Abstract
Pyflubumide is a novel carboxanilide acaricide discovered and developed by Nihon Nohyaku Co., Ltd., that exhibits excellent acaricidal activity against Tetranychus and Panonychus species, including strains that have developed resistance to conventional acaricides. Its safety profile against non-target arthropods is suitable for integrated pest management (IPM) programs. Pyflubumide was registered and launched in Japan in 2015 and Korea in 2017. This paper describes pyflubumide’s invention history, synthesis, exploratory synthesis, biological activity, toxicological profile, and mode of action.
Keywords: pyflubumide, carboxanilide, acaricide, mitochondrial complex II, IPM
Introduction
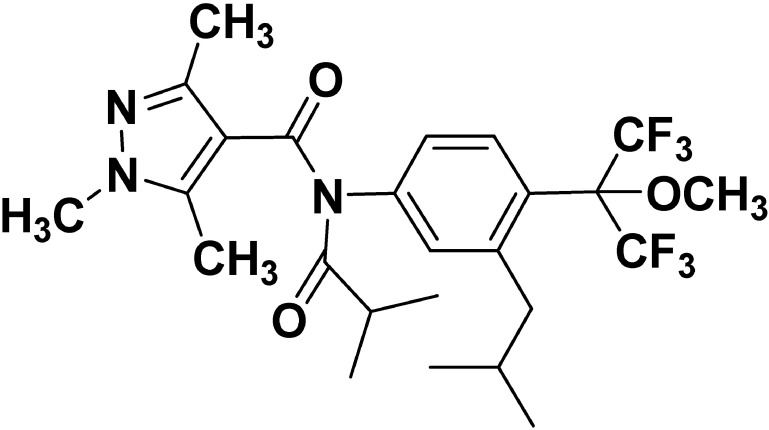
Pyflubumide, discovered and developed by Nihon Nohyaku Co., Ltd., is a novel carboxanilide acaricide that has a unique methoxyhexafluoroisopropyl substituent on the 4′-position (Fig. 1). It is the only acaricide with a carboxanilide structure, and it is classified in the new subgroup 25B (carboxanilide) in the IRAC (Insecticide Resistance Action Committee) MoA classification as a novel inhibitor of mitochondrial complex II on the respiratory chain. It shows a high control efficacy against phytophagous mites, including populations of spider mites that have developed resistance to conventional acaricides. Furthermore, pyflubumide is harmless to non-target arthropods, including beneficial insects and natural enemies, suggesting that it could be favorable for IPM programs.
Fig. 1. Chemical structure of pyflubumide.
Here, we describe pyflubumide’s invention history, synthesis, discovery, biological activity, toxicological profile, and mode of action.
1. History of invention
1.1. Discovery of the lead compound
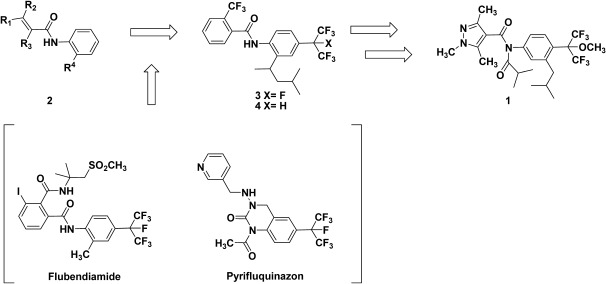
Pyflubumide was discovered from the research of carboxamide fungicides that inhibit mitochondrial complex II (Scheme 1). Since carboxin first appeared on the market in 1966, a number of mitochondrial complex II-inhibiting carboxamides have been developed. In particular, many broad-spectrum carboxamides 2 with bulky substituents on the 2′-position, e.g., boscalid and penthiopyrad, have been launched successively.1)
Scheme 1. Discovery of pyflubumide.
We were quite interested in the development of carboxamides with bulky substituent on the 2′-position in the late 1990s. Meanwhile, flubendiamide2) and pyrifluquinazon,3) novel insecticides having unique heptafluoroisopropyl group, were discovered by our colleagues in 1998 and 1999, respectively.
Continuous research with the goal of discovering a new pesticide by synthesizing compounds with this unique haloalkyl group has led to our synthesis of the 4′-heptafluoroisopropyl-substituted carboxamide 3. The compound only showed low fungicidal activity. This low fungicidal activity seemed to be mainly attributed to its high lipophilicity; hence, we designed the less lipophilic derivative 4. Although the fungicidal activity of the hexafluoroalkyl derivative 4 did not improve, it did show low acaricidal activity.
This unexpected acaricidal activity drew our strong attention because mites are quite problematic due to its rapid development of resistance to agrochemicals; therefore, novel acaricides have been highly and constantly demanded. Thus, we decided to start researching a new acaricide.
1.2. Optimization of the lead compound and the structure-activity relationship
Lead compound 4 was divided into four parts; acid moiety, aniline moiety, fluoroalkyl substituent, and amide modification for its optimization. The summary of the SAR is shown in Table 1.
Table 1. Summary of structure-activity relationship of carboxamides against Tetranychus urticae.
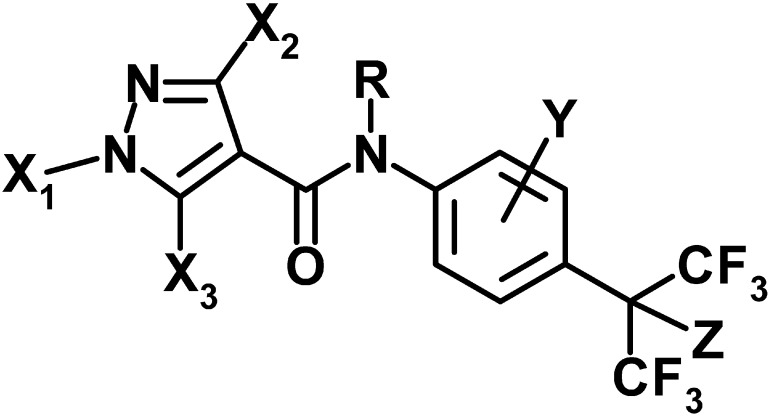 | |
|---|---|
| Substituents | Structure-activity relationship |
| X1 | CH3>n-Pr>H |
| X2 | CH3>Cl, H |
| X3 | CH3>Cl>H |
| Y | 2-(1,3-Me2-Bu), 3-i-Bu>2-(3-Me-Bu), 2-(1,3-Me2-Pentyl), 3-(2-Me-Bu)>3-O-i-Pr, 3-n-Pr≫2-i-Bu, H |
| Z | H, OCH3>OEt>O-n-Pr≫F |
| R | Alkyl carbonyl>H |
1.2.1. Optimization of the acid moiety
The acid moiety was examined by introducing the same acid moiety as that of mitochondrial complex II-inhibiting carboxamides, and we found that the furametpyr derivative showed good acaricidal activity. Further examination of the pyrazole substituents revealed that the 1,3,5-trimethyl pyrazole derivative showed excellent acaricidal activity.6)
1.2.2. Optimization of the anilino moiety
First, the effect of the substituent on the 2′-position was examined and the 1,3-dimethyl butyl group, the same substituent as that of penthiopyrad, was found to be the best substituent for the 2′-position.
The compound with the same substituent as that of mitochondrial complex II-inhibiting carboxamide fungicides showed excellent activity, and accordingly, we then examined the 3′-position because 3′-substituted carboxamide fungicides such as flutolanil also existed. The 3′-isopropyroxy derivative with the same substituent as that of flutolanil was examined. As expected, the flutolanil derivative showed acaricidal activity, although its activity level was moderate. We further investigated 3′-alkyl derivatives. As a result, we found that the 3′-isobutyl derivative showed activity comparable to that of the 2′-(1,3-dimethylbutyl) derivative.7)
From a manufacturing point of view, 3′-isobutyl derivatives are more advantageous because 3-isobutylaniline, the intermediate of the derivatives, can be easily prepared from less expensive materials and it has no chiral center. Accordingly, we then focused on optimizing 3′-isobutyl derivatives.
1.2.3. Optimization of the fluoroalkyl group
The optimization of the fluoroalkyl group revealed that hydrogen or a methoxy group was the best substituent, probably due to their favorable lipophilicity.
1.2.4. Optimization of the amide moiety
As a result of the amide modification, acyl derivatives showed excellent activity, and lower alkyl carbonyl derivatives showed especially superior activity. These results could be explained by considering acyl-substituted derivatives as propesticides. In agrochemistry, several compounds, including acaricides, have been already known to work as propesticides.8)
Among acyl derivatives, pyflubumide (1) was finally selected as the compound to develop.
2. Synthesis
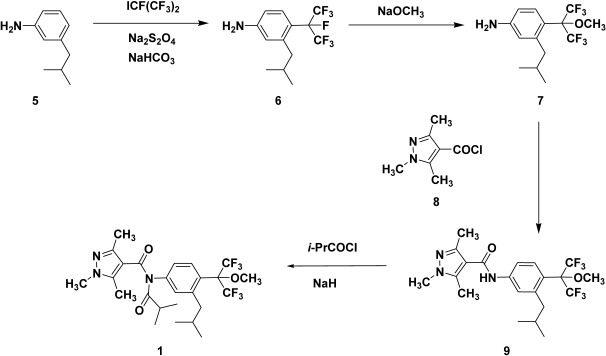
3-Isobutylaniline (5) was reacted with heptafluoroisopropyliodine under radical conditions to produce heptafluoroisopropyl derivative 6. The fluorine atom at the benzylic position of 6 was converted to the methoxy group by sodium methoxide to give aniline 7.4) The amidation of 7 with acid chloride 8 gave carboxanilide 9, followed by acylation to give pyflubumide (1, Scheme 2).
Scheme 2. Synthetic pathway of pyflubumide.
3. Physical and chemical properties
Common Name: Pyflubumide
Experimental code: NNI-0711
CAS registry No.: 926914-55-8
Chemical name: 3′-isobutyl-N-isobutyryl-1,3,5-trimethyl-4′-[2,2,2-trifluoro-1-methoxy-1-(trifluoromethyl)ethyl]pyrazole-4-carboxanilide
Molecular formula: C25H31F6N3O3
Molecular weight: 535.52
Appearance: White powder
Melting point: 86°C
Solubility in water: 0.27 mg/L (20°C)
Partition coefficient: log Po/w=5.34 (25°C)
4. Biological activity
4.1. Insecticidal and acaricidal spectrum of pyflubumide
The insecticidal and acaricidal spectrum of pyflubumide is shown in Table 2. Pyflubumide was specifically and highly active against all stages of agriculturally important spider mites, such as two-spotted spider mite, kanzawa spider mite and citrus red mite. It showed, in contrast, low activity against other pests, including tarsonemid and eriophyid mites and a broad range of insect species.
Table 2. Insecticidal and acaricidal spectrum of pyflubumide.
| Order | Common name | Test stages | LC50 (mg a.i./L) |
|---|---|---|---|
| Acarina | Two-spotted spider mite | Adult | 1.2 |
| Kanzawa spider mite | Adult | 1.9 | |
| Citrus red mite | Adult | 1.3 | |
| European red mite | Adult | 2.2 | |
| Pink citrus rust mite | Adult, Larva | >100 | |
| Tea rust mite | Adult, Larva | >100 | |
| Broad mite | Adult, Larva | >100 | |
| Hemiptera | Green peach aphid | Adult, Larva | >500 |
| Cotton whitefly | Egg | >100 | |
| Thysanoptera | Western flower thrips | 1st Larva | >500 |
| Diptera | Tomato leaf miner | Egg | >500 |
| Coleoptera | Rice weevil | Adult | >500 |
| Lepidoptera | Common cutworm | 3rd Larva | >500 |
4.2. Effects of pyflubumide on beneficial arthropods and natural enemies
As shown in Table 3, pyflubumide exhibited quite low toxicity against beneficial arthropods such as pollinators and natural enemies, including predatory mites and parasitoids that are commercially available and artificially introduced into various cultivation programs. Those results indicated that this carboxamide could be an excellent control agent against spider mites in horticulture, with high suitability to IPM programs.9)
Table 3. Effects of pyflubumide on beneficial arthropods and natural enemies.
| Common name | Scientific name | Test stage | LC30 (mg a.i./L) |
|---|---|---|---|
| Silkworm | Bombyx mori | 4th Larva | >100 |
| Honey bee | Apis mellifera | Adult | >200 |
| Hornfaced bee | Osmia cornifrons | Adult | >100 |
| Predatory mite | Phytoseiulus persimilis | Egg | >200 |
| Amblyseius californicus | Adult | >100 | |
| Amblyseius swirskii | Egg | >200 | |
| Lady beetle | Harmonia axyridis | Adult | >100 |
| Predatory midge | Aphidoletes aphidimyza | Larva | >100 |
| Parasite wasp | Apanteles glomeratus | Pupa | >200 |
| Encarsia formosa | Pupa | >200 | |
| Predatory bug | Orius strigicollis | Adult | >100 |
| Wolf spider | Pardosa pseudoannulata | Larva | >200 |
5. Toxicological properties
Pyflubumide showed quite low acute oral toxicity against mammals and was not classified as poisonous or deleterious compound. In addition, pyflubumide TGAI and its 20% SC showed no critical eye and skin irritation nor skin sensitization. Therefore, they were evaluated as “not classified” in GHS system. Various studies regarding carcinogenicity, mutagenicity, teratogenicity, reproduction, and so on confirmed that the compound would not cause human health concerns. Pyflubumide exhibited rather low toxicity toward aquatics, ready biodegradability in the environment, and low bioconcentration in fish, suggesting that the compound would have low environmental impact under GAP and administration for use.
6. Mode of action
Based on its structural similarity with the carboxamide fungicides, pyflubumide’s mode of action was expected to inhibit mitochondrial complex II. Thus, we investigated the inhibitory effects of pyflubumide on mitochondrial complex II. Pyflubumide did not show remarkable inhibitory activity; however, N-deisobutylated compound 9, i.e., the NH-form of pyflubumide, provided potent inhibitory activity against mitochondrial complex II from spider mites.10) Additionally, analytical chemical research revealed that pyflubumide can be metabolized rapidly to its NH-form in the spider mite homogenate. These results suggest that pyflubumide could be a propesticide, expressing its acaricidal activity through its metabolism to the active form in the spider mite’s body. On the other hand, it showed no inhibition against mitochondria from organisms other than spider mites (Table 4). Accordingly, inhibitory selectivity in vitro could account for the selective toxicity in vivo.
Table 4. Inhibitory activities of pyflubumide (1) and its NH-form (9) against mitochondrial complex II of a variety of species.
| Order | Common name | IC50 (µM) | |
|---|---|---|---|
| Compounds No. | |||
| 1 | 9 | ||
| Acarina | Two-spotted spider mite | >10 | 0.025 |
| Hymenoptera | Honey bee | NT | >1 |
| Lepidoptera | Common cutworm | NT | >10 |
| Diptera | Northern blowfly | NT | >10 |
| Salmoniformes | Rainbow trout | >10 | >10 |
| Rodentia | Norway rat | >1 | >10 |
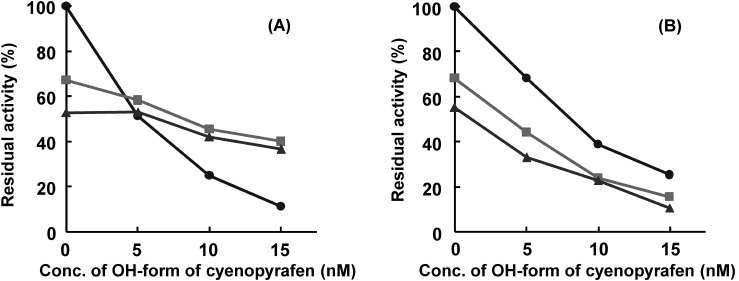
It has already been reported that beta-ketonitrile acaricides also inhibit mitochondrial complex II.11,12) We investigated the biochemical difference between pyflubumide and a beta-ketonitrile derivative by using a double-inhibitor titration assay. The inhibition of the NH-form of pyflubumide and the OH-form of cyenopyrafen were not additive (Fig. 2A), which is in contrast to the case using the same compound (the OH-form of cyenopyrafen vs. the OH-form of cyenopyrafen), in which almost additive inhibition was attained (Fig. 2B).
Fig. 2. The inhibition of complex II of spider mite mitochondria by the OH-form of cyenopyrafen in the presence of the NH-form of pyflubumide (A) or the OH-form of cyenopyrafen (B); (A) titration by the OH-form of cyenopyrafen in the presence of 0 (●), 20 (■), or 35 nM (▲) of the NH-form of pyflubumide; (B) titration by the OH-form of cyenopyrafen in the presence of 0 (●), 5 (■), or 6.5 nM (▲) of the OH-form of cyenopyrafen.
From the viewpoint of the above-mentioned results regarding the mode of action and the in vivo evaluation of its cross-resistance, mitochondrial complex II inhibitors recently launched in the market are categorized into the following separate subgroups: 25A (beta-ketonitrile derivatives; cyenopyrafen, cyflumetofen) and 25B (carboxanilides; pyflubumide) in classifying the mode of action by the Insecticide Resistance Action Committee (IRAC).13)
Conclusion
The usage of mitochondrial complex II-inhibiting carboxamides had been primarily limited to disease control. However, we have found a novel acaricidal activity by introducing unique fluoroalkyl groups, which finally led to the discovery of pyflubumide.
As of May 2017, pyflubumide was registered and commercialized in Japan/Korea, under the trade names of Dani-Kong, Noblesse® (single agent) and Double-Face (mixture with fenpyroximate). The registration of pyflubumide has been scheduled for approval as a pesticide in additional countries in the near future. It is also expected that pyflubumide, an excellent acaricide with unique characteristics, will be widely used to contribute to the success of agriculture in the world.
Acknowledgments
The authors are very thankful to the people who cooperated to the development of pyflubumide. The authors also wish to acknowledge Professor Hideto Miyoshi for the research into the mode of action of pyflubumide. Lastly, we also thank for the help of everyone in our company.
References
- 1).Y. Yanase, H. Katsuta, K. Tomiya, M. Enomoto and O. Sakamoto: J. Pestic. Sci. 38, 120–129 (2013), in Japanese. [Google Scholar]
- 2).M. Tohnishi, H. Nakao, T. Furuya, T. Shimizu, A. Seo, K. Sakata, S. Fujioka, H. Kodama, T. Hirooka and T. Nishimatsu: J. Pestic. Sci. 30, 354–360 (2005). [Google Scholar]
- 3).M. Uehara, M. Watanabe, M. Kimura, M. Morimoto and M. Yoshida (Nihon Nohyaku Co., Ltd.); Jpn Tokkyo Koho JP 4099622B (in Japanese).
- 4).T. Furuya, K. Machiya, A. Suwa and S. Fujioka (Nihon Nohyaku Co., Ltd.): PCT Int. Appl. WO2005/115994 (2005).
- 5).T. Furuya, H. Kanno, K. Machiya, A. Suwa, N. Yasokawa and S. Fujioka (Nihon Nohyaku Co., Ltd.): PCT Int. Appl. WO2007/020986 (2007).
- 6).T. Furuya, M. Yamaguchi, M. Tohnishi, A. Seo, M. Morimoto, T. Takemoto and S. Fujioka (Nihon Nohyaku Co., Ltd.): Pct. Int. Appl. WO2002/96882 (2002).
- 7).T. Furuya, A. Suwa, M. Nakano, S. Fujioka, N. Yasokawa and K. Machiya: J. Pestic. Sci. 40, 38–43 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).P. Jeschke: Pest Manag. Sci. 72, 210–225 (2016). [DOI] [PubMed] [Google Scholar]
- 9).T. Furuya, M. Nakano, A. Suwa, N. Yasokawa, S. Fujioka and K. Machiya: “Discovery and Synthesis of Crop Protection Products,” ACS symposium series, vol. 1204, eds. by P. Maienfisch, P. and T. M. Stevenson), American Chemical Society, Washington, DC, pp. 379–389 (2015).
- 10).M. Nakano, N. Yasokawa, A. Suwa, S. Fujioka, T. Furuya and K. Sakata: J. Pestic. Sci. 40, 15–24 (2015). [Google Scholar]
- 11).K. Nakahira, S. Takii and H. Murakami: Abstracts of papers, 11th IUPAC International Congress of Pesticide Chemistry, August, Kobe, Book of Abstract (2). II-1-i-23B (2006).
- 12).N. Hayashi, Y. Sasama, N. Takahashi and N. Ikemi: Pest Manag. Sci. 69, 1080–1084 (2013). [DOI] [PubMed] [Google Scholar]
- 13).http://www.irac-online.org/documents/moa-structures-poster-english/?ext=pdf (Accessed Mar 26, 2017)