiorino et al.58 also review animal models that show similar inhibition of sexual response when rats are placed in a novel environment, which is overcome when dopaminergic systems are sensitized using amphetamine.58 As yet, though, small‐scale human experiments have failed to replicate a decrease in inhibition proneness in a group of self‐identifying sex addicts. Whether HS in PD affects inhibition and/or excitation could be simply tested using the Bancroft approach and may lead to behavior modification pathways—both physical and psychological (see a previous work110).
Clinical and population surveys have found a correlation between impulsiveness and hypersexual behavior.52 This could suggest that hypersexual behavior is related to difficulties in regulating impulses per se, rather than particular regulation of sexual impulses. Theories around impulsivity have generally focused on the noradrenergic connections to the right inferior frontal cortex, and their role in prepotent motor disinhibition and delay discounting101, 111—which is linked with orbitofrontal circuitry and its serotonergic and dopaminergic control.108 There is limited systematic evidence for this outside attention deficit hyperactivity disorder, although patients with prefrontal lesions have been found to exhibit hypersexual behavior alongside an array of executive functioning in set‐shifting, emotional regulation, and planning.55, 56 The orbitofrontal cortex has been linked to abnormalities on the IGT,95 which were found in the new cases of HS reported here. As noted above, non‐neurological samples of “sexually addicted” patients also rate themselves as having general problems with impulsivity and self‐control,52 but, curiously, do not show objective executive function impairments.112
Finally, the high rate of comorbidity between anxiety disorders and HS, also observed in PD,88 may suggest an obsessive‐compulsive pathology, which is postulated to be a form of compulsive fantasizing used to relieve anxiety, which is gradually positively reinforced by the relief from anxiety. However, phenomenologically, there does seem to be discrepancy between the ego‐syntonic nature of sexual thoughts in hypersexual disorder, and the more ego‐dystonic obsessions of OCD. Schwartz and Abramowitz113 noted high levels of sexual arousal and pleasure from performing sexual acts among patients classified as having a nonparaphilic sexual addiction. This was in contrast to that reported by the patients with OCD.
The foregoing review leads us to the following conclusions and recommendations:
There appears to be relatively mixed data as to the prevalence of HS in the PD population, with a mean of approximately 3.5%,6 with variation possibly owing to the different criteria used, or cultural variation (e.g., previous works114, 115).
There are quite convincing demographic data on patients with HS, who seem to be often males on DAs (but see a previous work5) with higher LEDDs with earlier‐onset diseases; there is less information as to what processes underlie this.
The development of a standard tool for the assessment of HS, as exists in CG and CS, would undoubtedly advance the field.
Our new data, based on a small case series of 7 men, suggest that there are particular difficulties in decision making associated with negative consequences in HS PD patients, over and above other “executive” difficulties, and this requires replication. In fact, the only study to compare patients across ICDs found that those with HS were, if anything, more impaired on response inhibition and working memory.89
Greater sensitivity to the motor side effects of LEDDs was raised in one study84 and, if replicated, could be used to alert clinicians to vulnerability to HS in patients conforming to the relevant demographic profile.
At the neurobiological level, HS seems to overlap with other reward mechanisms and incentive salience (“wanting” rather than “liking”)93 and probably involves a wide interconnected brain network extending well beyond the basal ganglia, although the role of endogenous opioids may lead to novel therapeutic approaches.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
Research Project: The project was conceived of by ASD. DC did the bulk of the systematic reviewing. ASD and DC evaluated papers. PS examined and tested patients and compiled new data. (2) Statistical Analysis: Analysis was carried out by PS with review by ASD. (3) Manuscript: DC wrote the first draft, with extensive revisions by ASD. All authors contributed to the final draft.
D.C.: 1A, 1B, 3A, 3B
P.S.: 1C, 2A, 2B, 3B
A.S.D.: 1A, 1B, 2C, 3A, 3B
Disclosures
Funding Sources and Conflicts of Interest: A.S.D. is supported by the National Institute of Health Research (NIHR) Biomedical Research Center, awarded to the Institute of Psychiatry, King's College London and South London and Maudsley NHS Foundation Trust, London UK. The authors also acknowledge support from Parkinson's UK. The authors report no conflicts of interest.
Financial Disclosures for previous 12 months: The authors declare that there are no disclosures to report.
Supporting information
Appendix S1. Studies referring to hypersexuality in PD that failed to meet criteria for systematic review.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Voon V, Hassan K, Zurowski M, de Souza M, Thomsen T, Fox S, Lang AE, Miyasaki J. Prevalence of repetitive and reward‐seeking behaviours in Parkinson's disease. Neurology 2006;67:1254–1257. [DOI] [PubMed] [Google Scholar]
- 2. Okai D, Samuel M, Askey‐Jones S, David AS, Brown RG. Impulse control disorders and dopamine dysregulation in Parkinson's disease: a broader conceptual framework. Eur J Neurol 2011;18:1379–1383. [DOI] [PubMed] [Google Scholar]
- 3. Potenza MN. Should addictive disorders include non‐substance‐related conditions? Addiction 2006;101:142–151. [DOI] [PubMed] [Google Scholar]
- 4. Weintraub D, Koester J, Potenza MN et al. Impulse control disorders in Parkinson's disease: a cross‐sectional study of 3090 patients. Arch Neurol 2010;67:589–595. [DOI] [PubMed] [Google Scholar]
- 5. Cooper CA, Jadidian A, Paggi M et al. Prevalence of hypersexual behavior in Parkinson's disease patients: not restricted to males and dopamine agonist use. Int J Gen Med 2009;2:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Callesen MB, Scheel‐Krüger J, Kringelbach ML, Møller A. A systematic review of impulse control disorders in Parkinson's disease. J Parkinsons Dis 2013;3:105–138. [DOI] [PubMed] [Google Scholar]
- 7. Harvey NS. Serial cognitive profiles in levodopa‐induced hypersexuality. Br J Psychiatry 1988;153:833–836. [DOI] [PubMed] [Google Scholar]
- 8. Quinn NP, Toone B, Lang AE, Marsden CD, Parkes JD. Dopa dose‐dependent sexual deviation. Br J Psychiatry 1983;142:296–298. [DOI] [PubMed] [Google Scholar]
- 9. Riley DE. Reversible transvestic fetishism in a man with Parkinson's disease treated with selegiline. Clin Neuropharmacol 2002;25:234–237. [DOI] [PubMed] [Google Scholar]
- 10. Shapiro MA, Chang YL, Munson SK, Okun MS, Fernandez HH. Hypersexuality and paraphilia induced by selegiline in Parkinson's disease: report of 2 cases. Parkinsonism Relat Disord 2006;12:392–395. [DOI] [PubMed] [Google Scholar]
- 11. Cannas A, Solla P, Floris G et al. Hypersexual behaviour, frotteurism and delusional jealousy in a young parkinsonian patient during dopaminergic therapy with pergolide: a rare case of iatrogenic paraphilia. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:1539–1541. [DOI] [PubMed] [Google Scholar]
- 12. Odiyoor M, Kobylecki C, Hackett RJ, Silverdale MA, Kellett MW. Pramipexole and gender identity disorder: expanding the phenotype of hypersexuality in Parkinson's disease. Mov Disord 2009;24:2434–2435. [DOI] [PubMed] [Google Scholar]
- 13. Uitti RJ, Tanner CM, Rajput AH, Goetz CG, Klawans HL, Thiessen B. Hypersexuality with antiparkinsonian therapy. Clin Neuropharmacol 1989;12:375–383. [DOI] [PubMed] [Google Scholar]
- 14. Ballivet J, Marin A, Gisselmann A. Aspects of hypersexuality observed in parkinsonian patients treated by L‐dopa. Ann Med Psychol 1973;2:515–522. [PubMed] [Google Scholar]
- 15. Brown E, Brown GM, Kofman O, Quarrington B. Sexual function and affect in parkinsonian men treated with L‐dopa. Am J Psychiatry 1978;135:1552–1555. [DOI] [PubMed] [Google Scholar]
- 16. Romito LM, Raja M, Daniele A et al. Transient mania with hypersexuality after surgery for high frequency stimulation of the subthalamic nucleus in Parkinson's disease. Mov Disord 2002;17:1371–1374. [DOI] [PubMed] [Google Scholar]
- 17. Doshi P, Bhargava P. Hypersexuality following subthalamic nucleus stimulation for Parkinson's disease. Neurol India 2008;56:474–476. [DOI] [PubMed] [Google Scholar]
- 18. McKeon A, Josephs KA, Klos KJ et al. Unusual compulsive behaviors primarily related to dopamine agonist therapy in Parkinson's disease and multiple system atrophy. Parkinsonism Relat Disord 2007;13:516–519. [DOI] [PubMed] [Google Scholar]
- 19. Klos KJ, Bower JH, Josephs KA, Matsumoto JY, Ahlskog JE. Pathological hypersexuality predominantly linked to adjuvant dopamine agonist therapy in Parkinson's disease and multiple system atrophy. Parkinsonism Relat Disord 2005;11:381–386. [DOI] [PubMed] [Google Scholar]
- 20. Auyeung M, Tsoi TH, Tang WK et al. Impulse control disorders in Chinese Parkinson's disease patients: the effect of ergot derived dopamine agonist. Parkinsonism Relat Disord 2011;17:635–637. [DOI] [PubMed] [Google Scholar]
- 21. Djamshidian A, Cardoso F, Grosset D, Bowden‐Jones H, Lees AJ. Pathological gambling in Parkinson's disease – a review of the literature. Mov Disord 2011;26:1976–84. [DOI] [PubMed] [Google Scholar]
- 22. Kuzma JM, Black DW. Epidemiology, prevalence, and natural history of compulsive sexual behaviour. Psychiatr Clin North Am 2008;31:603–611. [DOI] [PubMed] [Google Scholar]
- 23. Coleman E. Is your patient suffering from compulsive sexual behavior? Psychiatry Ann 1992;22:320–325. [Google Scholar]
- 24. Krafft‐Ebbing R. Psychopathia Sexualis (1886). New York: Physicians and Surgeons Book Co; 1927. Translated by Rebman FJ. [Google Scholar]
- 25. Goodman A. Diagnosis and treatment of sexual addiction. J Sex Marital Ther 1993;19:225–251. [DOI] [PubMed] [Google Scholar]
- 26. Garcia FD, Thibaut F. Sexual addictions. Am J Drug Alcohol Abuse 2010;36:254–260. [DOI] [PubMed] [Google Scholar]
- 27. Kafka MP. Hypersexual disorder: a proposed diagnosis for DSM‐V. Arch Sex Behav 2010;39:377–400. [DOI] [PubMed] [Google Scholar]
- 28. Reid RC, Stein JA, Carpenter BN. Understanding the roles of shame and neuroticism in a patient sample of hypersexual men. J Nerv Ment Dis 2011;199:263–267. [DOI] [PubMed] [Google Scholar]
- 29. Giovannoni G, O'Sullivan JD, Turner K, Manson AJ, Lees AJL. Hedonistic homeostatic dysregulation in patients with Parkinson's disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry 2000;68:423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Domino EF, Sheng J. Relative potency and Efficacy of some dopamine agonists with varying selectivities for D1 and D2 receptors in MPTP‐induced hemiparkinsonian monkeys. J Pharmacol Exp Ther 1993;265:1387–1391. [PubMed] [Google Scholar]
- 31. Weintraub D, Papay K, Siderowf A. Screening for impulse control symptoms in patients with de novo Parkinson's disease: a case–control study. Neurology 2013;80:178–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lim S‐Y, O'Sullivan SS, Kotschet K et al. Dopamine dysregulation syndrome, impulse control disorders and funding after deep brain stimulation surgery for Parkinson's disease. J Clin Neurosci 2009;16:1148–1152. [DOI] [PubMed] [Google Scholar]
- 33. Voon V, Gao J, Brezing C et al. Dopamine agonists and risk: impulse control disorders in Parkinson's disease. Brain 2011;134:1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Voon V, Pessiglione M, Brezing C et al. Mechanisms underlying dopamine‐mediated reward bias in compulsive behaviors. Neuron 2010;65:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Joutsa J, Martikainen K, Niemelä S et al. Increased medial orbitofrontal [18F] fluorodopa uptake in Parkinsonian impulse control disorders. Mov Disord 2012;27:778–782. [DOI] [PubMed] [Google Scholar]
- 36. Voon V, Reynolds B, Brezing C et al. Impulsive choice and response in dopamine agonist‐related impulse control behaviors. Psychopharmacology 2010;207:645–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Villa C, Martinez‐Horta S, Pascual‐Sedano B et al. Behavioral profile in Parkinson's disease with impulse control disorders. Before and after dopaminergic medication management. Mov Disord 2011;26:118. [Google Scholar]
- 38. Santangelo G, Vitale C, Trojano L, Verde F, Grossi D, Barone P. Cognitive dysfunctions and pathological gambling in patients with Parkinson's disease. Mov Disord 2009;24:899–905. [DOI] [PubMed] [Google Scholar]
- 39. Djamshidian A, O'Sullivan SS, Sanotsky Y et al. Decision making, impulsivity, and addictions: do Parkinson's disease patients jump to conclusions? Mov Disord 2012;27:1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nagy H, Bódi N, Moustafa A et al. The relationship between reward learning and personality: a follow‐up study on the effects of dopamine agonists in young Parkinson's patients. Eur J Neurol 2009;16:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Eimeren T, Ballanger B, Pellecchia G, Miyasaki JM, Lang AE, Strafella AP. Dopamine agonists diminish value sensitivity of the orbitofrontal cortex: a trigger for pathological gambling in Parkinson's disease. Neuropsychopharmacology 2009;34:2758–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu K, O'Sullivan S, Politis M, Bose S, Lees A, Piccini P. Rewarding visual cues increase dopamine neurotransmission in Parkinson's patients with impulse control disorders: a PET study. J Neurol Neurosurg Psychiatry 2010;81:29. [Google Scholar]
- 43. Bódi N, Keri S, Nagy H et al. Reward‐learning and the novelty‐seeking personality: a between – and within – subjects study of the effects of dopamine agonists on young Parkinson's patients. Brain 2009;132:2385–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Claassen DO, van den Wildenberg WP, Ridderinkhof KR et al. The risky business of dopamine agonists in Parkinson disease and impulse control disorders. Behav Neurosci 2011;125:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Siri C, Cilia R, De Gaspari D, Villa F et al. Psychiatric symptoms in Parkinson's disease assessed with the SCL‐90R self‐reported questionnaire. Neurol Sci 2010;31:35–40. [DOI] [PubMed] [Google Scholar]
- 46. de Chazeron I, Pereira B, Llorca P‐M, Defrost P, Durif F. Assessing hypersexuality In Parkinson's disease: validation of a screening instrument. Parkinsonism Relat Disord 2012;2:37. [DOI] [PubMed] [Google Scholar]
- 47. Goodwin FK. Psychiatric side effects of levodopa in man. JAMA 1971;218:1915–1920. [PubMed] [Google Scholar]
- 48. Evans AH, Strafella AP, Weintraub D, Stacy M. Impulsive and compulsive behaviors in Parkinson's disease. Mov Disord 2009;24:1561–1570. [DOI] [PubMed] [Google Scholar]
- 49. Courty E, Durif F, Zenut M, Courty P, Lavarenne J. Psychiatric and sexual disorders induced by apomorphine in Parkinson's disease. Clin Neuropharmacol 1997;20:140–147. [DOI] [PubMed] [Google Scholar]
- 50. Korpelainen JT, Hiltunen P, Myllylä VV. Moclobemide‐induced hypersexuality in patients with stroke and Parkinson's disease. Clin Neuropharmacol 1998;21:251–254. [PubMed] [Google Scholar]
- 51. Sohtaoglu M, Demiray DY, Kenangil G, Özekmekçi S, Erginöz E. Long term follow‐up of Parkinson's disease patients with impulse control disorders. Parkinsonism Relat Disord 2010;16:334–337. [DOI] [PubMed] [Google Scholar]
- 52. Reid RC, Karim R, McCrory E, Carpenter BN. Self reported differences on measures of executive function and hypersexual behaviour in a patient and community sample of men. Int J Neurosci 2010;120:120–127. [DOI] [PubMed] [Google Scholar]
- 53. Bancroft J, Graham CA, Janssen E, Sanders SA. The dual control model: current status and future directions. J Sex Res 2009;46:121–142. [DOI] [PubMed] [Google Scholar]
- 54. Georgiadis JR, Kringelbach ML, Pfaus JG. Sex for fun: a synthesis of human and animal neurobiology. Nat Rev Urol 2012;9:486–498. [DOI] [PubMed] [Google Scholar]
- 55. Rogers RD, Everitt BJ, Baldacchino A et al. Dissociable deficits in the decision‐making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan‐depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology 1999;20:322–339. [DOI] [PubMed] [Google Scholar]
- 56. Rees PM, Fowler J, Maas CP. Sexual function in men and women with neurological disorders. Lancet 2007;369:512–525. [DOI] [PubMed] [Google Scholar]
- 57. Bancroft J, Vukadinovic Z. Sexual addiction, sexual compulsivity, sexual impulse disorder or what? Towards a theoretical model. J Sex Res 2004;41:225–234. [DOI] [PubMed] [Google Scholar]
- 58. Fiorino DF, Phillips AG. Facilitation of sexual behavior in male rats following d‐amphetamine induced behavioral sensitization. Psychopharmacology 1999;142:200–208. [DOI] [PubMed] [Google Scholar]
- 59. Bartlik B, Kaplan P, Kaplan H. Psychostimulants apparently reverse sexual dysfunction secondary to selective serotonin re‐uptake inhibitors. J Sex Marital Ther 1995;21:264–271. [DOI] [PubMed] [Google Scholar]
- 60. Ernst E, Pittler MH. Yohimbine for erectile dysfunction: a systematic review and meta‐analysis of randomized clinical trials. J Urol 1998;159:433–6. [DOI] [PubMed] [Google Scholar]
- 61. Bowers MB, van Woert M, Davis L. Sexual behavior during L‐dopa treatment for Parkinsonism. Am J Psychiatry 1997;127:1691–1693. [DOI] [PubMed] [Google Scholar]
- 62. Shapiro SK. Hypersexual behavior complicating levodopa (L‐dopa) therapy. Minn Med 1973;56:58–59. [PubMed] [Google Scholar]
- 63. Walsh RA, Lang AE. Multiple impulse control disorders developing in Parkinson's disease after initiation of amantadine. Mov Disord 2012;27:326–327. [DOI] [PubMed] [Google Scholar]
- 64. d'Orsi G, Demaio V, Specchio LM. Pathological gambling plus hypersexuality in restless legs syndrome – a new case. Neurol Sci 2011;32:707–709. [DOI] [PubMed] [Google Scholar]
- 65. Pineau F, Schüpbach M, Corvol JC, Flamand‐Rouvière C, Vidailhet M, Roze E. Long‐standing paraphilia induced by dopamine agonists in Parkinson's disease. Mov Disord 2010;25:963–965. [DOI] [PubMed] [Google Scholar]
- 66. Fernández MF, González TM. Pathological gambling and hypersexuality due to dopaminergic treatment in Parkinson's disease. Actas Esp Psiquiatr 2009;37:118–122. [PubMed] [Google Scholar]
- 67. Kataoka H, Shinkai T, Inoue M, Satoshi U. Increased medial temporal blood flow in Parkinson's disease with pathological hypersexuality. Mov Disord 2009;24:471–473. [DOI] [PubMed] [Google Scholar]
- 68. Pinggera GM, Pichler R, Rehder P, Kerschbaumer A, Buttazzoni A, Zangerl F, Mitterberger M. Penile strangulation in a patient with Parkinson's disease: a case report. Cases J 2009;2:9379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nielssen OB, Cook RJ, Joffe R, Meagher LJ, Silberstein P. Paraphilia and other disturbed behavior associated with dopamimetic treatment for Parkinson's disease. Mov Disord 2009;24:1091–1092. [DOI] [PubMed] [Google Scholar]
- 70. Bach J‐P, Oertel WH, Dodel R, Jessen F. Treatment of hypersexuality in Parkinson's disease with carbamazepine – a case report. Mov Disord 2009;24:1241–1242. [DOI] [PubMed] [Google Scholar]
- 71. Munhoz RP, Fabiani G, Becker N, Teive HA. Increased frequency and range of sexual behavior in a patient with Parkinson's disease after use of pramipexole: a case report. J Sex Med 2009;6:1170–1180. [DOI] [PubMed] [Google Scholar]
- 72. Solla P, Floris G, Taeconi P, Cannas A. Paraphilic behaviours in a parkinsonian patient with hedonistic homeostatic dysregulation. Int J Neuropsychopharmacol 2006;9:767–768. [DOI] [PubMed] [Google Scholar]
- 73. Kanovský P, Bares M, Pohanka M, Rektor I. Penile erections and hypersexuality induced by pergolide treatment in advanced, fluctuating Parkinson's disease. J Neurol 2002;249:112–114. [DOI] [PubMed] [Google Scholar]
- 74. Weinman E, Ruskin PE. Levodopa dependence and hypersexuality in an older Parkinson's disease patient. Am J Geriat Psychiatry 1995;3:81–83. [DOI] [PubMed] [Google Scholar]
- 75. Vogel HP, Schiffter R. Hypersexuality – a complication of dopaminergic therapy in Parkinson's disease. Pharmacopsychiatry 1983;16:107–110. [DOI] [PubMed] [Google Scholar]
- 76. Vitale C, Santangelo G, Erro R et al. Impulse control disorders induced by rasagiline as adjunctive therapy for Parkinson's disease: report of 2 cases. Parkinsonism Relat Disord 2013;19:483–484. [DOI] [PubMed] [Google Scholar]
- 77. Tschopp L, Salazar Z, Gomez Botello MT, Roca CU, Micheli F.. Impulse control disorder and piribedil: report of 5 cases. Clin Neuropharmacol 2010;33:11–3. [DOI] [PubMed] [Google Scholar]
- 78. Foley SR, Arthur K, Kelly BD. Psychiatric sequelae of Parkinson's disease: a case report. Eur Psychiatry 2006;21:211–213. [DOI] [PubMed] [Google Scholar]
- 79. Ahlskog JE. Pathological behaviors provoked by dopamine agonist therapy of Parkinson's disease. Physiol Behav 2011;104:168–172. [DOI] [PubMed] [Google Scholar]
- 80. Poletti M, Bonuccelli U. Impulse control disorders in Parkinson's disease: the role of personality and cognitive status. J Neurol 2012;259:2269. [DOI] [PubMed] [Google Scholar]
- 81. Wu K, Politis M, Piccini P. Impulse control disorders in Parkinson's disease: a review. Curr Psychiatry Rev 2012;8:235–246. [Google Scholar]
- 82. Fenu S, Wardas J, Morelli M. Impulse control disorders and dopamine dysregulation syndrome associated with dopamine agonist therapy in Parkinson's disease. Behav Pharmacol 2009;20:363–379. [DOI] [PubMed] [Google Scholar]
- 83. Fan W, Ding H, Ma J, Chan P. Impulse control disorders in Parkinson's disease in a Chinese population. Neurosci Lett 2009;465:6–9. [DOI] [PubMed] [Google Scholar]
- 84. Solla P, Cannas A, Floris GL et al. Behavioral, neuropsychiatric and cognitive disorders in Parkinson's disease patients with and without motor complications. Prog Neuropsychopharmacol Biol Psychiatry 2011;35:1009–1013. [DOI] [PubMed] [Google Scholar]
- 85. Evans AH, Lees AJ. Dopamine dysregulation syndrome in Parkinson's disease. Curr Opin Neurol 2004;17:393–398. [DOI] [PubMed] [Google Scholar]
- 86. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Health Disorders. 4th ed. Washington, DC: USA; 1994. [Google Scholar]
- 87. Lee J‐Y, Kim J‐M, Kim JW et al. Association between the dose of dopaminergic medication and the behavioral disturbances in Parkinson's disease. Parkinsonism Relat Disord 2010;16:202–207. [DOI] [PubMed] [Google Scholar]
- 88. Voon V, Sohr M, Lang AE et al. Impulse control disorders in Parkinson disease: a multicenter case–control study. Ann Neurol 2011;69:986–996. [DOI] [PubMed] [Google Scholar]
- 89. Vitale C, Santangelo G, Trojano L et al. Comparative neuropsychological profile of pathological gambling, hypersexuality, and compulsive eating in Parkinson's disease. Mov Disord 2011;26:830–836. [DOI] [PubMed] [Google Scholar]
- 90. Milner B. Effect of different brain lesions on card sorting. Arch Neurol 1963;9:90–100. [Google Scholar]
- 91. Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Perceptual and motor skills. Am Sci 1958;8:271–6. [Google Scholar]
- 92. Dimoska‐Di Marco A. A meta‐analysis of response inhibition and Stroop interference control deficits in adults with traumatic brain injury (TBI). J Clin Exp Neuropsychol 2011;33:471–485. [DOI] [PubMed] [Google Scholar]
- 93. Politis M, Loane C, Wu K et al. Neural response to visual sexual cues in dopamine treatment‐linked hypersexuality in Parkinson's disease. Brain 2013;136:400–411. [DOI] [PubMed] [Google Scholar]
- 94. Mamikonyan E, Siderowf AD, Duda JE et al. Long‐term follow‐up of impulse control disorders in Parkinson's disease. Mov Disord 2008;23:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science 1997;275:1293–1295. [DOI] [PubMed] [Google Scholar]
- 96. Burgess PW, Shallice T. The Hayling and Brixton Tests. Oxford: Pearson Assessment; 1997. [Google Scholar]
- 97. Bechara A, Damasio H. Decision‐making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia 2002;40:1675–1689. [DOI] [PubMed] [Google Scholar]
- 98. Kobayakawa M, Koyama S, Mimura M, Kawamura M. Decision making in Parkinson's disease: analysis of behavioural and physiological patterns in the Iowa Gambling Task. Mov Disord 2008;4:547–552. [DOI] [PubMed] [Google Scholar]
- 99. Rossi M, Gerschcovich ER, de Achaval D et al. Decision‐making in Parkinson's disease patients with and without pathological gambling. Eur J Neurol 2010;17:97–102. [DOI] [PubMed] [Google Scholar]
- 100. Bentivoglio AR, Baldonero E, Ricciardi L, De Nigris F, Daniele A. Neuropsychological features of patients with Parkinson's disease and impulse control disorders. Neurol Sci 2013;34:1207–1213. [DOI] [PubMed] [Google Scholar]
- 101. Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev 2006;26:379–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. O'Sullivan SS, Wu K, Politis M et al. Cue‐induced striatal dopamine release in Parkinson's disease‐associated impulsive‐compulsive behaviours. Brain 2011;134:969–978. [DOI] [PubMed] [Google Scholar]
- 103. Kor A, Fogel YA, Reid RC, Potenza MN. Should hypersexual disorder be classified as an addiction? Sex Addict Compulsivity 2013;20:27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Blackburn JR, Pfaus JG, Phillips AG. Dopamine functions in appetitive and defensive behaviours. Prog Neurobiol 1992;39:247–279. [DOI] [PubMed] [Google Scholar]
- 105. Guo G, Tong Y, Xie CW, Lange LA. Dopamine transporter, gender, and number of sexual partners among young adults. Eur J Hum Genet 2007;15:279–287. [DOI] [PubMed] [Google Scholar]
- 106. Guo G, Tong YY. Age at first sexual intercourse, genes, and social context: evidence from twins and the dopamine D4 receptor gene. Demography 2006;43:747–769. [DOI] [PubMed] [Google Scholar]
- 107. Leeman RF, Potenza MN. Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacology 2012;219:469–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Probst CC, van Eimeren T. The functional anatomy of impulse control disorders. Curr Neurol Neurosci Rep 2013;13:386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Voon V, Mehta AR, Hallett M. Impulse control disorders in Parkinson's disease: recent advances. Curr Opin Neurol 2011;24:324–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Okai D, Askey‐Jones S, Samuel M et al. Trial of CBT for impulse control behaviors affecting Parkinson patients and their caregivers. Neurology 2013;80:792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rubia K, Smith AB, Brammer MJ, Taylor E. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. NeuroImage 2003;20:351–358. [DOI] [PubMed] [Google Scholar]
- 112. Reid RC, Garos S, Carpenter BN, Coleman E. A surprising finding related to executive control in a patient sample of hypersexual men. J Sex Med 2001;8:2227–2236. [DOI] [PubMed] [Google Scholar]
- 113. Schwartz SA, Abramowitz JS. Are nonparaphilic sexual addictions a variant of obsessive‐compulsive disorder? A pilot study Cogn Behav Pract 2003;10:372–377. [Google Scholar]
- 114. Tan Z‐K, Lor TL, Mohamad H et al. Impulsive‐compulsive behaviours in an Asian Parkinson's disease cohort. Mov Disord 2011;26:116. [DOI] [PubMed] [Google Scholar]
- 115. Chiang H‐L, Huang Y‐S, Chen S‐T, Wu Y‐R. Are there ethnic differences in impulsive/compulsive behaviors in Parkinson's disease? Eur J Neurol 2012;19:494–500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Studies referring to hypersexuality in PD that failed to meet criteria for systematic review.


