Abstract
Purpose
In this study, we report results from a 5-year surveillance for noncandidal yeast species causing invasive infections from 65 hospitals in China.
Materials and methods
Species identification was carried out by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) supplemented by rDNA sequencing, and fluconazole and voriconazole susceptibilities of yeasts were determined by Clinical and Laboratory Standards Institute (CLSI) disk diffusion methods.
Results
Overall, 884 noncandidal isolates belonging to 38 species were collected. Cryptococcus neoformans was the most common (75.6%), which also comprised 96.5% of the isolates from cerebrospinal fluid (CSF) and 62.6% from blood, followed by Trichosporon asahii (6.9%) and Rhodotorula mucilaginosa (5.1%). Fluconazole susceptibility and resistant rates were 74.1% and 9.7% for C. neoformans and 81.0% and 5.2% for T. asahii. Voriconazole exhibited good activity in comparison to these two species (99.5% and 98.3% of the isolates, were susceptible). However, 100% of the R. mucilaginosa isolates were resistant to both azoles. Other noncandidal yeast species showed reduced susceptibility to fluconazole (53.3%) but most were susceptible to voriconazole (94.3%). Over the 5 years, a decrease in the proportion of fluconazole-susceptible isolates was observed for C. neoformans (90%–67%, P<0.001) and other noncandidal yeast species (91%–66%, P<0.001). Moreover, the prevalence of azole-resistant R. mucilaginosa increased from 1% to 7% (P<0.001).
Conclusion
The shift in azole susceptibilities in mainland China calls for continued surveillance for noncandidal yeasts.
Keywords: invasive fungal infections, noncandidal yeasts, epidemiology, azole susceptibility, China
Introduction
Invasive yeast infections are a major threat to patients, particularly the immunocompromised and critically ill, with high morbidity and mortality.1–5 Although Candida species remain the major cause of such infections, noncandidal yeast species are increasingly encountered as pathogens.1,5–8 However, knowledge of the clinical characteristics and epidemiology of these pathogens remains relatively limited.1,6,9 Moreover, data on antifungal susceptibility profiles of noncandidal yeasts are relatively few. Even where antifungal susceptibility was performed, there are no clinical breakpoints (CBPs) established using standard broth microdilution methods to guide interpretation.10,11 These limitations result in uncertainty in clinical management and best practice use of antifungal drugs.1,6
China, as one of the most fast developing countries, also suffers from the challenges of relative lack of epidemiology and drug resistance data for invasive yeast infections.7,12 To close this knowledge gap and to assist clinical management, the China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study was initiated in 2009, focusing on both invasive candidiasis (IC) and noncandidal infections.7 Up to the fifth surveillance year (2014), 65 hospitals from 27 of the 34 provinces in China participated, enabling over 8,000 yeast isolates being collected.
In this study, we summarize the overall comparative species distribution of noncandidal yeast isolates and their antifungal susceptibility to fluconazole and voriconazole as determined by the Clinical and Laboratory Standards Institute (CLSI) disk diffusion methodology.10,13
Materials and methods
Study design and isolates
The CHIF-NET study is a prospective, laboratory-based, multicenter study of invasive yeast infections.7 This study comprised data from August 1, 2009, to July 31, 2014, and study inclusion criteria have been described previously.7 Each surveillance year, all non-repetitive yeast isolates from eligible patients with invasive infections were forwarded to the central laboratory, the Department of Clinical Laboratory, Peking Union Medical College Hospital, for species confirmative identification and antifungal susceptibility testing. The study was approved by the Human Research Ethics Committee of Peking Union Medical College Hospital (S-263). The quality control strains for identification and antifungal susceptibility testing were Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258.
Species identification
All yeast isolates were identified to the species level in the central laboratory by sequencing of the fungal rDNA internal transcribed spacer (ITS) regions in year 17 or by an algorithm of matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS, Vitek MS system; bioMérieux, Marcy-l’Étoile, France) supplemented by ITS sequencing.14
Antifungal susceptibility testing
Susceptibility to fluconazole and voriconazole was determined using the CLSI disk diffusion method,6,10 and the results were interpreted as per the CLSI M44-S3 document (for fluconazole, susceptible, ≥19 mm; susceptible dose-dependent [SDD], 15–18 mm; resistant, ≤14 mm; for voriconazole, susceptible, ≥17 mm; SDD, 14–16 mm; resistant, ≤13 mm).13
Statistical analyses
All comparisons were performed using SPSS software version 12.0 (SPSS Inc., Chicago, IL, USA). Comparisons of continuous variables were performed by using the Mann– Whitney test, and comparisons of categorical variables were performed by using a chi-squared test or Fisher’s exact test, as appropriate. A P-value of 0.05 was significant.
Results
Isolates and patients
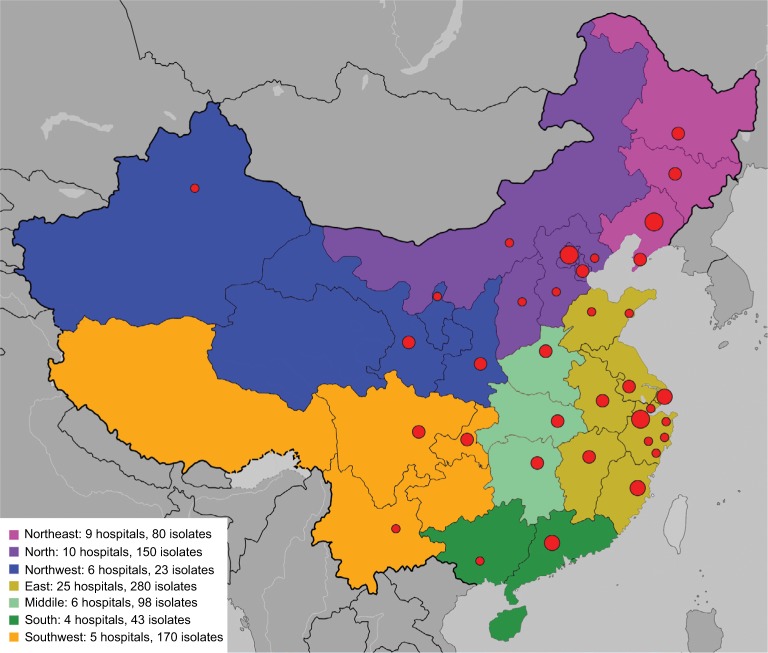
Fifty-five of 65 participating hospitals submitted a total of 884 non-repetitive noncandidal yeast isolates from separate patients (the remaining 10 hospitals identified no episodes of noncandidal yeast infection cases during the study period) (Figure 1). Of the isolates, 300 (35.5%) were cultured from female patients and 544 (64.5%) from male patients. Patient age ranged from 0 to 91 years (median 58, IQR 30–44).
Figure 1.
Noncandidal invasive yeast isolates collected in the five-year China Hospital Invasive Fungal Surveillance Net study.
Note: Seven geographic regions in China were labeled by different colors in the map, and locations of participant hospitals were labeled by red dots.
A total of 38 noncandidal yeast species were identified (Table 1). By genera, Cryptococcus species was most common (77.5% or 654/844 isolates), followed by Trichosporon species (74/844, 8.8%), Rhodotorula species (44/844, 5.2%), and other uncommon genera (<4%). At the species level, Cryptococcus neoformans was predominant, accounting for over 75.6% of the isolates (638/844), but Cryptococcus gattii was rare (7/844, 0.8%). Trichosporon asahii was the second commonest species (58/844, 6.9%), followed by Rhodotorula mucilaginosa (43/844, 5.1%), Kodamaea ohmeri (26/844, 3.1%), and Saccharomyces cerevisiae (16/844, 1.9%) (Table 1).
Table 1.
Species distribution of noncandidal yeast isolates causing invasive infections and azole susceptibility of each species
| Species | Total | % | Antifungal susceptibility (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Fluconazole | Voriconazole | |||||||
| S | SDD | R | S | SDD | R | |||
| Cryptococcus spp. | 654 | 77.5 | 73.7 | 16.4 | 9.9 | 99.4 | 0.5 | 0.2 |
| Cryptococcus neoformans | 638 | 76.4 | 74.1 | 16.1 | 9.7 | 99.5 | 0.5 | |
| Cryptococcus gattii | 7 | 0.8 | 57.1 | 28.6 | 14.3 | 100 | ||
| Cryptococcus laurentii | 4 | 0.5 | 50.0 | 25.0 | 25.0 | 100 | ||
| Cryptococcus curvatus | 3 | 0.4 | 66.7 | 33.3 | 100 | |||
| Cryptococcus arboriformis | 1 | 0.1 | 100 | 100 | ||||
| Cryptococcus humicola | 1 | 0.1 | 100 | 100 | ||||
| Trichosporon spp. | 74 | 8.8 | 77.0 | 16.2 | 6.8 | 97.3 | 2.7 | |
| Trichosporon asahii | 58 | 6.9 | 81.0 | 13.8 | 5.2 | 98.3 | 1.7 | |
| Trichosporon mucoides | 3 | 0.4 | 66.7 | 33.3 | 100 | |||
| Trichosporon japonicum | 3 | 0.4 | 33.3 | 33.3 | 33.3 | 66.7 | 33.3 | |
| Trichosporon asteroides | 3 | 0.4 | 100 | 100 | ||||
| Trichosporon inkin | 3 | 0.4 | 33.3 | 33.3 | 33.3 | 100 | ||
| Trichosporon dermatis | 1 | 0.1 | 100 | 100 | ||||
| Trichophyton interdigitale | 1 | 0.1 | 100 | 100 | ||||
| Trichosporon jirovecii | 1 | 0.1 | 100 | 100 | ||||
| Trichosporon montevideense | 1 | 0.1 | 100 | 100 | ||||
| Rhodotorula spp. | 44 | 5.2 | 100 | 100 | ||||
| Rhodotorula mucilaginosa | 43 | 5.1 | 100 | 100 | ||||
| Rhodotorula diobovatum | 1 | 0.1 | 100 | 100 | ||||
| Other yeast spp. | 72 | 8.5 | 51.4 | 23.6 | 25.0 | 95.8 | 2.8 | 1.4 |
| Kodamaea ohmeri | 26 | 3.1 | 38.5 | 42.3 | 19.2 | 100 | ||
| Saccharomyces cerevisiae | 16 | 1.9 | 87.5 | 12.5 | 93.8 | 6.3 | ||
| Dipodascus capitatus | 7 | 0.8 | 71.4 | 28.6 | 100 | |||
| Pichia caribbica | 5 | 0.6 | 20.0 | 60.0 | 20.0 | 80.0 | 20.0 | |
| Arthrographis kalrae | 2 | 0.2 | 100 | 100 | ||||
| Aureobasidium pullulans | 1 | 0.1 | 100 | 100 | ||||
| Cyberlindnera rhodanensis | 1 | 0.1 | 100 | 100 | ||||
| Debaryomyces nepalensis | 1 | 0.1 | 100 | 100 | ||||
| Trichomonascus ciferrii | 1 | 0.1 | 100 | 100 | ||||
| Hanseniaspora opuntiae | 1 | 0.1 | 100 | 100 | ||||
| Kazachstania telluris | 1 | 0.1 | 100 | 100 | ||||
| Pichia fabianii | 1 | 0.1 | 100 | 100 | ||||
| Pichia jadinii | 1 | 0.1 | 100 | 100 | ||||
| Pichia kluyveri | 1 | 0.1 | 100 | 100 | ||||
| Pichia manshurica | 1 | 0.1 | 100 | 100 | ||||
| Pichia sydowiorum | 1 | 0.1 | 100 | 100 | ||||
| Pseudozyma antarctica | 1 | 0.1 | 100 | 100 | ||||
| Pseudozyma spp. | 1 | 0.1 | 100 | 100 | ||||
| Quambalaria cyanescens | 1 | 0.1 | 100 | 100 | ||||
| Rhodosporidiobolus fluvialis | 1 | 0.1 | 100 | 100 | ||||
| Sporidiobolus spp. | 1 | 0.1 | 100 | 100 | ||||
| Total | 844 | 100 | 68.2 | 16.1 | 15.6 | 93.7 | 0.6 | 5.7 |
Note: Bold data represented as summarized data.
Abbreviations: R, resistant; S, susceptible; SDD, susceptible dose-dependent.
Although Cryptococcus spp. was predominant in all seven geographic regions, its frequency varied from 56.3% in northeast China to 89.4% in southwest China (Table 2). Trichosporon spp. was more prevalent in northwest and south China regions (frequency 21.7% and 20.9%, respectively), Rhodotorula spp. was more commonly seen in north and northeast regions (12.5% and 11.3%, respectively), whereas other noncandidal yeast species had a frequency of 16.3% and 15%, respectively, in the south and north China regions (Table 2).
Table 2.
Geographic distribution of noncandidal yeast genera in mainland China
| Geographic region | Number of isolates (%) | |||
|---|---|---|---|---|
| Cryptococcus spp. | Trichosporon spp. | Rhodotorula spp. | Other yeast spp. | |
| East | 230 (82.1) | 15 (5.4) | 13 (4.6) | 22 (0.1) |
| Middle | 80 (81.6) | 8 (8.2) | 1 (1.0) | 9 (0.1) |
| North | 106 (70.7) | 19 (12.7) | 17 (11.3) | 8 (0.1) |
| Northeast | 45 (56.3) | 13 (16.3) | 10 (12.5) | 12 (0.2) |
| Northwest | 16 (69.6) | 5 (21.7) | 0 (0) | 2 (0.1) |
| South | 25 (58.1) | 9 (20.9) | 2 (4.7) | 7 (0.2) |
| Southwest | 152 (89.4) | 5 (2.9) | 1 (0.6) | 12 (0.1) |
Species distribution by hospital service
Overall, only 7.1% (60/844) and 6.8% (57/844) of the noncandidal yeast isolates were collected from emergency departments and outpatient clinics, respectively, and the majority of isolates (727/844, 86.1%) were cultured from patients in inpatient wards.
Of these 727 isolates, 487 (67.0%) were from inpatients in medical wards, 95 (13.1%) from surgical wards, 92 (12.7%) from patients in intensive care units (ICUs), and 53 (7.3%) from other ward types. Of note, the variation in specimen distribution among different inpatient departments largely stemmed from the proportions of the most common organism, C. neoformans, 67.2% (429/638) of which were isolated from medical wards. In comparison, isolate rates of other species from medical, surgical wards, and ICUs exhibited less variation (28%–32.9%).
Species distribution by specimen types
In this study, over 50% of the isolates (433/844) were cultured from cerebrospinal fluid (CSF), followed by blood (31.4%), ascitic fluid (4.1%), pus (3.7%), tissue (3%), venous catheter (2.5%), and pleural fluid (1.9%) (Table 3). The specimen distribution in noncandidal yeast infections was notably different to that in IC, among which blood samples predominated (3,858/8,829 isolates, 43.7% during the same period of time in CHIF-NET), and CSF samples only accounted for <2% (162/8,829) of the collection (Xiao M et al, unpublished data).
Table 3.
Species distribution by specimen types
| Specimen type | Number of isolates (%) | ||||
|---|---|---|---|---|---|
| Total | Cryptococcus spp. | Trichosporon spp. | Rhodotorula spp. | Other yeast spp. | |
| Cerebrospinal fluid | 433 (51.3) | 428 (98.8) | 4 (0.9) | 1 (0.2) | |
| Blood | 265 (31.4) | 169 (63.8) | 28 (10.6) | 34 (12.8) | 34 (12.8) |
| Ascitic fluid | 35 (4.1) | 9 (25.7) | 13 (37.1) | 2 (5.7) | 11 (31.4) |
| Pus | 31 (3.7) | 11 (35.5) | 10 (32.3) | 2 (6.5) | 8 (25.8) |
| Tissue | 25 (3.0) | 21 (84.0) | 1 (4.0) | 3 (12.0) | |
| Venous catheter | 21 (2.5) | 3 (14.3) | 11 (52.4) | 2 (9.5) | 5 (23.8) |
| Pleural fluid | 16 (1.9) | 8 (50.0) | 3 (18.8) | 5 (31.3) | |
| Bronchoalveolar lavage fluid | 5 (0.6) | 2 (40.0) | 2 (40.0) | 1 (20.0) | |
| Hydrarthrosis | 5 (0.6) | 3 (60.0) | 2 (40.0) | ||
| Peritoneal dialysate | 4 (0.5) | 2 (50.0) | 2 (50.0) | ||
| Bone marrow | 3 (0.4) | 2 (66.7) | 1 (33.3) | ||
| Bile | 1 (0.1) | 1 (100) | |||
There was a high frequency of Cryptococcus spp. in CSF samples (428/433 isolates, 98.8%) (Table 3), whereas other species were rarely recovered from CSF (four isolates of T. asahii and one isolate of Sporidiobolus spp.) (Table 3). Cryptococcus spp. were also the most common pathogens identified in blood, pus, tissue, and pleural fluid samples (Table 3). However, non-CSF clinical samples comprised a broader range of noncandidal pathogens, with a total of 19 species identified from blood and 16 and 12 species from ascitic fluid and pus samples, respectively.
Antifungal susceptibilities
Overall, 576/844 (68.2%) isolates were susceptible to fluconazole, and 15.6% of the isolates (132/844) were fluconazole-resistant (Table 1). In comparison, voriconazole exhibited superior activity, with 93.7% (791/844) of the isolates being susceptible to the agent and resistance only occurred in 5.7% (48/844) of the cases (Table 1).
The azole susceptibilities varied between different species and for both the azoles tested. For the most common species, C. neoformans and T. asahii, 74.1% and 81.0% of the isolates were susceptible to fluconazole, respectively, while both species had susceptibility rates of >98% to voriconazole (Table 1). However, all isolates of R. mucilaginosa were cross-resistant to fluconazole and voriconazole (Table 1). Only around half of the other uncommon noncandidal yeast isolates (37/72, 51.4%) were susceptible to fluconazole, and one-fourth (18/72, 25.0%) were fluconazole resistant. However, these species were susceptible to voriconazole (69/72, 95.8%).
Five-year trends
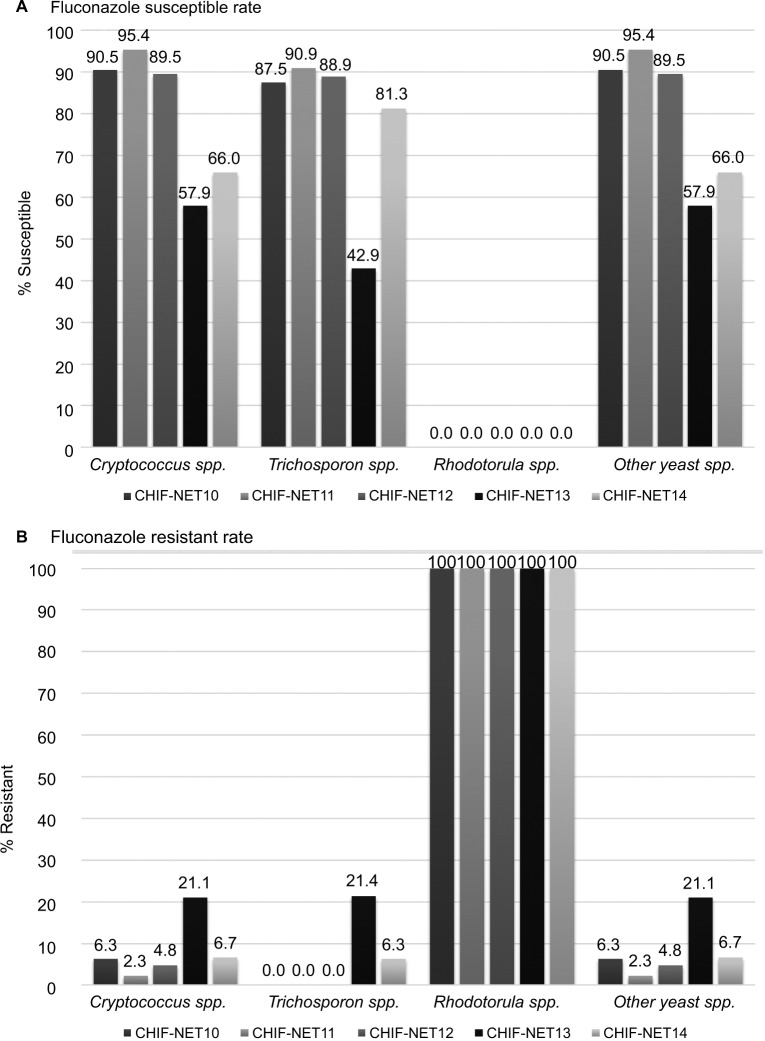
Over 5 years, the frequency of isolation of Cryptococcus spp., Trichosporon spp., and other noncandidal yeast spp. varied between 73.6%–82.1%, 5.8%–11.3%, and 6.6%–10.5%, respectively, with no significant trend. However, Rhodotorula spp. increased significantly from 1.3% in year 1 to 7.0% in year 5 (P<0.001).
Cryptococcus spp. exhibited a significantly decreased fluconazole susceptibility from 90.5% in year 1 to 66.0% in year 5 (P<0.001) (Figure 2). In addition, there were no fluconazole-resistant Trichosporon species strains in years 1–3, but 21.4% of the strains were resistant in year 4, and 6.3% in year 5 (P<0.001) (Figure 2). Other noncandidal species also exhibited decreased susceptibility from 90.5% in year 1 to 66.0% in year 5 (P<0.001) (Figure 2).
Figure 2.
Trends of fluconazole susceptibility over 5 years.
Notes: (A) Trends of fluconazole susceptible rate. (B) Trends of fluconazole resistant rate.
Abbreviation: CHIF-NET, China Hospital Invasive Fungal Surveillance Net.
Discussion
The growing population of immunosuppressed patients and increase in medical interventions have resulted in the rise of invasive fungal infections and emergence of novel opportunistic pathogen species.1,4,5,15 Although epidemiology and antifungal susceptibility data on IC, which account for a large proportion of invasive fungal infections, are well established, knowledge of infections caused by noncandidal yeasts remain limited in China.
Of note, the significance of antifungal resistance is well acknowledged in non-Candida albicans species and increasing trend in species distribution from C. albicans to non-C. albicans species.1,16,17 However, similar issues in noncandidal yeast species have been relatively understudied, despite the fact that noncandidal yeast species may be less susceptible to antifungal drugs.1,8,18,19 One difficulty in assigning susceptibility is the absence of CBPs for noncandidal yeast species based on broth microdilution methods,10,11 with epidemiologic cutoff values (ECVs) only developed for C. neoformans.20 In the CHIF-NET study, CLSI disk diffusion methods were employed, as interpretative criteria have been well studied and verified in the ARTEMIS global surveillance program and provide a less expensive and more flexible antifungal susceptibility testing alternative.6,10,17 Disk diffusion assays have exhibited good correlation with broth microdilution methods.21,22
Of the 844 isolates collected, C. neoformans was the most common organism (>75%), predominating in both CNS (>98%) and bloodstream infections (~63%). In comparison, non-C. neoformans species, including C. gattii, were sporadically discovered (<1%). Although globally Cryptococcus spp. cause infections mainly in HIV/AIDS patients,5 a large proportion of cryptococcal infections occur in non-HIV infected patients in China23 and are predominantly caused by C. neoformans ST5/VNI/α genotype.24 Although as shown in our previous reports, Cryptococcus spp. remained highly susceptible to amphotericin B and 5-flucytosine (>98% of the isolates had wild-type phenotype to these two agents),24 as azoles are still the mainstay of treatment for c ryptococcosis,25,26 the decreasing trend of susceptibility to fluconazole observed in this study is clinically relevant.
Trichosporon spp. was the third most common noncandidal yeast genus reported in the ARTEMIS global study,6 and in this study, the second most common. The genus can been found in the environment and is associated with summer-type hypersensitivity pneumonitis mostly reported in Japan.1,27 Invasive fungal infections caused by Trichosporon spp., particularly fungemia, most commonly affect patients with hematological diseases.1,19 Although the Trichosporon spp. was all formerly classified as Trichosporon beigelii, molecular assays had reclassified the genus, and T. asahii remained most common human pathogenic species.19,27
Rhodotorula species are also emerging opportunistic pathogens, with a higher prevalence in the Asia-pacific regions (17%) than in other regions (5%–14%).6 As found in this study, fungemia is typically the predominant clinical manifestation for Rhodotorula infection,28 although the species can also cause central nervous system infection.29 The major risk factors for Rhodotorula infection include patient immunosuppression and the presence of a central venous catheter.28,29 In addition, the genus is notable because of its intrinsic resistance to both echinocandins, fluconazole, and often to other azoles.1,8,29 Although susceptibility to voriconazole may be variable,1 in this study, all Rhodotorula isolates were cross-resistant to voriconazole. The frequency of Rhodotorula spp., over 95% of which were R. mucilaginosa, significantly increased over 5 years.
Other noncandidal yeast species, although accounting for less than 4% of the collection, also exhibited decreased fluconazole susceptibility (susceptible rate of around 50% overall), but remaining susceptible to voriconazole. There are no robust guidelines to inform antifungal therapy for these infections. Better diagnostics coupled with surveillance data such as that from the CHIF-NET study could benefit selection of initial antifungal therapy.
As a limitation of this study, CBPs used for CLSI disk diffusion testing were not species-specific adjusted for non-candidal yeast species, as previously noted.6 In addition, only two azole agents were studied, as the CLSI disk diffusion methodology was only established for fluconazole and voriconazole when the CHIF-NET study was initiated.6,17 Although many noncandidal yeast species, eg, Cryptococcus, Trichosporon, and Rhodotorula species, are echinocandin-resistant, data on susceptibility profiles to a broad range of antifungal agents remain a guide to antifungal therapy testing for such susceptibility, including the newer azoles, echino-candins, amphotericin, and flucytosine, will be undertaken in the next stage of the CHIF-NET study.
Conclusion
Our surveillance provided accurate epidemiology and robust antifungal susceptibility data on noncandidal yeast causing invasive infections in China, which was useful for guiding the selection of adequate antifungal therapy. In addition, the notable trends of decreased fluconazole susceptibility in noncandidal yeast species warranted further continued surveillance and essential stewardship interventions.
Acknowledgments
China Hospital Invasive Fungal Surveillance Net (CHIF-NET) Study Group (CHIF-NET 2010–2014): list of participated hospitals and co-principle investigators are as follows: 1) Ying-Chun Xu, He Wang, Peking Union Medical College Hospital, Beijing; 2) Mei Kang, Yu-Ling Xiao, West China Hospital of Sichuan University, Chengdu, Sichuan Province; 3) Zi-Yong Sun, Zhong-Ju Chen, Tongji Hospital, Tongji Medical College Huazhong University of Science and Technology, Wuhan, Hubei Province; 4) Kang Liao, Peng-Hao Guo, The First Affiliated Hospital of Zhongshan University, Guangzhou, Guangdong Province; 5) Yan-Ping Luo, Li-Yan Ye, The General Hospital of People’s Liberation Army, Bei-jing; 6) Zhi-Dong Hu, Na Yue, General Hospital of Tianjin Medical University, Tianjin; 7) Ya-Ning Mei, Gen-Yan Liu, Jiangsu Province Hospital, Nanjing, Jiangsu Province; 8) Da-Wen Guo, Shu-Lan Chen, The First Clinic College of Harbin Medical University, Harbin, Heilongjiang Province; 9) Ruo-Yu Li, Zhe Wan, Peking University First Hospital, Beijing; 10) Yu-Hong Pan, Lan-Mei Gao, Fujian Medical University Union Hospital, Fuzhou, Fujian Province; 11) Yun-Zhuo Chu, Fu-Shun Li, First Hospital of China Medical University, Shenyang, Liaoning Province; 12) Yun-Song Yu, Jie Lin, Sir Run Run Shaw Hospital, Hangzhou, Zhejiang Province; 13) Xian-Ju Feng, Hui Xu, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province; 14) Qing Yang, The First Affiliated Hospital of Zhe-jiang University School of Medicine, Hangzhou, Zhejiang Province; 15) Hai-Feng Shao, General Hospital of Nanjing Military Area Command, Nanjing, Jiangsu Province; 16) Wen-En Liu, Hong-Ling Li, Xiangya Hospital Central South University, Changsha, Hunan Province; 17) Huo-Xiang Lv, Qu-Hao Wei, Zhejiang Province People’s Hospital, Hang-zhou, Zhejiang Province; 18) Yong Wang, Yan Jin, Shandong Provincial Hospital, Qingdao, Shandong Province; 19) Li-Wen Liu, The People’s Hospital of Liaoning Province, Shenyang, Liaoning Province; 20) Dan-Hong Su, The First Affiliated Hospital of Guangzhou Medical Collage, Guangzhou, Guangdong Province; 21) Yu-Xing Ni, Shanghai Ruijin Hospital, Shanghai; 22) Gui-Ling Zou, Xue-Fei Du, The Fourth Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang Province; (23) Xin-Lan Hu, Ning Li, Fujian Provincial Hospital, Fuzhou, Fujian Province; 24) Ling Ma, Shuai-Xian Du, Union Hospital Tongji Medical College of Huazhong University of Science and Technology, Wuhan, Hubei Province; 25) Xiu-Lan Song, The First Hospital of Jiaxing, Jiaxing, Zhejiang Province; 26) Hua Yu, Xiang-Ning Huang, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, Chengdu, Sichuan Province; 27) Tie-Li Zhou, Qing Wu, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province; 28) Wei-Jia, Gang Li, The General Hospital Affiliated to Ningxia Medical University, Yinchuan, Ningxia Province; 29) Qiang-Qiang Zhang, Huashan Hospital, Fudan University, Shanghai; 30) Zhi-Jie Zhang, The Second Hospital Affiliated to China Medical University, Shenyang, Liaoning Province; 31) Zhi-Yong Zhang, Southwest Hospital Affiliated to the Third Military Medical University, Chongqing; 32) Rong Zhang, Hong-Wei Zhou, The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, Zhejiang Province; 33) Xiu-Li Xu, Xiao Chen, Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi Province; 34) Li-Ping Zhang, Li Yan, The First Affiliated Hospital of Chongqing Medical Hospital, Chongqing; 35) Xue-Song Xu, Wei Li, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province; 36) Tie-Ying Hou, Li-Yan Zhang, Guangdong Provincial People’s Hospital, Guangzhou, Guangdong Province; 37) Lin-Qiang Deng, Hui Chen, Jiangxi Province People’s Hospital, Nanchang, Jiangxi Province; 38) Ke-Cheng Li, Fei Xia, Third Affiliated Hospital of Wenzhou Medical College, Wenzhou, Zhejiang Province; 39) Wei Song, Yong-Xin Shi, The Affiliated Qin-gdao Municipal Hospital of Qingdao University Medical College, Qingdao, Shandong Province; 40) Yuan-Hong Xu, Ji-Lu Shen, The First Affiliated Hospital of AnHui University of Science And Technology, Hefei, Anhui Province; 41) Xiao-Min Xu, Ningbo Second Hospital, Ningbo, Zhejiang Province; 42) Guo-Xiong Li, Hui Ding, Central Hospital of Lishui City, Lishui, Zhejiang Province; 43) Rong Tang, Xing Ding, Shanghai first People’s Hospital, Shanghai; 44) Jian-Hong Zhao, Dong-Yan Shi, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei Province; 45) Jing Wang, Xiao-Guang Xiao, First Affiliated Hospital of Dalian Medical University, Dalian, Liaoning Province; 46) Ling Meng, Second Affiliated Hospital of Lanzhou University, Lanzhou, Gansu Province; 47) Xiao-Ming Wang, Xu-Feng Ji, The First Hospital of Jilin University, Changchun, Jilin Province; 48) Su-Fei Yu, Chun-Yan Xu, Zhejiang Taizhou Hospital, Taizhou, Zhejiang Province; 49) Qiong Zhang, Ping Ji, The First Hospital of Xinjiang Medical University, Urumchi, Xinjiang Province; 50) Long-Hua Hu, Bai-Ling Zhang, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi Province; 51) Bin Yang, Yu-Lan Lin, First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian Province; 52) Jin-E Lei, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, Shaanxi Province; 53) Hai-Bin Wang, Jing Zhu, First Affiliated Hospital of PLA General Hospital, Beijing; 54) Hong-Jie Liang, The First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi Province; 55) Xiao-Ling Ma, Huai-Wei Lu, Anhui Provincial Hospital, Hefei, Anhui Province; 56) Wen-Cheng Xue, General Hospital of Shenyang Military Area, Shenyang, Liaoning Province; 57) Bin Shan, Yan Du, The First Affiliated Hospital, Kunming Medical University, Kunming, Yunnan Province; 58) Xiang-Yang Chen, People’s Hospital of Zhengzhou, Zhengzhou, Henan Province; 59) Run-Mei Zhang, Jian-Bang Kang, The Second Hospital of Shanxi Medical University, Taiyuan, Shanxi Province; 60) Jian-Lei Zhang, Tianjin First Center Hospital, Tianjin; 61) Wei Cao, The Second Xiangya Hospital of Central South University, Changsha, Hunan Province; 62) Jun-Lin Zhang, Quang Fu, The Affiliated Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia Province; 63) Yan-Ping Fan, Dalian Municipal Central Hospital, Dalian, Liaoning Province; 64) Lian-Hua Wei, Feng-Mei Zou, Gansu Province People’s Hospital, Lanzhou, Gansu Province; and 65) Yan-Yan Guo, Tangshan Gongren Hospital, Tangshan, Hebei Province. This work was supported by a National Natural Science Foundation of China (81572057), a Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2016-I2M-1–014) and a Beijing Innovation Base Cultivation and Development Special Fund (Z171100002217068).
Footnotes
Author contributions
All authors contributed toward data analysis, drafting, and revising the study and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Miceli MH, Díaz JA, Lee SA. Emerging opportunistic yeast infections. Lancet Infect Dis. 2011;11(2):142–151. doi: 10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz S, Kontoyiannis DP, Harrison T, Ruhnke M. Advances in the diagnosis and treatment of fungal infections of the CNS. Lancet Neurol. 2018;17(4):362–372. doi: 10.1016/S1474-4422(18)30030-9. [DOI] [PubMed] [Google Scholar]
- 3.Pande A, Non LR, Romee R, Santos CAQ. Pseudozyma and other non-Candida opportunistic yeast bloodstream infections in a large stem cell transplant center. Transplant Infectious Disease. 2017;19(2):e12664. doi: 10.1111/tid.12664. [DOI] [PubMed] [Google Scholar]
- 4.Kullberg BJ, Arendrup MC. Invasive Candidiasis. N Engl J Med. 2015;373(15):1445–1456. doi: 10.1056/NEJMra1315399. [DOI] [PubMed] [Google Scholar]
- 5.Limper AH, Adenis A, Le T, Harrison TS. Fungal infections in HIV/ AIDS. Lancet Infect Dis. 2017;17(11):e334–e343. doi: 10.1016/S1473-3099(17)30303-1. [DOI] [PubMed] [Google Scholar]
- 6.Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: 10.5-year analysis of susceptibilities of noncandidal yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J Clin Microbiol. 2009;47(1):117–123. doi: 10.1128/JCM.01747-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang H, Xiao M, Chen SC, et al. In vitro susceptibilities of yeast species to fluconazole and voriconazole as determined by the 2010 National China Hospital Invasive Fungal Surveillance Net (CHIF-NET) study. J Clin Microbiol. 2012;50(12):3952–3959. doi: 10.1128/JCM.01130-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spiliopoulou A, Anastassiou ED, Christofidou M. Rhodotorula fungemia of an intensive care unit patient and review of published cases. Mycopathologia. 2012;174(4):301–309. doi: 10.1007/s11046-012-9552-9. [DOI] [PubMed] [Google Scholar]
- 9.Pfaller MA, Diekema DJ. Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol. 2004;42(10):4419–4431. doi: 10.1128/JCM.42.10.4419-4431.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CLSI. M60 . Performance Standards for Antifungal Susceptibility Testing of Yeasts. First Edition. Wayne, PA: Clinical and Laboratory Standards Institute (CLSI); 2018. p. 28. [Google Scholar]
- 11.EUCAST Antifungal Agents, Breakpoint Tables for Interpretation of MICs, Version 8.1. 2017. [Accessed September 13, 2018]. Available from: http://www.eucast.org/clinical_breakpoints/
- 12.Guo F, Yang Y, Kang Y, et al. Invasive candidiasis in intensive care units in China: a multicentre prospective observational study. J Antimicrob Chemother. 2013;68(7):1660–1668. doi: 10.1093/jac/dkt083. [DOI] [PubMed] [Google Scholar]
- 13.CLSI. M44-S3 Zone Diameter Interpretive Standards, Corresponding Minimal Inhibitory Concentration (MIC) Interpretive Breakpoints, and Quality Control Limits for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Third Informational Supplement Wayne, PA: Clinical and Laboratory Standards Institute (CLSI); 2011 [Google Scholar]
- 14.Zhang L, Xiao M, Wang H, et al. Yeast identification algorithm based on use of the Vitek MS system selectively supplemented with ribosomal DNA sequencing: proposal of a reference assay for invasive fungal surveillance programs in China. J Clin Microbiol. 2014;52(2):572–577. doi: 10.1128/JCM.02543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borman AM, Linton CJ, Miles SJ, Johnson EM. Molecular identification of pathogenic fungi. J Antimicrob Chemother. 2008;61(Suppl 1):i7–i12. doi: 10.1093/jac/dkm425. [DOI] [PubMed] [Google Scholar]
- 16.Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The global problem of antifungal resistance: prevalence, mechanisms, and management. Lancet Infect Dis. 2017;17(12):e383–e392. doi: 10.1016/S1473-3099(17)30316-X. [DOI] [PubMed] [Google Scholar]
- 17.Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida Species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. 2010;48(4):1366–1377. doi: 10.1128/JCM.02117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denning DW. Echinocandin antifungal drugs. Lancet. 2003;362(9390):1142–1151. doi: 10.1016/S0140-6736(03)14472-8. [DOI] [PubMed] [Google Scholar]
- 19.de Almeida Júnior JN, Hennequin C. Invasive Trichosporon Infection: a Systematic Review on a Re-emerging Fungal Pathogen. Front Microbiol. 2016;7:1629. doi: 10.3389/fmicb.2016.01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller MA, Castanheira M, Diekema DJ, Messer SA, Jones RN. Wild-type MIC distributions and epidemiologic cutoff values for fluconazole, posaconazole, and voriconazole when testing Cryptococcus neoformans as determined by the CLSI broth microdilution method. Diagn Microbiol Infect Dis. 2011;71(3):252–259. doi: 10.1016/j.diagmicrobio.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Espinel-Ingroff A, Canton E, Gibbs D, Wang A. Correlation of Neo-Sensitabs tablet diffusion assay results on three different agar media with CLSI broth microdilution M27-A2 and disk diffusion M44-A results for testing susceptibilities of Candida spp. and Cryptococcus neoformans to amphotericin B, caspofungin, fluconazole, itraconazole, and voriconazole. J Clin Microbiol. 2007;45(3):858–864. doi: 10.1128/JCM.01900-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao M, Fan X, Chen SC, et al. Antifungal susceptibilities of Candida glabrata species complex, Candida krusei, Candida parapsilosis species complex and Candida tropicalis causing invasive candidiasis in China: 3 year national surveillance. J Antimicrob Chemother. 2015;70(3):802–810. doi: 10.1093/jac/dku460. [DOI] [PubMed] [Google Scholar]
- 23.Feng X, Yao Z, Ren D, Liao W, Wu J. Genotype and mating type analysis of Cryptococcus neoformans and Cryptococcus gattii isolates from China that mainly originated from non-HIV-infected patients. FEMS Yeast Res. 2008;8(6):930–938. doi: 10.1111/j.1567-1364.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- 24.Fan X, Xiao M, Chen S, et al. Predominance of Cryptococcus neoformans vargrubii multilocus sequence type 5 and emergence of isolates with non-wild-type minimum inhibitory concentrations to fluconazole: a multi-centre study in China. Clin Microbiol Infect. 2016;22(10):887.e1–887.e9. doi: 10.1016/j.cmi.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Chen SC, Sorrell TC, Chang CC, Paige EK, Bryant PA, Slavin MA. Consensus guidelines for the treatment of yeast infections in the haematology, oncology and intensive care setting, 2014. Intern Med J. 2014;44(12b):1315–1332. doi: 10.1111/imj.12597. [DOI] [PubMed] [Google Scholar]
- 26.Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O, European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, European Confederation of Medical Mycology ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect. 2014;20(Suppl 3):76–98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- 27.Sugita T, Ikeda R, Nishikawa A. Analysis of Trichosporon isolates obtained from the houses of patients with summer-type hypersensitivity pneumonitis. J Clin Microbiol. 2004;42(12):5467–5471. doi: 10.1128/JCM.42.12.5467-5471.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuon FF, Costa SF. Rhodotorula infection. A systematic review of 128 cases from literature. Rev Iberoam Micol. 2008;25(3):135–140. doi: 10.1016/s1130-1406(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 29.Tsiodras S, Papageorgiou S, Meletiadis J, et al. Rhodotorula mucilaginosa associated meningitis: A subacute entity with high mortality. Case report and review. Med Mycol Case Rep. 2014;6:46–50. doi: 10.1016/j.mmcr.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]