Abstract
Careful, often cumbersome, screening is a fundamental part of DBS evaluation in Parkinson's disease (PD). It often involves a brain MRI, neuropsychological testing, neurological, surgical, and psychiatric evaluation, and “ON/OFF” motor testing. Given that DBS has now been a standard treatment for advanced PD, with clinicians’ improved comfort and confidence in screening and referring patients for DBS, we wondered whether we can now streamline our lengthy evaluation process. We reviewed all PD patients evaluated for DBS at our center between 2006 and 2011 and analyzed the reasons for exclusion and for dropping out despite passing the screening process. A total of 223 PD patients who underwent DBS evaluation had complete charting. Only 131 (58.7%) patients were successfully implanted. Sixty‐one (27.3%) patients were excluded after screening because of significant cognitive decline (32.7%), early disease with room for medication adjustment (29.5%), behavioral dysfunction (21.3%), suspected secondary parkinsonism or atypical parkinsonism syndrome (13.1%), PD, but with poor levodopa response (11.4%), unrealistic goals (9.8%), PD with predominant axial symptoms (6.5%), significant comorbidities (6.5%), or abnormal brain imaging (3.2%). In addition, 31 (13.9%) patients were cleared for surgery, but either chose not have it (18 patients), were lost to follow‐up (12 patients), or were denied by medical insurance (1 patient). Through careful screening, a significant percentage of surgical candidates continue to be identified as less suitable because of a variety of reasons. This underscores the continued need for a comprehensive, multidisciplinary screening process.
Keywords: deep brain stimulation, screening, Parkinson
DBS of the STN or the globus pallidus interna (GPi) has been established as an effective treatment option for selected patients with advanced Parkinson's disease (PD).1 It is mainly indicated for PD patients who, despite optimization of medical treatment, continue to suffer from motor fluctuations, disabling dyskinesias, or for PD patients with medication‐resistant tremors. Patients with profound cognitive deficits and those with predominant gait and balance dysfunction are believed to do poorly after DBS surgery2 and thus are generally excluded from surgical candidacy in most DBS centers. It is generally believed that DBS can improve levodopa‐responsive PD symptoms, as well as tremors.
To optimize the outcome of DBS surgery, most centers have adopted screening procedures to ensure appropriate patient selection for surgery. At the Cleveland Clinic Center for Neurological Restoration (Cleveland, OH), screening includes evaluation by a movement disorders neurologist (to clarify diagnosis and assess medication optimization), “ON/OFF” l‐dopa testing (to document which symptoms improve and to what degree), brain MRI (to rule out structural lesions), neuropsychological evaluation (to evaluate cognitive integrity), neurosurgical evaluation (to assess for surgical contraindications), and psychiatric evaluation (to evaluate behavioral health). The final decision for each patient is then determined by the collective agreement of a multidisciplinary team during a patient management conference. This “committee” includes neurosurgery, neurology, psychiatry, nursing, neuropsychology, and medical ethics. This is often a lengthy evaluation process and can be time‐consuming. This process has previously been described with discussion of the ethical importance.3 There have been several attempts to streamline or shorten the lengthy process of DBS screening.4, 5, 6
We wanted to evaluate which elements of the screening process contributed least to excluding inappropriate surgical candidates (and therefore could potentially be removed in an effort to streamline the lengthy screening process) and how many of those deemed as appropriate surgical candidates eventually went on to receive DBS surgery at our center. We therefore conducted a retrospective study looking at the reasons for exclusion and the dropout rate in all PD patients who were fully evaluated for DBS at our center.
Patients and Methods
A retrospective chart review of PD patients evaluated for DBS at the Center for Neurological Restoration of the Cleveland Clinic, over a 5‐year period, from 2006 to early 2011, was conducted. We identified all patients who were not implanted after being fully evaluated. The reasons for excluding patients from surgical candidacy as well as reasons for patient dropout after being cleared for surgery were determined and categorized.
The following patient scenarios were not included in this study: patients who did not complete the full process of DBS screening; patients who were evaluated for electrode revision; patients who were evaluated outside the predetermined time period and those who were thought to need more workup or medical management, but were later rediscussed and considered to be good candidates; and patients who were cleared for surgery, but decided to have it at another center. We chose to include only patients who completed the full screening process to avoid contaminating the sample with patients who were clearly unsuitable for surgery or wrongly triaged. Those included patients who were referred at very early stages of disease who were not on l‐dopa or on just starting doses of l‐dopa, patients without parkinsonism (e.g., essential tremors, depression, and hypothyroidism), and patients who came in for clinical evaluation and were wrongly triaged as DBS candidates. In those scenarios, the provider at the first encounter simply canceled the DBS screening process. We then classified the reasons for exclusion under nine major categories: cognitive dysfunction; behavioral dysfunction; early PD and/or room for medication adjustment; PD but with poor response to l‐dopa; suspected secondary parkinsonism or atypical parkinsonism syndrome; PD but with predominant axial symptoms (gait and/or balance dysfunction); unrealistic patient surgical goals (in addition to/or other than improvement of gait and balance dysfunction); medical/surgical comorbidities; and abnormal brain imaging. Clearly, some of these categories were overlapping; however, we tried to outline clear borders for each category as much as possible. We included all memory, attention, visuospatial, and executive dysfunctions under the cognitive category and all the mood and perceptual dysfunctions under the behavioral category. All patients underwent full neuropsychological battery interpreted by one of two dedicated neuropsychologists with special expertise in the process of DBS screening. All patients who were excluded on the basis of psychiatric or behavioral factors were evaluated by a dedicated movement disorder psychiatrist and underwent full medication trial for their mood, perceptual, or behavioral problem including one or more follow‐up visits with the psychiatrist. Only patients who had persistent symptoms despite full trial of psychiatric treatment were excluded from surgery on that basis.
This was a minimal risk study utilizing existing data through chart review and not requiring any direct patient evaluation for the purpose of the study. Data were deidentified, informed consent was waived, and the study was exempt from review by the Cleveland Clinic Foundation Institutional Review Board.
Results
A total of 223 PD patients were identified as having had a full DBS evaluation with available records during the predetermined period and meeting criteria for inclusion. A total of 131 (58.7%) patients (38 females and 93 males; mean age: 63.2 years) got implanted in the STN or the GPi after the evaluation process. A total of 92 patients (41.2%) were not implanted after completing the full screening process (25 females and 67 males; mean age: 65.3 years). Of the 92 patients who were not implanted, 31 were cleared for surgery, but ended up not having it. Eighteen of these patients chose not to have the surgery and 12 of them were lost in follow‐up without stating their intentions. Only 1 patient was cleared for surgery, but did not have it because of insurance denial.
A total of 61 patients (27.3% of the total patients evaluated) were recognized as poor candidates for surgery by the evaluating multidisciplinary team and were thus excluded from the procedure. The reasons of exclusion were variable (Table 1). A total of 18 patients (29.5% of all excluded patients) had multiple exclusion factors, with the most common factor combination being combined cognitive and behavioral dysfunctions.
Table 1.
Exclusion factors for DBS for PD determined at the time of screening
| Exclusion Categories | Number of Patients | Percentage |
|---|---|---|
| Cognitive | 20 | 32.7 |
| Early PD/room for medication adjustment | 18 | 29.5 |
| Psychiatric/behavioral | 13 | 21.3 |
| Suspected secondary parkinsonism or atypical parkinsonism | 8 | 13.1 |
| Poor l‐dopa response | 7 | 11.4 |
| Unrealistic or unfeasible patient surgical goals | 6 | 9.8 |
| Predominant axial symptoms | 4 | 6.5 |
| Medical/surgical comorbidities | 4 | 6.5 |
| Abnormal brain imaging | 2 | 3.2 |
| Lack of family support | 1 | 1.6 |
| Total | 83 | 135.6 |
The total number of exclusion factors (83) is greater than the number of patients excluded (61), and the total percentage is above 100% because 18 patients had multiple exclusion factors.
The most common exclusion categories in a descending order of frequency were: cognitive (20 patients; 32.7%); early PD/potentially manageable without surgery (18 patients; 29.5%); behavioral (13 patients; 21.3%); suspected secondary parkinsonism or atypical parkinsonism (8 patients; 13.1%); poor l‐dopa response (7 patients; 11.4%); unrealistic or unfeasible surgical goals (6 patients; 9.8%); predominant axial symptoms (4 patients; 6.5%); medical or surgical comorbidities (4 patients; 6.5%); and abnormal brain imaging (2 patients; 3.2%). Lack of family support was a cofactor in 1 excluded patient.
In the cognitive category, the most common causes of exclusion were as follows: frank dementia (9 patients); multidomain mild cognitive impairment (MCI; 9 patients); single‐domain MCI (language; 1 patient); and low IQ at baseline in 1 patient. Only patients with MCI patterns suggestive of a cortical dysfunction (e.g., language, memory, and so on) were among excluded patients. Patients with predominantly subcortical patterns of MCI (executive function, processing speed, and so on) were not routinely excluded during the screening process.
In the behavioral category, the most common causes of exclusion were uncontrolled anxiety (6 patients), depression (4 patients), impulse control disorder (4 patients, with punding in 2 of them), persistent psychosis (4 patients), and personality disorder (1 patient). These characteristics were thought to place the patients at too high a risk of burden either intra‐ or postoperatively.
The most common secondary or atypical parkinsonism syndromes resulting in patient exclusion were probable MSA (5 patients), vascular parkinsonism (2 patients), and drug‐induced parkinsonism (1 patient).
The most common surgical goals that the team considered to be less realistic were desires to improve gait and balance that did not improve with l‐dopa, improve confusion, improve fatigue, improve l‐dopa‐resistant loss of hand dexterity, improve dyspnea and head pressure, and complete cure or complete resolution of symptoms.
The medical or surgical comorbidities resulting in patient exclusion were metastatic prostate cancer, severe scoliosis impairing respiratory function, liver cirrhosis, and history of repeated infections.
Abnormal findings on brain imaging leading to patient exclusion were bilateral basal ganglia calcification and multiple brain infarcts in a patient with clinical features suggestive of vascular parkinsonism.
Discussion
Our study looked at the rate of patient exclusion and patient dropout during the process of preoperative screening for DBS. Several recent publications have tried to provide guidelines regarding proper case selection and surgical contraindications.2, 4 Increasing awareness among general neurologists has likely resulted in more favorable cases being referred for DBS evaluation in the most recent years. Despite this, our multidisciplinary team approach for DBS screening excluded approximately one third of all cases referred to our center over the 5‐year period of the study. This process can indeed be laborious, and several attempts have been made to shorten this process by different centers.4, 5, 6 The reasons of exclusion, however, varied greatly in our cohort. Cognitive dysfunction remains the most important exclusion factor in our cohort, as it is in the majority of DBS literature. Cognitive impairment has been consistently blamed for suboptimal surgical outcomes not only in the long term, but also in the immediate postoperative period.7 The most common cognitive causes of exclusion in our cohort were dementia and multidomain MCI. Patients with “subcortical” patterns of cognitive impairment were not as frequently excluded during the screening process. We are currently investigating whether particular patterns of cognitive impairment are, in fact, predictive of outcome.
The large portion of “early PD” (or nonoptimized medication regimen) being the second‐most common exclusion factor (29.5%) in our cohort suggests that health care providers may now have a lower threshold in considering DBS for their patients and tend to refer earlier and milder cases of PD in recent years. It may also reflect the increasing role of self‐referral, the favorable public perception of DBS, or the clinicians’ increasing comfort with the procedure. However, this also underscores the need for optimizing medical management before taking the patient through the complete and expensive DBS screening process. Indeed, by doing so, close to 30% of negative screening—and its associated cost—can be avoided. However, the optimal timing for DBS implant remains a topic of discussion, with a recent trial reporting benefit from DBS in PD patients with early motor complications.8
Some factors that were prominent in our cohort have not been prominent in earlier reports.9 For example, unrealistic or unfeasible surgical goals were the basis of exclusion for a considerable number of our patients. Not uncommonly, patients and family expressed different surgical goals during the neurological and ‐surgical evaluations. Thus, we found having separate comprehensive neurological and ‐surgical evaluations to be helpful in prioritizing patient goals and exploring their expectations. Moreover, having these evaluations early on during the screening process can allow exclusion of such patients before more time‐ and resource‐consuming interventions, such as brain MRI, neuropsychiatry evaluation, and behavioral evaluation, are performed.
Another previously under‐represented exclusion factor is the role of anxiety coming as the most common psychiatric cause of exclusion. Most previous reports have focused on the role of depression. Excessive anxiety may have negative implications both intra‐ and postoperatively, and we believe it should be well managed before considering surgery, particularly for the awake surgeries.10
Interestingly, we also noted a significant rate of patient dropout after being cleared for surgery (19.1% of all cleared patients). The majority of these patients stated their wish not to have the surgery whereas some of them were simply lost to follow‐up. Learning more about the details of surgery, such as its noncurative nature, risks, long‐term commitment to programming sessions, and cost, may have accounted for this.
Unique to our center is the integration of a professional bioethicist as part of our DBS committee. Beyond providing input during patient management meetings, formal bioethical consult was requested for 5 of our patients during the study period to help in decision making regarding surgery. Of those five, 3 went forward with DBS with the support of the ethicist and agreement of the patient.
The majority of DBS referrals to our center were made by general neurologists. Only a small number of patients were self‐referred for DBS evaluation. Although our center gets frequent referrals from primary care doctors, those are often referrals for clinical evaluations and not for DBS screening per se. There were no referrals from outside movement disorder specialists, neurosurgeons, or psychiatrists. The ratio of self‐referred patients to those referred from general neurologists is too small in our cohort to allow for any meaningful comparative study between both groups. It is to be mentioned that our center does not utilize any prescreening tool to identify potential DBS candidates, and we are unaware whether any of these tools were used by the referring physicians.
In 2002, Lopiano et al. conducted a study looking at the exclusion causes of PD patients undergoing STN DBS evaluation at their center.9 Their smaller cohort consisted of 98 evaluated patients, of whom 29 patients were excluded (29.6%). The percentage of excluded patients was very comparable to our cohort (27.3%). Their first two causes of exclusion were identical to ours: cognitive/psychiatric dysfunction and modest clinical picture. Abnormal brain imaging was more represented in their group (31%), compared to 3.2% in ours. This may be a reflection of the increased awareness among the referring health care providers, as discussed earlier. Ten percent of their patients were excluded because of poor motivation for surgery. Those patients might correspond to our dropout group and our group with unrealistic expectations. Medical comorbidity was the cause of exclusion in 6.9% of their patients, similar to our percentage of 6.5%. Patient expectations, l‐dopa response, atypical parkinsonism, and axial symptoms were not represented in their cohort.
More recently, Katz et al. reported the outcome of DBS referrals in their center.11 They noted that, over the period between 2005 to 2009, the yearly percentage of referred patient deemed as “good candidates” by their multidisciplinary team decreased, whereas the percentage of patients deemed as “possible future candidates” and “poor candidates” increased. They attributed this change in pattern to the referring clinicians’ greater confidence in the procedure. The exclusion percentage in our cohort was more or less the same over the years. Regardless, we feel that this is one example where increasing familiarity and confidence with the procedure should not be a reason to relax the screening process.
As with all retrospective reviews, a weakness of this study lies in the extrapolation of data from notes. There were no explicit minutes taken of the multidisciplinary team, and documentation varied between providers’ notes. Nonetheless, impressions from each step of the DBS screening process were available through our electronic medical record system, and only patients who underwent the full DBS screening were included in this study. Furthermore, the study does not include all patients discussed in our meeting because records were fully available for a subset of individuals and some patients were rediscussed at subsequent meetings after further workup. Finally, although this study highlights the importance of a comprehensive screening process in view of variable exclusion factors, it lacks a comparison group and there is no direct evidence that the outcome of DBS after our traditional selection procedure is better than after a different, less‐stringent selection.
In conclusion, multiple factors should be considered when screening PD patients for DBS. Based on our cumulative experience, we still recommend a thorough, comprehensive screening process for DBS evaluation, including a separate neurological and ‐surgical evaluation, a full neurocognitive testing, and brain imaging. Each step in our current screening process was responsible for identifying a percentage of nonideal surgical candidates. Abbreviating the evaluation process may result in improper selection of PD patients for DBS surgery. However, especially in the current context of reducing unnecessary expenses, this screening process can be staged to help reduce costs and improve the efficiency of the process (Fig. 1). Patient should first undergo a thorough clinical evaluation from the neurologist to ascertain the diagnosis and optimize medical management given that 42.6% of our patients who were refused surgery either had a non‐PD parkinsonism or were not yet optimized medically. Patients should then discuss at length with the neurologist and neurosurgeon to assess their understanding of the risk/benefits ratio, given that 19.1% of our patients who were deemed good surgical candidates refused surgery. If the patients then understand this ratio and wish to proceed, the ON/OFF testing can be performed, owing to the fact that 17.9% of our patients who were refused surgery had poor l‐dopa response or axial symptoms. At that point, if the patient is still considered a good candidate for surgery, brain MRI, neuropsychological testing, and psychiatry evaluation would be pursued. We are aware that following this algorithm, though decreasing the cost of medical expenses, would increase the patient's inconvenience and direct cost given that it might imply multiple trips to a health care facility. This could be decreased by scheduling all the procedures and visits within a 48‐hour window and in the order suggested in the algorithm. If, at any step in the process, the patient is found not to be a good DBS candidate, the process is terminated and the remaining visits or procedures are cancelled. Each DBS performing institution should examine this approach and adapt it to its specific circumstances as well as those of each individual patient. In addition, we recommend paying close attention to potential “social factors” (such as family support, travel and distance from the center, financial stability, and so on) when screening patients because they can be responsible for a significant number of dropouts even among PD patients who are cleared for surgery.
Figure 1.
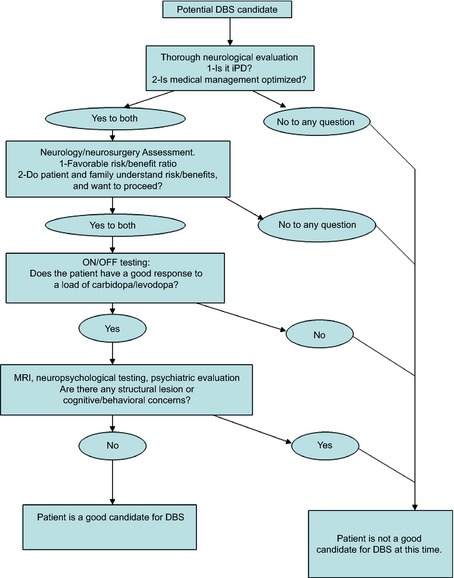
Suggested staging of the screening process for patients with PD being evaluated for DBS.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
H.A.: 1A, 1B, 1C, 3A, 3B
R.M.: 1B, 3B
A.M.: 3B
A..A.: 3B
M.G.: 3B
S.C.: 3B
I.I.: 3B
P.S.: 3B
M.P.: 3B
C.K.: 3B
D.F.: 3B
P.J.F.: 3B
H.H.F.: 1A, 1B, 3B
Disclosures
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interests.
Financial Disclosures for previous 12 months: H.A. received an educational grant from Teva Pharmaceuticals. R.M. is a site subinvestigator for the following studies: CD reflex (2% effort) and Mysticol (2% effort). A.M. receives fees of $5,000 or more per year as a paid consultant, speaker, or member of an advisory committee for Monteris Medical, Inc., and he may receive future financial benefits from the Cleveland Clinic for inventions or discoveries commercialized through the following companies: Autonomic Technologies, Inc., CardioNomic, and IntElect Medical, Inc. P.S. is on the speaker bureau for UCB and Teva Pharmaceuticals. M.P. receives fees of $5,000 or more per year as a paid consultant, speaker, or member of an advisory committee for Lundbeck. Dr. Fernandez has received research support from Abbott, Acadia, Biotie Therapeutics, EMD‐Serono, the Huntington Study Group, Merck, the Michael J. Fox Foundation, the International and Parkinson Movement Disorder Society (MDS), the National Parkinson Foundation, the National Institutes of Health/National Institute of Neurological Disorders and Stroke, Novartis, the Parkinson Study Group, Synosia, and Teva, but has no owner interest in any pharmaceutical company; has received honoraria from Advanced Health Media, Cleveland Clinic CME, Medical Communications Media, MDS, and Vindico Medical Education as a speaker in CME events; has received honoraria from Ipsen, Merz Pharmaceuticals, Pfizer, Teva Neuroscience, and Zambon Pharmaceuticals as a speaker and/or consultant; and has received royalty payments from Demos Publishing for serving as a book author/editor. The Cleveland Clinic has contracts with EMD Serono, Abbott, and Merz Pharmaceuticals for Dr. Fernandez’ role as a member of the Global Steering Committee for Safinamide and LCIG studies and Head Principal Investigator for the Xeomin Registry Study. Dr. Fernandez also serves as the Chair of the Publication Committee for Xeomin Studies (Merz Pharmaceuticals); a member of the Publication Committee for Dysport studies (Ipsen Pharmaceuticals); and a consultant for Prostrakan/KyowaHakko, Britannia, Knopp, and US WorldMeds, but he does not receive any personal compensation for these roles. Dr. Fernandez has received a stipend from MDS for serving as medical editor of the MDS website.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009;301:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bronstein JM, Tagliati M, Alterman RA, et al. Deep brain stimulation for Parkinson disease an expert consensus and review of key issues. Arch Neurol 2011;68:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ford PJ, Kubu CS. Stimulating debate: ethics in a multidisciplinary functional neurosurgery committee. J Med Ethics 2006;32:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wachter T, Minguez‐Castellanos A, Valldeoriola F, Herzog J, Stoevelaar H. A tool to improve pre‐selection for deep brain stimulation in patients with Parkinson's disease. J Neurol 2011;258:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Okun MS, Fernandez HH, Pedraza O, et al. Development and initial validation of a screening tool for Parkinson disease surgical candidates. Neurology 2004;63:161–163. [DOI] [PubMed] [Google Scholar]
- 6. Oyama G, Rodriguez RL, Jones JD, et al. Selection of DBS candidates in private neurology practices: referral may be simpler than a computerized triage system. Neuromodulation 2012;15:246–250. [DOI] [PubMed] [Google Scholar]
- 7. Mikos A, Pavon J, Bowers D, et al. Factors related to extended hospital stays following deep brain stimulation for Parkinson's disease. Parkinsonism Relat Disord 2010;16:324–328. [DOI] [PubMed] [Google Scholar]
- 8. Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson's disease with early motor complication. N Engl J Med 2013;368:610–622. [DOI] [PubMed] [Google Scholar]
- 9. Lopiano L, Rizzone M, Bergamasco B, Tavella A, Torre E, Perozzo P, Lanotte M. Deep brain stimulation of the subthalamic nucleus in PD: an analysis of the exclusion causes. J Neurol Sci 2002;195:167–170. [DOI] [PubMed] [Google Scholar]
- 10. Ford PJ, Boulis N, Montgomery E, Rezai AR. A patient revoking consent during awake craniotomy: an ethical challenge. Neuromodulation 2007;10:329–332. [DOI] [PubMed] [Google Scholar]
- 11. Katz M, Kilbane C, Rosengard J, Alterman RL, Tagliati M. Referring patients for deep brain stimulation: an improving practice. Arch Neurol 2011;68:1027–1032. [DOI] [PubMed] [Google Scholar]


