ABSTRACT
To develop a safe and efficacious heat-stable rotavirus vaccine, new lyophilized formulations were developed using rotavirus serotypes constituting RotaTeq®. A series of formulation compositions, differing in buffering agents, bulking agents, cryoprotectants, amino acids and divalent cations, were screened for their ability to provide stability to rotavirus serotypes during lyophilization and when stored under elevated temperatures for extended periods. Lead formulations and lyophilization cycles were further optimized. Stability profiles of thus optimized formulations showed their ability to retain the potency of rotavirus for > 36 months at 5°C, 20 months at 37°C, and 7 months at 45°C. The heat-stable lyophilized rotavirus formulations developed met the all critical quality attributes for appearance, heat-stability during storage, moisture content as well as pH, viability and stability after reconstitution and has great potential to be used as vaccine candidates for improving access in low-income countries.
KEYWORDS: Heat-stable vaccines, lyophilization, stability, rotavirus vaccine, cold chain, development, rotavirus
Introduction
Rotavirus (RV) is highly contagious that infects nearly every child by the age of 3–5 years and is the leading cause of diarrhea worldwide.1 Vaccination by rotavirus vaccines (RVVs) remains the most effective way to address the disease,2 consequently World Health Organization (WHO) recommends introducing RVVs worldwide to reduce the heavy burden of RV caused under-5 years diarrheal mortality.1 Although few RVVs are licensed for local use, as of this writing three RVVs were licensed globally; two monovalent vaccines Rotarix®, and Rotavac® and a pentavalent vaccine RotaTeq.®3 These vaccines have been proven safe and efficacious, however, they require cold-chain storage at or below 2o to 8oC before use.4-5 The need for introduction of RVVs by many low-income countries is high, but several limitations such as price, adequate supply as well as insufficient cold-chain capacity at distant delivery points, have slowed their introduction. Consequently, development of an affordable, heat-stable RVVs could accelerate its adoption, improve coverage in countries already using vaccine, and improve equity by improving access primarily in the hardest to reach populations.
Various methods such as lyophilization, spray drying, foam drying, spray coating, spray freeze drying have been used for formulating stable vaccines. These methods work on principle of removal of water from formulation slowing down the physical and chemical degradation of vaccine and permits the extended shelf life and storage outside the cold chain.6 Lyophilization is widely used due to its advantages over other methods such as the minimal thermal damage to the actives as well as high technology adaptability by manufacturers in low and middle-income countries.7-10
Therefore, the objective of our work was to develop oral, heat-stable and affordable lyophilized rotavirus formulations based on RotaTeq® antigens useful as vaccine candidates. Developing lyophilized vaccine can have several challenges that are unique to the antigen. These challenges include preserving antigen structure critical for vaccine efficacy from freezing and dehydration stresses as well as designing optimal cycle time to obtain product with required critical quality attributes. Here, several different stabilizing ingredient combinations (formulations) were screened for their ability to minimize process losses, provide long term stability as well as support optimal lyophilization. The rational screening strategy employed herein used experiments that allowed stepwise identification and optimization of formulations that provides protection to virus during freezing, in liquid state, during dehydration, heat stability in solid state and upon reconstitution. The heat stable formulations thus obtained and described here has potential to become a safe and efficacious RVV to improve access in developing world.
Results
Selection of stabilizers: Generally Regarded As Safe (GRAS) excipients frequently used in the formulation of vaccines or in injectable preparations were selected based on literature. These excipients can be classified as buffer (HEPES: 5 (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid); protectant (Sucrose), bulking agent (PVP: Polyvinyl pyrrolidone), salt (NaCl: sodium chloride), amino acids (L-arginine, glycine), activating agent (CaCl2.2H2O and ZnCl2), dispersant (Tween 20). The formulations, coded HSRV00 to HSRV18, varying in concentrations of stabilizing excipients were prepared (Table 1).
Table 1.
Formulation compositions evaluated using freeze-thaw-stability studies. The potency values were obtained for untreated control samples, freeze-thawed samples and freeze-thawed samples incubated at 25oC for one week (freeze-thawed-stability). LOQ represent Limit of Quantification i.e. virus not detected since viral titer losses exceeded limit of quantification of the assay. Error represent SD propagated from six replicates each of samples used for calculation.
| Formulation | Hepes (mM) | NaCl (mM) | Ratio Sucrose (g/100 mL)/ PVP K-25 (g/100 mL) | L- Arginine (mM) | Glycine (mM) | CaCl2.2H2O (mM) | ZnCl2 (mM) | Tween-20 (%w/v) | Titer loss in freeze-thawed samples (Log10 IU/mL) | Titer loss in freeze-thawed-stability samples(Log10 IU/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| HSRV00 | 10 | 50 | 1.2/4.8 | 100 | 0 | 2 | 0 | 0.02 | 0.06 ± 0.14 | 0.90 ± 0.13 |
| HSRV01 | 25 | 25 | 3/3 | 50 | 50 | 1 | 1 | 0.01 | 0.00 ± 0.10 | 1.20 ± 0.31 |
| HSRV02 | 10 | 0 | 1.2/4.8 | 10 | 0 | 0 | 0 | 0 | 0.07 ± 0.10 | <LOQ |
| HSRV03 | 10 | 50 | 4/2 | 10 | 0 | 2 | 2 | 0 | <LOQ | <LOQ |
| HSRV04 | 50 | 0 | 4/2 | 100 | 0 | 2 | 0 | 0 | 0.00 ± 0.10 | 0.50 ± 0.21 |
| HSRV05 | 50 | 50 | 1.2/4.8 | 10 | 100 | 2 | 0 | 0 | 0.00 ± 0.14 | 0.50 ± 0.09 |
| HSRV06 | 10 | 0 | 1.2/4.8 | 100 | 100 | 2 | 2 | 0 | 0.12 ± 0.19 | <LOQ |
| HSRV07 | 50 | 50 | 4/2 | 10 | 0 | 0 | 0 | 0.02 | 0.00 ± 0.09 | <LOQ |
| HSRV08 | 50 | 50 | 1.2/4.8 | 100 | 0 | 0 | 2 | 0 | 0.15 ± 0.24 | <LOD |
| HSRV09 | 50 | 0 | 1.2/4.8 | 10 | 0 | 2 | 2 | 0.02 | 0.19 ± 0.11 | 1.80 ± 0.28 |
| HSRV10 | 10 | 0 | 4/2 | 10 | 100 | 2 | 0 | 0.02 | 0.06 ± 0.09 | 1.30 ± 0.30 |
| HSRV11 | 50 | 50 | 4/2 | 100 | 100 | 2 | 2 | 0.02 | 0.03 ± 0.08 | <LOQ |
| HSRV12 | 50 | 0 | 4/2 | 10 | 100 | 0 | 2 | 0 | 0.06 ± 0.21 | <LOQ |
| HSRV13 | 10 | 50 | 4/2 | 100 | 100 | 0 | 0 | 0 | 0.05 ± 0.14 | 0.20 ± 0.11 |
| HSRV14 | 50 | 0 | 1.2/4.8 | 100 | 100 | 0 | 0 | 0.02 | 0.09 ± 0.10 | <LOQ |
| HSRV15 | 10 | 50 | 1.2/4.8 | 10 | 100 | 0 | 2 | 0.02 | 0.10 ± 0.19 | 0.50 ± 0.08 |
| HSRV16 | 25 | 25 | 3/3 | 50 | 50 | 1 | 1 | 0.01 | 0.26 ± 0.13 | <LOQ |
| HSRV17 | 10 | 0 | 4/2 | 100 | 0 | 0 | 2 | 0.02 | 0.00 ± 0.10 | <LOQ |
| HSRV18 | 25 | 25 | 3/3 | 50 | 50 | 1 | 1 | 0.01 | 0.15 ± 0.17 | <LOQ |
Formulation screening: To mimic the thermal stress conditions experienced by the RVs during lyophilization and to unravel the impact of excipients on potency losses during processing and storage, freeze-thaw-stability studies were conducted using serotype G1 as model strain.
The results summarized in Table 1 show that the formulations HSRV00, HSRV04, HSRV05, HSRV13 and HSRV15 showed titer losses <1.0 log and were selected as formulations potentially offering best protection to virus during lyophilization. To understand if absolute concentration of ingredients in formulation influence virus protection and quality of cake , formulations that had highest(HSRV11), intermediate (HSRV01) and lowest (HSRV02) concentration of excipients were also selected for lyophilization even though titer losses exceeded 1.0 log in freeze thaw stability studies. The Tg’ values of selected formulations were found to be HSRV00: −33.1 ± 0.3, HSRV01: −31.4 ± 0.5, HSRV02: −26.0 ± 0.1, HSRV04: −31.6 ± 0.3, HSRV05: −31.8 ± 0.1, HSRV11: −32.5 ± 0.3, HSRV13: −33.1 ± 0.6 and HSRV15: −31.0 ± 0.3. To accommodate these variances in Tg’, that may influence cake quality and potency, initially, selected formulations were lyophilized with G1 serotype at a concentration of Log10 7.3 IU/mL, under gentle conditions (−35oC to −42oC for primary drying and +20oC for secondary drying (Table S1)). The cakes were evaluated for appearance, %MC, potency and stability upon storage at 5°C and 37°C for at least 4 weeks.
The formulations HSRV00 and HSRV04 showed elegant cakes with %MC of 0.75% and 0.84% respectively. However, collapsed cakes were obtained for formulations HSRV05, HSRV11, HSRV13 and HSRV15 with %MC of 4.0% to 5.0% even though titer losses were less than 0.3 log. The titer losses for HSRV00, HSRV04, HSRV05 and HSRV13 were observed to be within 0.3 log, however, HSRV01 and HSRV02 showed titer losses > 0.3 log during stability testing at both 5°C and 37oC. Considering the cake appearance, moisture content ,the potency and stability data, HSRV00 and HSRV04 were selected for subsequent studies.
The formulations and their variants described here do not offer any ANC. Therefore, we developed a reconstitution buffer based on RotaTeq® but providing palatability to the final 2.0 mL reconstituted product. This palatable reconstitution buffer consisted of 20 % w/v sucrose, 108 mM sodium dihydrogen phosphate monohydrate and 215.9 mM trisodium citrate, in water. The reconstituted formulation using reconstitution buffer exhibited an ANC of 0.70 to 0.80 mEq/2.0 mL reconstituted dose. All lyophilized cakes dissolved within 10 ± 5 seconds in reconstitution buffer (2.0 mL) forming a clear liquid with a pH of 6.10 ± 0.10. This buffer is stable for at least 30 months when stored at controlled room temperatures (Supplementary Information (SI) Table S2).
Lyophilization and stability studies: To test the ability of formulations HSRV00 and HSRV04 to confer stability to remaining serotypes, the formulations were prepared with five RV serotypes, each at a concentration of Log10 7.0 IU/mL and lyophilized (two batches; 99h lyophilization cycle). Formulations HSRV00 and HSRV04 showed a %MC of 0.72 and 0.75 respectively. The resulting cakes were incubated at 5°C and 37oC. The cumulative loss (process loss + loss after incubation) per month of the formulations HSRV00 and HSRV04 for G1, G2, G3, G4 and P1A[8] were found to be 0.12 ± 0.10, 0.07 ± 0.03, 0.11 ± 0.04, 0.01 ± 0.01, 0.29 ± 0.10 and 0.05 ± 0.01, 0.00 ± 0.01, 0.16± 0.10, 0.06 ± 0.01, 0.12± 0.02 respectively. No losses were observed at 5°C.
Another batch for both the formulations HSRV00 and HSRV04 was prepared using the same cycle but at double the rotavirus concentration (Log10 7.3 IU/mL). The cumulative loss per month of the formulations HSRV00 and HSRV04 for G1, G2, G3, G4 and P1A[8] were obtained to be 0.27 ± 0.13, 0.29 ± 0.08, 0.11 ± 0.05, 0.12 ± 0.05, 0.30 ± 0.07 and 0.13 ± 0.04 , 0.10 ± 0.07, 0.09 ±0.10, 0.02 ± 0.06, 0.20 ± 0.20 respectively. Thus, both the formulations met specification of titer losses for all serotypes not exceeding 0.3 logs each at 37°C for 1 month at single or double the RVV concentrations.
Optimization of cycle for process efficiency: To further optimize process and formulation one of the lead formulation HSRV04 was lyophilized with G1, G2, G3, G4 and P1A[8] at the concentrations of 6.81, 6.92, 6.91, 6.78 and 6.83 Log10 IU /mL dose equivalent respectively. These concentrations are 0.5 log higher than minimal potency specifications for RotaTeq.®5 The primary drying temperature based on the Tg’ (−31.7°C) was set at −35oC and −33oC resulting in 57 h and 63 h cycles respectively. The, 57h cycle however resulted in collapsed cakes in centrally placed vials but 63h cycle yielded elegant cakes with homogenous drying across the shelf characterized by average %MC below 1.5% . These vials were tested for stability at 5°C, 37°C and 45°C. With the criteria of cumulative losses not exceeding 0.5 logs for each serotype, the formulation was observed to be stable for 12 weeks at each condition, consistently across 3 batches. The P1A[8] strain was found to be most susceptible to potency loss at high temperatures. Attempts at reducing cycle time below 63h using −33oC as primary drying temperature resulted in inconsistent cakes (data not shown).
Optimization of formulation for process efficiency: To further optimize the process with the aim to reduce cycle time without compromising on above stability profile, composition of HSRV04 was tweaked by either (i) replacing PVP K-25 with PVP K-40, (ii) increasing the bulking agent concentration or (iii) changing the sugar: bulking agent ratio ( Table 2 ) while keeping the RV concentrations same. The changes (ii) and (iii) resulted in increasing in solid content of pre-lyophilized liquid from 9.0 % in HSRV04 to a maximum of 13.0 %. This approach is known to increase Tg’ allowing increase in primary drying temperature, improving sublimation rate resulting in primary drying time reduction. This hypothesis was in confirmation with the Tg’ data obtained for these formulations (Table 2 ). It was shown that higher MW PVP was more efficient in inhibiting sucrose crystallization and by stabilizing glassy structures of the sugar may improve the stability of co-lyophilized proteins and peptides.11 However, HSRV04E, prepared by replacing the PVP K-25 with PVP K-40 showed no improvement in Tg’.
Table 2.
Formulations prepared by varying excipients. The stabilizing buffer solutions were coded as HSRV04B, HSRV04C, HSRV04D and HSRV04E. The formulations were prepared by mixing 85% of stabilizing buffer by volume, adjusted to have concentrations indicated, with 15% by volume of mixed rotavirus bulk. The Tg’ is shown as mean ± SD of three samples.
| Ingredients | HSRV04 | HSRV04B | HSRV04C | HSRV04D | HSRV04E |
|---|---|---|---|---|---|
| HEPES (mM) | 50 | 50 | 50 | 50 | 50 |
| Sucrose (%) | 4.0 | 4.0 | 4.0 | 6.0 | 4.0 |
| PVP K-25 (%) | 2.0 | 4.0 | 6.0 | 4.0 | 0 |
| PVP K-40 (%) | 0 | 0 | 0 | 0 | 2.0 |
| L-Arginine (mM) | 100 | 100 | 100 | 100 | 100 |
| CaCl2.2H2O (mM) | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Tg’ (oC) | −31.6 ± 0.3 | −29.0 ± 0.8 | −28.7 ± 0.4 | −29.8 ± 0.2 | −31.5 ± 0.0 |
These formulations were lyophilized using 54 h cyle as outlined in SI. As expected from the respective Tg’ values, formulations HSRV04B, HSRV04C, HSRV04D yielded elegant cakes with %MC below 1.0 % but HSRV04E showed collapsed cakes. The formulations HSRV04B, HSRV04C and HSRV04D were tested for the stability at 5oC and 37°C. The results (Table 3 ) show that although HSRV04C showed comparatively higher cumulative loss, titer losses for all tested formulations did not exceed 0.3 log for 30 days at both the temperatures tested.
Table 3.
Summary of the process loss, stability loss and the cumulative loss of pentavalent composition in trial formulations at 37°C and 5oC. Process loss was measured as potency difference between samples before and after lyophilization. Stability loss was calculated as a difference in potencies at day zero and 1 month. Cumulative loss is sum of Process and Stability losses. Error represent SD propagated from six replicates each of samples used for calculation.
| |
Rotavirus Serotype |
HSRV04B |
HSRV04C |
HSRV04D |
|||
|---|---|---|---|---|---|---|---|
| Moisture content (%) | 0.7 % | 0.3 % | 0.3 % | ||||
| Process Loss (Log10 IU) | G1 | 0.21 ± 0.07 | 0.00 ± 0.02 | 0.03 ± 0.08 | |||
| G2 | 0.13 ± 0.09 | 0.11 ± 0.04 | 0.16 ± 0.07 | ||||
| G3 | 0.00 ± 0.09 | 0.20 ± 0.10 | 0.00 ± 0.02 | ||||
| G4 | 0.03 ± 0.02 | 0.02 ± 0.03 | 0.10 ± 0.03 | ||||
| P1A[8] | 0.02 ± 0.05 | 0.11 ± 0.05 | 0.04 ± 0.08 | ||||
| Storage Condition |
|
37°C |
5 oC |
37°C |
5 oC |
37°C |
5 oC |
| Stability Loss after 1 month (Log10 IU) | G1 | 0.09 ± 0.03 | 0.00 ± 0.04 | 0.20 ± 0.07 | 0.20 ± 0.10 | 0.20 ± 0.08 | 0.16 ± 0.10 |
| G2 | 0.00 ± 0.05 | 0.00 ± 0.05 | 0.02 ± 0.07 | 0.02 ± 0.03 | 0.02 ± 0.05 | 0.00 ± 0.05 | |
| G3 | 0.02 ± 0.08 | 0.03 ± 0.04 | 0.00 ± 0.05 | 0.02 ± 0.04 | 0.11 ± 0.08 | 0.03 ± 0.05 | |
| G4 | 0.00 ± 0.02 | 0.00 ± 0.04 | 0.11 ± 0.10 | 0.09 ± 0.10 | 0.11 ± 0.05 | 0.02 ± 0.03 | |
| P1A[8] | 0.00 ± 0.06 | 0.03 ± 0.00 | 0.10 ± 0.02 | 0.00 ± 0.05 | 0.07 ± 0.10 | 0.00 ± 0.05 | |
| Cumulative loss after 1 month (Log10 IU) | G1 | 0.30 ± 0.08 | 0.21 ± 0.08 | 0.20 ± 0.07 | 0.20 ± 0.10 | 0.23 ± 0.11 | 0.19 ± 0.13 |
| G2 | 0.13 ± 0.10 | 0.13 ± 0.09 | 0.12 ± 0.08 | 0.13 ± 0.05 | 0.18 ± 0.09 | 0.16 ± 0.09 | |
| G3 | 0.02 ± 0.10 | 0.03 ± 0.10 | 0.20 ± 0.11 | 0.22 ± 0.11 | 0.11 ± 0.08 | 0.03 ± 0.05 | |
| G4 | 0.03 ± 0.03 | 0.03 ± 0.04 | 0.13 ± 0.10 | 0.11 ± 0.10 | 0.17 ± 0.06 | 0.10 ± 0.04 | |
| P1A[8] | 0.00 ± 0.08 | 0.05 ± 0.05 | 0.21 ± 0.05 | 0.11 ± 0.07 | 0.11 ± 0.13 | 0.04 ± 0.09 | |
To test the consistency of one of the better performing exemplified formulation HSRV04D additional batches (N2 and N3) were prepared. The lyophilized product appeared as white/ yellowish-white elegant cake with %MC ≤ 1.0%. The longitudinal stability studies were conducted under the storage conditions of 5°C and 37°C. Linear regression of real-time data at 5°C and 37°C showed that titer losses were < 0.5 logs for at least 24 months and 3 months respectively ( Fig 1 ). The loss rates obtained from linear regression of the real-time stability data are shown in (SI Table S3). The process losses for all serotypes in N1, N2 and N3 were less than 0.1 log (Fig S1).
Figure 1.
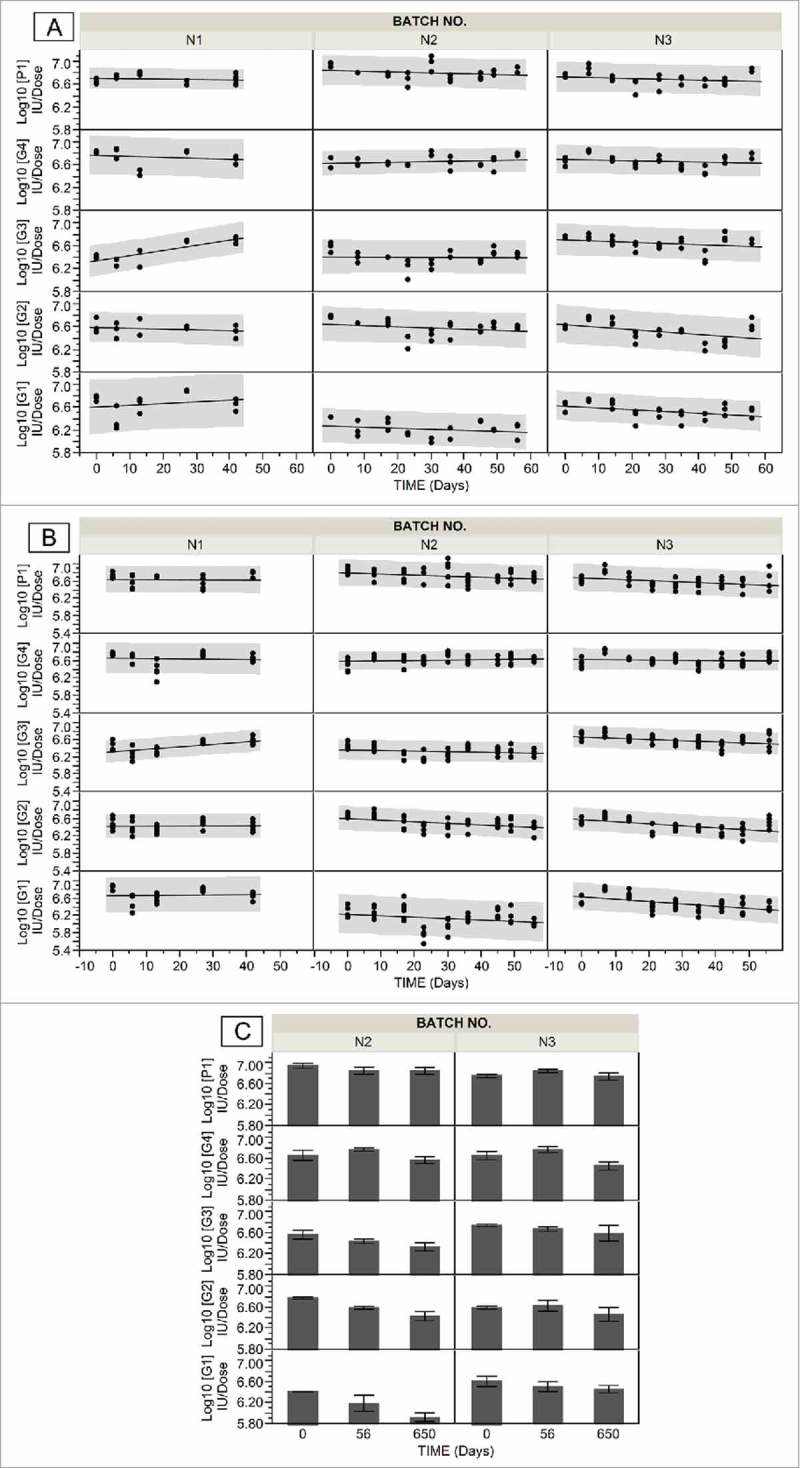
Stability data of pentavalent lyophilized formulation HSRV04D in three independent manufacturing batches (N1, N2 and N3). Panels A and B show stability data of HSRV04D containing G1, G2, G3, G4 and P1A[8] rotavirus serotypes incubated at 5oC and 37oC respectively for indicated time. The potency was determined by MQPA assay and expressed as Log10 IU/ dose. The solid line through the data is linear fit. Confidence of prediction (95%) is indicated in shaded grey. Panel C shows extended stability data of batches N2 and N3 that were incubated at 5oC for 650 days. The error bars represent SD for 6 replicates analyzed.
To explore the feasibility of manufacturing multi-dose vials, the formulation HSRV04D5 was formulated by incorporating rotavirus strains at five-fold higher concentrations to HSRV04D (G1:7.51; G2:7.62; G3:7.61; G4:7.47 and P1A[8]:7.52 in Log10 IUs/mL). Three consecutive batches (T1, T2 and T3) were manufactured using 54h cycle. The average %MC of resulting cakes were 1.2 ± 0.3, 1.2 ±0.3 and 1.0 ± 0.1 respectively. The process losses for all serotypes in T1, T2 and T3 were less than 0.1 log (Fig S1). Modulated DSC showed Tg as a reversing event at 61.0°C. This value indicated that the formulation HSRV04D5 with %MC ≤ 1.2 may be stable at the temperatures below 61oC.
The longitudinal stability studies for HSRV04D5 were conducted at storage conditions of 5°C, 37°C and 45°C (Fig. 2 ). The loss rates obtained from linear regression of the real-time stability data showed that HSRV04D5 is stable for > 36 months at 5°C, 20 months at 37°C and 7 months at 45°C with criteria of titer loss <0.5 log for any serotype (SI Table S3). It was observed that P1A[8] was the most labile serotype with highest loss rates at 37°C and 45°C.
Figure 2.
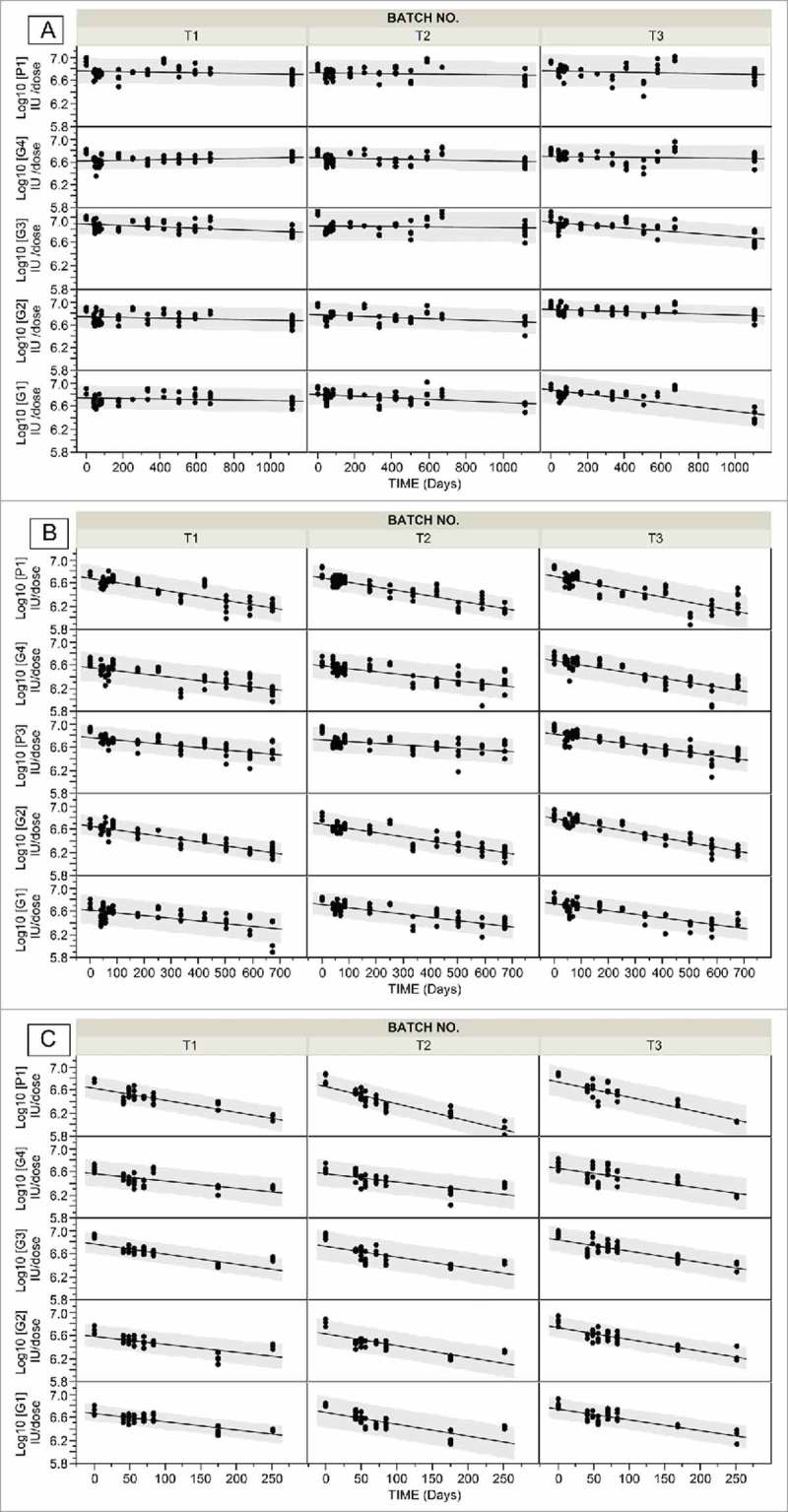
Stability data of pentavalent lyophilized formulation HSRV04D5 in three independent manufacturing batches (T1, T2 and T3). Panels A, B and C show stability data of HSRV04D5 containing G1, G2, G3, G4 and P1A[8] rotavirus serotypes incubated at 5oC, 37 oC and 45 oC respectively. The potency was determined by MQPA assay and expressed as Log10 IU/ dose. The solid line is linear fit to the data. Confidence of prediction (95%) is indicated in shaded grey.
Post Reconstitution Stability determination: Stability after reconstitution was determined for HSRV04D5. As shown in Fig. 3 the reconstituted HSRV04D5 showed a potency loss of less than 0.1 log when stored for 24 hours under different storage conditions.
Figure 3.
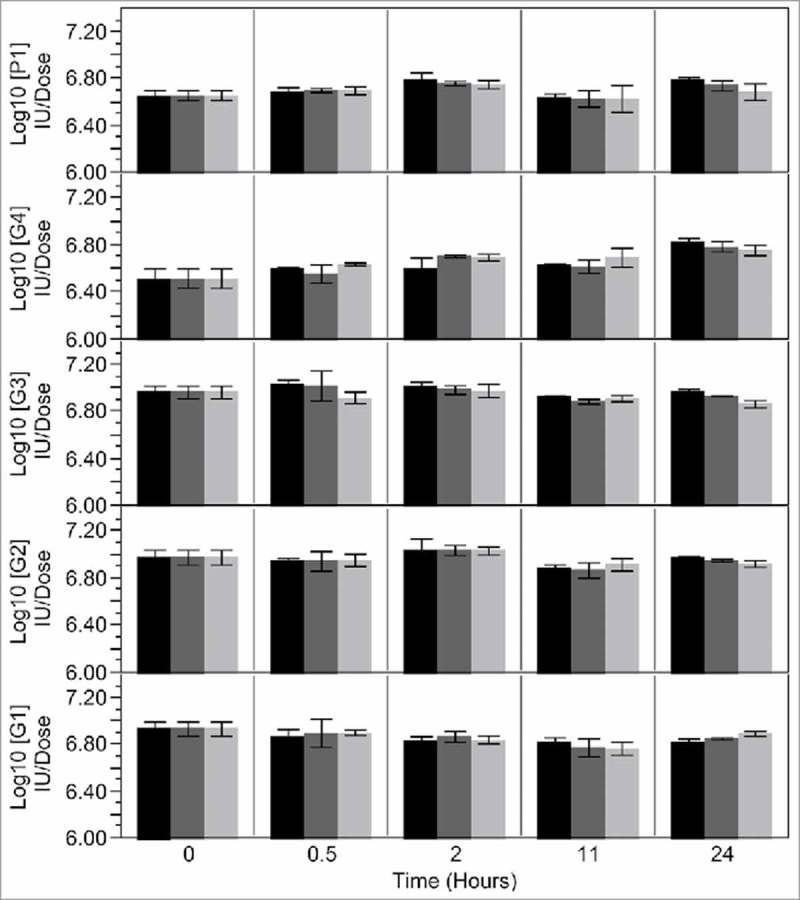
Post reconstitution stability data of pentavalent lyophilized formulation HSRV04D5. The lyophilized HSRV04D5 containing G1, G2, G3, G4 and P1A[8] rotavirus serotypes was reconstituted with 2.0 mL of reconstitution buffer and stoppered. The reconstituted samples were incubated at -70 oC (black bars), 5 oC (dark grey bars) and at 25 oC ± 3 oC (60 % RH ± 3 % RH) (light grey bars). Samples were withdrawn at indicated time points and analyzed for potency with MQPA assay and expressed as Log10 IU/ dose. The data represent mean of potency ± SD of at least three samples.
Discussion
Lesser economic resources to pay for the vaccine including its storage, distribution and the training to properly handle and administer is adversely impacting the morbidity and mortality amongst infants and children in the developing countries.12 To address this major problem, it is imperative to develop cost-effective, sustainable and heat-stable vaccines.
With this objective, we prepared lyophilized RVV formulations using optimized concentrations of cost-effective, GRAS excipients that imparted varying degree of heat-stability to RV serotypes. An empirical Freeze-Thaw-Stability approach was used as a preliminary screen to identify excipients combinations that offer protection to G1 serotype. The selection of formulations from above studies and testing them by lyophilization helped in further elimination of formulations that require iterative lyophilization process optimization to obtain optimal product. Overall, this approach although lacked robustness of a DoE methodology, but had an advantage in quickly and cost- effectively identifying stabilizers that offered protection to RVV during freezing as well as storage at room temperatures upon reconstituted.
Amorphous sugar glass forming disaccharide, sucrose was used as a principal stabilizer in our formulations as it is known to be most effective in stabilizing proteinaceous products during and after lyophilization as compared to crystalline excipients.
Other excipients were also selected to improve key quality attributes of the formulations. For example, RV are acid-labile and gets rapidly inactivated with a half-life of less than 12 minutes at pH 2.0.13 Since RVVs are intended to be administered orally, it is crucial to protect RV from getting inactivated by gastric acid. The effective way for administering such vaccines is to pre- or co-administer antacids or neutralizing buffers. In later approach, the palatability of the formulations is an important formulation factor14-15 for pediatric vaccines. RotaTeq® uses phosphate- citrate buffer for protection of viruses from gastric acid. This buffering system on itself results in tart solution and if lyophilized, sodium phosphate precipitates during freezing resulting in drastic pH changes which may affect stability.16 Additionally, citrate is known chelator of divalent ions. Previous studies suggest that divalent metals may be necessary for structural integrity17-18 with high calcium concentration enhancing the infectivity of several rotavirus strains.19 Thus, citrate may affect stability if incorporated as lyophilized vaccine constituent. To address above issues, we incorporated phosphate- citrate buffer system supplemented with 20 % sucrose for palatablity separately from lyophilized components that are supplemented with calcium or zinc.
It was also shown that addition of small amounts of surfactants protects proteins from both freeze- and surface-induced denaturation as well as inhibit aggregation upon reconstitution.20 Our data show that surfactant (Tween 20) addition is not obligatory since process losses (includes any freeze- and surface-induced denaturation) were minimal and the post reconstitution stability data (includes any aggregation upon reconstitution) showed that reconstituted HSRV04D5, lacking Tween 20, retains its potency during storage not only at 5°C and at controlled room temperatures (≤25°C) but also when frozen at −70°C and thawed.
Our post reconstitution data is also supports non- cold chain dependent transport of pre-reconstituted vaccine to the point of administration to minimize time of administration. However, WHO currently do not recommend storage of reconstituted vaccine outside 2°C to 8°C for more than 6 h to avoid contamination.21 This risk is particularly high for live attenuated vaccines like RVVs which have to be formulated without preservatives. One of the major sources of introducing contamination is open reconstitution process wherein vaccine constituents provided in vials, ampoules and syringes are mixed with syringes or adapters. To minimize this risk and to take advantage of the extended heat-stability post reconstitution in field setting (e.g. reduction in vaccine preparation time by reconstituting beforehand22), integrated reconstitution devices that minimize the risks of contamination associated with open reconstitution are being developed in our labs.
Lyophilization, is an expensive process due to long drying cycles requiring high vacuums as well as comprising stages with both low and high temperatures for condensation and sublimation respectively, we attempted reduction in cycle times guided by critical temperatures. The Tg’ as a determinant of collapse temperature (Tc) provided an indication of upper limit of the product temperature during primary drying and was used to design a cycle suitable for obtaining stable product with acceptable appearance. It is recommended to keep Tg’ > −40°C for economical process. Since formulation composition determines Tc, we optimized formulations enabling increase in Tg’ of predominantly sucrose based formulation to −29°C (Tg’ for pure sucrose is −32°C). While this is small gain in Tg’, it allowed for reduction in cycle time to 54 h and showed a feasible path for further reduction in cycle time. Further reduction in cycle time is possible by setting shelf temperature higher than product temperature (Tp). As the energy is drawn from the system during sublimation, the product temperature can remain lower than Tg’ at early stages of primary drying. It should be noted that reduction in cycle time using above approach is likely to be lyophilizer specific and may require reoptimization when transferred to different equipment and scales. Storing below Tg, protected from moisture reuptake, is important for lyophilized products in order to maintain the rigid-glass structure and hence stability of the product. Onset of Tg at 61oC obtained for HSRV04D5 is well above the intended storage temperatures of the formulation and observed extended stability at higher temperatures could be the direct result Tg > storage temperatures.
For economical process it is also crucial that the virus titer losses be kept at minimum during processing. This is a function of stabilizing excipients as well as process conditions. We have shown that formulations and process described here incur negligible process losses. This is significant since stresses during lyophilization process usually causes up to 40 % loss in virus potencies.23
Lyophilization adds on capital equipment and recurring process costs that are not incurred for liquid RVV formulations. Thus, incorporating lyophilization must be weighed against need for thermostable vaccine, resulting cost per dose and final costs per person immunized. Several studies show that thermostability has a positive impact by way of lowering loss of vaccine potency, reducing wastage, improving reach as well as reducing cold-chain and logistics cost.24-26 Reduction in process losses, particularly for pentavalent, multidose formulations described herein, will minimize cost-impact and favor incorporation of lyophilization for heat stabilization of RVV. Furthermore, a heat stable vaccine with stability profile of HSRV04D and HSRV04D5 will require less antigen overfill to compensate for titer losses incurred during shelf life storage compared to a liquid vaccine. A formal cost-effectiveness evaluation will be required before specific conclusions in this regard can be made.
Interestingly, we also showed that formulations tested can support stability of serotypes at higher concentrations allowing for heat-stable multi-dose vials. This indicate that mass ratio of stabilizer to virus is adequate for later, a crucial determinant of stability during and long-term stability.27 Although from a safety point of view a single dose presentation is preferred, public sector immunization programs particularly in low- and middle-income countries, tend to rely on multi-dose vials since they offer lower prices per purchased dose as well as minimize cold chain storage and distribution requirements.28-29 Modelling studies show that vial size is dependent on characteristics of the vaccine, the vaccine supply chain, immunization session size, and goals of decision makers with optimal vial size may vary among locations within a country.30 Thus, ability of same quantity of stabilizers to confer stability at different RV concentrations offers flexibility in manufacturing, process design, reduction in cost of raw materials and manufacturing as well as simplicity in regulatory pathway.
Our studies have obtained a cost-effective, heat-stable formulation which is stable not only for 20 months at 37°C but also for 7 months at temperatures as high as 45oC. WHO recommends that all the childhood vaccines are to be stored at 2oC – 8oC except the oral polio vaccine during their distribution within a country.31 However, it is very difficult to maintain the temperature at the vaccine stores and during cold chain transit, particularly in developing countries, resulting in large amount of vaccine being wasted.32 For example, in India, with largest Universal Immunization Program (UIP) in the world, vaccines spend at least nine months from manufacturer to recipients,33 putting huge burden on cold chain which has issues of space, quality and maintenance at all levels.34-36 Since cold chain is the most important component to ensure the quality of vaccine, developing a heat stable vaccine that could be transported and stored outside cold chain for extended period of time is the high priority for healthcare sector.37 The time-temperature stability profile shown by us has potential to obviate cold chain requirements typical in developing world.
It is particularly noteworthy that heat stability profile of HSRV04D5 is a significant improvement not only over liquid RotaTeq® (24 months at 2–8°C, 9–25°C for 48 h or 26–30°C for 12 h)38 but also over previous efforts to heat stabilize RotaTeq® serotypes by lyophilization18 and in liquid state (VVM 7).39 HSRV04D5 also has improved stability compared to lyophilized Rotarix® (stable for 36 months 2–8°C and 7 days at 37°C), and Rotavac (storage at -20°C and 6 months at 5°C ± 3°C) while it is comparable to ROTASIIL® (stable for 36 months at 2–8°C and 25°C and 18 months at 40°C).40
We recognize that there will be several issues in leveraging the heat stability of a vaccine in a system designed for cold chain dependent vaccines. In this regard, recent efforts by many countries to pilot or adopt protocols allowing vaccines storage at ambient temperatures for a specific period with an intent to minimize logistical challenges and to reduce or eliminate the need for cold chain supplies41-45 are encouraging. Thus, during early adaption, availability of heat stable vaccines can facilitate less reliance on exact storage timing and refrigeration if not complete elimination of cold chain.
In summary, the heat-stable formulations described here were prepared using GRAS excipients and the serotypes constituting RotaTeq®; a safe and efficacious oral RVV used since 2006. Although diversity of serotypes may change from time to time, these five serotypes account for >90% of rotavirus disease worldwide.46 The M-QPA potency assay used in this study forms the basis of RotaTeq® potency setting, evaluation and release. Taken together, we expect that formulations described here would be clinically non-inferior to Rotateq® (manuscript under preparation) and provide a heat-stable, safe and efficacious RVV for use in developing world.
Methods
Materials: The five live, bovine-human reassortant rotavirus strains (G1, G2, G3, G4 and P1A[8]) were obtained from Merck & Co. Inc., USA. Sodium dihydrogen phosphate monohydrate, trisodium citrate dihydrate, Hepes, Sucrose, polyvinyl pyrrolidone K-25 (PVP K-25), L-Arginine and Calcium chloride dihydrate were purchased from Merck KGaA, Germany. The PVP K-40, Sodium chloride (NaCl), glycine and Zinc chloride (ZnCl2) was purchased from Sigma- Aldrich, USA. Tween-20 was obtained from SD Fine Chemicals, India. Glass vials (Schott 2R, 16.0 ± 0.15 mm body diameter) were purchased from Schott-Kaisha, India and rubber stoppers from West Pharmaceuticals, USA.
Virus quantification: Multivalent qPCR-based potency assay (M-QPA) was used to determine rotavirus potency and was essentially similar to described earlier.47 Briefly, confluent Vero cell monolayers in 96-well plates were inoculated with serial dilutions of test samples, a pentavalent reassortant rotavirus reference standard and assay controls, followed by incubation for 24 h. The cells were lysed with a Triton X-100 solution and the lysates assayed by RT-qPCR to quantitate viral nucleic acid produced during replication. The RT-qPCR uses primer/probe sets specific to each virus reassortant and the potencies of each sample were determined relative to the reference standard. The assay was validated for non-interference from all formulation ingredients used in this study. The limit of quantification using in-house standards used in this study was determined as described earlier47 and was found to be Log10 5.70 IU/ mL giving quantitation range of 2 log. The lyophilized formulations were tested for potency under different storage conditions as indicated.
Freeze-thaw studies: Formulation ingredients were dissolved in the indicated quantities in Milli-Q water. The pH was adjusted to 6.1 ± 0.1 with 1.0N hydrochloric acid. This solution was mixed with G1 serotype in a ratio of 85: 15 v/v to generate ‘Pre lyophilized liquid’ with virus concentration of Log10 7.0 IU/ mL. Aliquots of 1.0 mL each were prepared in Type-I clear glass vials of 3.0 mL capacity and were subjected to five consecutive freeze-thaw cycles at −50oC and +20oC (at a cooling /heating rate of 0.60oC/minute) using Virtis Advantage Plus XL-70 (SP Scientific) lyophilizer. The potency of at least 6 replicates was analyzed by M-QPA assay at the end of the 5th cycle (T = 0) and after one week (T+7) storage at 25°C. All lyophilization experiments were carried out in Type-I clear glass vials of 3.0 mL capacity using indicated cycles.
Stability studies: The samples were incubated in stability chamber (Memmert, Germany) for indicated times and temperatures of 37°C ± 2°C (RH 75% ± RH 5%) henceforth 37°C, 45°C ± 2°C (RH 75% ± RH 5%) henceforth 45°C and 5°C ± 3°C henceforth 5°C. A titer loss specification of ≤ 0.3 log and ≤ 0.5 log was set for selecting formulations during screening experiments involving stability studies for less and more than 30 days respectively. Statistical analysis was performed using JMP 10.
Thermal analysis: The glass transition temperature of the freeze concentrated solute (Tg’) was determined by Differential Scanning Calorimeter (DSC) (DSC 8000, PerkinElmer). Data was analyzed using Pyris™ software. Aluminum pans of 30 µL capacity containing pre-lyophilized liquid and water as reference were placed in the sample holders. Pans were frozen from 4°C to −70oC with at a rate of 1°C/min followed by heating from −70oC to +50oC at a rate of 50oC per min. The nitrogen flow rate was set to 20 mL/min. The Tg’ was recognized as an endothermic shift of the baseline of the heating curve. Modulated DSC (DSC 8000; Perkin Elmer) was used to determine the Glass transition temperature (Tg) determination values of lyophilized cakes. The samples were precooled to -10oC followed by heating to +90oC at a heating rate of 20oC/minute. The isothermal period was set to 1 minute and the number of repetitions to achieve the final desired ending temperature were set as 100. The Tg was recognized on the reversing heat flow curve as an endothermic shift of the baseline and determined as the onset point.
Percent moisture content (%MC): Karl-Fischer titration (Metrohm, Switzerland) method was used, as per the European pharmacopeia 2.5.32.
Physicochemical properties, Dissolution time and pH: Appearance and color of the lyophilized cake were evaluated visually. Time required to obtain a clear solution visually, when 2.0 mL of reconstitution buffer was added to the vial containing lyophilized cake, was noted as the dissolution time. The pH of reconstituted vaccine was recorded and the results are reported as Mean ± SD.
Acid Neutralization Capacity (ANC): The ANC of reconstituted vaccine was evaluated as described in USP <38> NF 33.
Post reconstitution stability studies: The lyophilized cakes were reconstituted with reconstitution buffer and were incubated under −70°C, 5°C ± 3°C and 25°C storage conditions for 24h. The samples were withdrawn at specified time intervals and were analyzed for the potency.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
All authors are employees of MSD Wellcome Trust Hilleman Labs Pvt. Ltd, who funded the research.
References
- 1.World Health Organization, Rotavirus Vaccines, WHO Position Paper , 1st FEBRUARY 2013. [Google Scholar]
- 2.Tate JE, Burton AH, Boschi-Pinto C, Parashar UD. World health organization–coordinated global rotavirus surveillance network. global, regional, and national estimates of rotavirus mortality in children <5 years of age, 2000–2013.Clin Infect Dis. 2016 May 1;62 (2):S96–S105. doi: 10.1093/cid/civ1013. [DOI] [PubMed] [Google Scholar]
- 3.Kirkwood CD, Ma LF, Carey ME, Steele AD. The rotavirus vaccine development pipeline. Vaccine. 2017 Apr 7. pii: S0264-410X (17)30410-3. doi: 10.1016/j.vaccine.2017.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rotarix Product Insert [accessed 2018 March 30] available athttps://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Rotarix/pdf/ROTARIX-PI-PIL.PDF.
- 5.RotaTeq Product insert [accessed 2018 March 30] http://www.merck.com/product/usa/pi_circulars/r/rotateq/rotateq_pi.pdf available at.
- 6.Smallshaw JE, Vitetta ES. A lyophilized formulation of RiVax, a recombinant ricin subunit vaccine, retains immunogenicity. Vaccine. 2010 Mar 11;28(12):2428–35. doi: 10.1016/j.vaccine.2009.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang BS, Fischer NL. Development of an efficient single-step freeze-drying cycle for protein formulations. Pharm Res, 1995. 12(6):831–7. [DOI] [PubMed] [Google Scholar]
- 8.Franks F. Freeze-drying of bioproducts: putting principles into practice. Eur J Pharm Biopharm. 1998 May;45(3):221–9. [DOI] [PubMed] [Google Scholar]
- 9.Jameel F, Pikal MJ. Design of a Formulation for Freeze Drying, in Formulation and Process Development Strategies for Manufacturing Biopharmaceuticals. New Jersey, United States of America: John Wiley & Sons, Inc; 2010. p. 457–92. [Google Scholar]
- 10.Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000 Aug 10;203(1-2):1–60. [DOI] [PubMed] [Google Scholar]
- 11.Zeng XM, Martin GP, Marriott C. Effects of molecular weight of polyvinylpyrrolidone on the glass transition and crystallization of co-lyophilized sucrose. Int J Pharm. 2001 May 7;218(1-2):63–73. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen D, Zaffran M, Designing vaccines for developing-country populations: ideal attributes, delivery devices, and presentation formats. Procedia in Vaccinology. 2010;2(2):119–23. [Google Scholar]
- 13.Weiss C, Clark HF. Rapid inactivation of rotaviruses by exposure to acid buffer or acidic gastric juice. J Gen Virol. 1985 Dec;66 (Pt 12):2725–30. [DOI] [PubMed] [Google Scholar]
- 14.European Medical Agency http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/07/WC500147002.pdf Guideline on pharmaceutical development of medicines for paediatric use [accessed 2018 March 30] available at.
- 15.European Medical Agency, Reflection Paper http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003782.pdf Formulations of Choice For The Paediatric Population [accessed 2018 March 30] available at.
- 16.Pikal-Cleland KA, Rodríguez-Hornedo N, Amidon GL, Carpenter JF. Protein denaturation during freezing and thawing in phosphate buffer systems: monomeric and tetrameric beta-galactosidase. Arch Biochem Biophys. 2000 Dec 15;384(2):398–406 [DOI] [PubMed] [Google Scholar]
- 17.Truong-Le V, Yee L, Lechuga-Ballesteros D, Ohtake S Formulations for preservation of rotavirus, Patent WO2009042202 A2 [Google Scholar]
- 18.Burke CJ, Volkin DB, Rotavirus vaccine formulations, Patent US5932223A [Google Scholar]
- 19.Pando V, Isa P, Arias CF, López S. Influence of calcium on the early steps of rotavirus infection. Virology. 2002 Mar 30;295(1):190–200 [DOI] [PubMed] [Google Scholar]
- 20.Chang BS, Kendrick BS, Carpenter JF, Surface-induced denaturation of proteins during freezing and its inhibition by surfactants. J Pharm Sci. 1996 Dec;85(12):1325–30. [DOI] [PubMed] [Google Scholar]
- 21.WHO Policy Statement : Multi-dose Vial Policy (MDVP), Handling of Multi-Dose Vaccine Vials After Opening, 2014, [accessed 2018 March 30] available at http://apps.who.int/iris/bitstream/handle/10665/135972/WHO_IVB_14.07_eng.pdf.;jsessionid=5767DC9B1AE29D39E6FDA755E720C8CD?sequence = 1
- 22.De Coster I FX, Faure C, Ziani E, Nicolas L, Soubeyrand B, Van Damme P. Assessment of preparation time with fully-liquid versus non-fully liquid paediatric hexavalent vaccines. A time and motion study. Vaccine. 2015 Jul 31;33(32):3976–82. doi: 10.1016/j.vaccine.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Hansen LJJ, Daoussi R, Vervaet C, Remon JP, De Beer TRM. Freeze-drying of live virus vaccines: a review. Vaccine. 2015 Oct 13;33(42):5507–19. doi: 10.1016/j.vaccine.2015.08.085. [DOI] [PubMed] [Google Scholar]
- 24.Lee BY, Cakouros BE, Assi TM, Connor DL, Welling J, Kone S, Djibo A, Wateska AR, Pierre L, Brown ST. The impact of making vaccines thermostable in Niger's vaccine supply chain. Vaccine. 2012;30:5637–43. doi: 10.1016/j.vaccine.2012.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin A, Levin C, Kristensen D, Matthias D. An economic evaluation of thermostable vaccines in Cambodia, Ghana and Bangladesh. Vaccine 2007;25:6945–57. doi: 10.1016/j.vaccine.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 26.Lydon P, Zipursky S, Tevi-Benissan C, Djingarey MH, Gbedonou P, Youssouf BO, Zaffran M. Economic benefits of keeping vaccines at ambient temperature during mass vaccination: the case of meningitis A vaccine in Chad. Bull World Health Organ. 2014;92:86–92. doi: 10.2471/BLT.13.123471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpenter JF, Chang BS, Garzon-Rodriguez W, Randolph TW. Rationale design of stable lyophilized protein formulations: theory and practice. In Carpenter JF, Manning MC, editors. Rationale Design of Stable Protein Formulations-theory and Practice. New York: Kluwer Academic/Plenum publishers; 2002. p. 109–33 [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Trends in use of multi-dose vaccine vials in UNICEF procuring countries, 2012, [accessed 2018 March 30] available athttp://www.who.int/immunization/sage/meetings/2012/april/consultation_INC4_MDVuse_JLiu_20120401.pdf
- 29.Heaton A, Krudwig K, Lorenson T, Burgess C, Cunningham A, Steinglass R. Doses per vaccine vial container: an understated and underestimated driver of performance that needs more evidence. Vaccine. 2017 Apr 19;35(17):2272–8. doi: 10.1016/j.vaccine.2016.11.066. [DOI] [PubMed] [Google Scholar]
- 30.Haidari LA, Wahl B, Brown ST, Privor-Dumm L, Wallman-Stokes C, Gorham K, Connor DL, Wateska AR, Schreiber B, Dicko H, et al. . One size does not fit all: the impact of primary vaccine container size on vaccine distribution and delivery. Vaccine. 2015 Jun 22;33(28):3242–7. doi: 10.1016/j.vaccine.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Department of Health & Family Welfare, Government of India , Handbook for Vaccine & Cold Chain Handlers. available at http://www.nccvmtc.org/PDF2/2_070.pdf, 2010.
- 32.Murhekar MV, Dutta S, Kapoor AN, Bitragunta S, Dodum R, Ghosh P, Swamy KK, Mukhopadhyay K, Ningombam S, Parmar K, et. al. Frequent exposure to suboptimal temperatures in vaccine cold-chain system in India: results of temperature monitoring in 10 states. Bull World Health Organ. 2013 Dec 1;91(12):906–13. doi: 10.2471/BLT.13.119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Cold Chain and Vaccine Management Resource Centre (NCCVMRC) , India, United Nations Children´s Fund and National Institute of Health & Family Welfare (NIHFW), India, National Cold Chain Assessment India. September 2014. [Google Scholar]
- 34.VACCINE WASTAGE ASSESSMENT , India, [accessed 2018 March 30] avaialble at http://www.itsu.org.in/repository-resources/26.Vaccine%20wastage%20assessment%20-2010.pdf. 2010.
- 35.National Institute of Epidemiology, I.C.M.R. , Chennai, Temperature monitoring of the vaccine cold chain to assess the level of freezing. 2012: [accessed 2018 March 30] avaialble at http://www.itsu.org.in/repository-resources/17.Temperature%20Monitoring%20(to%20assess%20freezing)%20-%20ICMR-UNICEF-2012.pdf.
- 36.National Cold Chain Vaccine Management Resource Center (NCCVMRC) at NIHFW, India, National Effective Vaccine Management Assessment, 2013. [accessed 2018 March 30] avaialble at http://www.itsu.org.in/repository-resources/15.National%20EVM%20Assessment%20-%20UNICEF,%202013.pdf.
- 37.Levine MM, "IDEAL" vaccines for resource poor settings. Vaccine. 2011 Dec 30;29 (4):D116–25. doi: 10.1016/j.vaccine.2011.11.090. [DOI] [PubMed] [Google Scholar]
- 38.RotaTeq Product information REVISED October 2008 http://www.who.int/immunization_standards/vaccine_quality/RotaTeq_Product_Insert.pdf [accessed 2018 March 30] available at.
- 39.Evans RK, Strable EL, Isopi L, Thermally stable rotavirus vaccine formulations and methods of use thereof, Patent WO2017106115 [Google Scholar]
- 40.Naik SP, Zade JK, Sabale RN, Pisal SS, Menon R, Bankar SG, Gairola S, Dhere RM. Stability of heat stable, live attenuated Rotavirus vaccine (ROTASIIL®). Vaccine. 2017 May 19;35(22):2962–9. doi: 10.1016/j.vaccine.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 41.Quiroga R, Halkyer P, Gil F, Nelson C, Kristensen D. A prefilled injection device for outreach tetanus immunization by Bolivian traditional birth attendants. Rev Panam Salud Publica. 1998 Jul;4(1):20–5 [DOI] [PubMed] [Google Scholar]
- 42.Sutanto A, Suarnawa IM, Nelson CM, Stewart T, Soewarso TI. Home delivery of heat-stable vaccines in Indonesia: outreach immunization with a prefilled, single-use injection device. Bull World Health Organ. 1999;77(2):119–26. [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L, Li J, Chen H, Li F, Armstrong GL, Nelson C, Ze W, Shapiro CN. Hepatitis B vaccination of newborn infants in rural China: evaluation of a village-based, out-of-cold-chain delivery strategy. Bull World Health Organ. 2007 Sep;85(9):688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Halm A, Yalcouyé I, Kamissoko M, Keïta T, Modjirom N, Zipursky S, Kartoglu U, Ronveaux O. Using oral polio vaccine beyond the cold chain: a feasibility study conducted during the national immunization campaign in Mali. Vaccine. 2010 Apr 26;28(19):3467–72. doi: 10.1016/j.vaccine.2010.02.066. [DOI] [PubMed] [Google Scholar]
- 45.Zipursky S, Boualam L, Cheikh DO, Fournier-Caruana J, Hamid D, Janssen M, Kartoglu U, Waeterloos G, Ronveaux O, Assessing the potency of oral polio vaccine kept outside of the cold chain during a national immunization campaign in Chad. Vaccine. 2011 Aug 5;29(34):5652–6. doi: 10.1016/j.vaccine.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Goveia MG, Nelson CB, Ciarlet M. Rotateq: progress toward developing world access. J Infect Dis. 2010 Sep 1;202 Suppl:S87–92. doi: 10.1086/653546. [DOI] [PubMed] [Google Scholar]
- 47.Ranheim T, Mathis PK, Joelsson DB, Smith ME, Campbell KM, Lucas G, Barmat S, Melissen E, Benz R, Lewis JA, et al. . Development and application of a quantitative RT-PCR potency assay for a pentavalent rotavirus vaccine (RotaTeq). J Virol Methods. 2006 Feb;131(2):193–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


