Abstract
Background
Traumatic brain injury (TBI) is a common healthcare problem related to disability. An easy-to-use trauma scoring system informs physicians about the severity of trauma and helps to decide the course of management. The purpose of this study is to use the combination of both physiological and anatomical assessment tools that predict the outcome and develop a new modified prognostic scoring system in TBIs.
Patients and methods
A total of 181 subjects admitted to the emergency department (ED) of Sanglah General Hospital were documented for both Marshall CT scan classification score (MCTC) and Revised Trauma Score (RTS) upon admission. Glasgow Outcome Scale (GOS) was then documented at six months after brain injury. A new Modified Revised Trauma–Marshall score (m-RTS) was developed using statistical analytic methods.
Results
The total sample enrolled for this study was 181 patients. The mean RTS upon admission was 10.2±1.2. Of the 181 subjects, 110 (60.8%) were found to have favorable GOS (GOS score >3). Best Youden’s index results were obtained with any of the RTS of ≤10 with area under receiver operating characteristic (ROC) curve of 0.2542 and with risk ratio of 2.9 (95% CI=1.98−4.28; P=0.001); and Marshall score ≤2 with area under ROC curve of 0.2249 with risk ratio of 3.9 (95% CI=2.52−5.89; P=0.001). The RTS–Marshall combination has higher sensitivity with risk ratio of 4.5 (CI 95%=2.55−8.0; P=0.001) for screening tools of unfavorable outcome. The Pearson’s correlation between RTS and Marshall classification is 0.464 (P<0.001).
Conclusion
Combination of physiological and anatomical score improves the prognostic of outcome in moderate and severe TBI patients, formulated in this accurate, simple, applicable and reliable m-RTS prognostic score model.
Keywords: head injury, CT scan, revised score, prognostic score
Introduction
Traumatic brain injury (TBI) is a major global public health problem. In the USA alone, the Centers for Disease Control and Prevention reports that at least 1.7 million people sustain TBIs each year.1 Out of these, 50,000 will die and many more will join the estimated 5.3 million Americans living with TBI-related disabilities.2 Associated long-term physical, cognitive, and psychological disturbances can affect survivors’ ability to live and work independently.
Trauma is a time-sensitive condition and recognized as one of the main challenges in modern healthcare.3 Easy-to-use trauma scoring systems inform physicians of the severity of the trauma in patients and help them to decide the course of trauma management.4 Scoring systems can also be used for clinical decision-making when a trauma patient has just arrived at the emergency department (ED). Trauma scoring systems can also be used to prepare the patient for surgery.3
Several trauma scoring systems are already in use. One is the Revised Trauma Score (RTS). It uses three specific physiologic parameters: Glasgow Coma Scale (GCS), blood pressure, and respiration rate.3,4
Neuroimaging is essential in defining the anatomical extent of intracranial injury and managing patients with acute brain injury. It is important to understand the critical pathophysiological derangements that happen and that are also responsible for the poor outcome. The Marshall classification of traumatic brain injury (MCTC) is a CT-scan derived metric using only a few features and has been shown to predict outcome in patients with traumatic brain injury. This system was first published in 1992 and remains one of the most commonly used systems for grading acute traumatic brain injury on the basis of CT findings.5,6
The aim of this study was to see if combining both physiological (RTS) and anatomical (MCTC) factors better predict the outcome.
Patients and methods
This is a prospective observational study approved by the Committee of Ethical Study of Sanglah General Hospital in Denpasar, Indonesia. All subjects involved in this study have provided written informed consent signed by their parents, next of kin, or legal guardians.
Subjects were taken from all eligible patients admitted to the ED of Sanglah General Hospital in 2017. Selection criteria include moderate or severe TBI case, aged 18 years old without respiratory distress, cardiologic problems, or multiple trauma upon admission. Both MCTC and RTS were documented during the first 60 minutes after the patient was admitted to ED. The Glasgow Outcome Scale (GOS) was observed six months after the injury took place.
We calculated the predictive power of each RTS, MCTC, and the combination of both to generate the highest Youden’s index. Youden’s index is a single statistic that captures the performance of a dichotomous diagnostic test. Pearson’s correlation was then calculated to determine the correlation between RTS and MCTC. We used logistic regression analysis to determine the independent association of unfavorable outcomes. Analysis was carried out using SPSS statistics 16.0, and a P-value of <0.05 was considered statistically significant.
Results
The total sample size for this study was 181 patients. Main recorded characteristics were age, sex, time of management, intracranial lesion type found in CT scan, type of management chosen, and pupil conditions. Subject characteristics are summarized in Table 1. Mean age was 39.4 years. The diagnosis of TBI included intracranial bleeding (n=57), epidural hematoma (n=41), subdural hematoma (n=48), and diffuse axonal injury (n=17). Twenty patients came with anisocoric pupils, and 21 patients came with late response to light stimuli.
Table 1.
Subject characteristics
| Variables | n=181 |
|---|---|
|
| |
| Age (year), mean±SD | 39.4±18.3 |
| Sex, n (%) | |
| ○ Male | 131 (72.4) |
| ○ Female | 50 (27.6) |
| Intracranial lesion, n (%) | |
| ○ Epidural hematoma | 41 (22.7) |
| ○ Subdural hematoma | 48 (26.5) |
| ○ Intracranial hemorrhage | 57 (31.5) |
| ○ Subarachnoid hemorrhage | 17 (9.4) |
| ○ Diffuse axonal injury | 17 (9.4) |
| ○ Intra ventricular hemorrhage | 1 (0.5) |
| Management, n (%) | |
| ○ Conservative | 91 (50.3) |
| ○ Surgical | 90 (49.7) |
| Diameter of pupils, n (%) | |
| ○ Isochoric | 155 (85.6) |
| ○ Anisochoric | 20 (11.1) |
| ○ Bilateral dilated | 6 (3.3) |
| Pupillary light reflex, n (%) | |
| ○ Response | 151 (83.4) |
| ○ Late response | 21 (11.6) |
| ○ Unresponsive | 9 (5) |
| Mechanism of injury, (%) | |
| ○ Traffic accident | 151 (83.4) |
| ○ Workplace accident nor related to traffic | 11 (6.1) |
| ○ Falls | 9 (4.9) |
| ○ Unknown | 5 (2.8) |
| ○ Others | 5 (2.8) |
Distributions of RTS, MCTC, and GOS assessments on the subjects during the study is shown in Table 2. The mean RTS score upon admission was 10.2±1.2. Of the 181 subjects, 110 (60.8%) were found to have favorable GOS.
Table 2.
RTS, Marshall score, and GOS assessment characteristics
| Variables | n=181 |
|---|---|
|
| |
| GCS, mean±SD | 8.3±2.8 |
| 0 (3) | 13 (7.2%) |
| 1 (4–5) | 29 (16%) |
| 2 (6–8) | 17 (9.4%) |
| 3 (9–12) | 122 (67.4%) |
| Respiratory rate, mean±SD | 21.4±3.5 |
| 3 (>29 x/minute) | 20 (11.1%) |
| 4 (10–29 x/minute) | 161 (88.9%) |
| Systolic blood pressure, mean±SD | 122.3±25.1 |
| 2 (50–75 mmHg) | 4 (2.2%) |
| 3 (76–89 mmHg) | 2 (1.1%) |
| 4 (>89 mmHg) | 175 (96.7%) |
| RTS, mean±SD | 10.2±1.2 |
| Marshall classification CT scan, n (%) | |
| 1 (Non evacuated mass lesion) | 11 (6.1) |
| 2 (Diffuse injury IV/evacuated mass) | 61 (33.7) |
| 3 (Diffuse injury III) | 36 (19.9) |
| 4 (Diffuse injury II) | 60 (33.1) |
| 5 (Diffuse injury I) | 13 (7.2) |
| GOS, n (%) | |
| 1 (Death) | 66 (36.5) |
| 2 (Vegetative state) | 1 (0.6) |
| 3 (Severe disability) | 4 (2.2) |
| 4 (Moderate disability) | 17 (9.4) |
| 5 (Good recovery) | 93 (51.4) |
| GOS, n (%) | |
| Good–moderate (favorable) | 110 (60.8) |
| Severe, vegetative and death (unfavorable) | 71 (39.2) |
Abbreviations: GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; RTS, Revised Trauma Score.
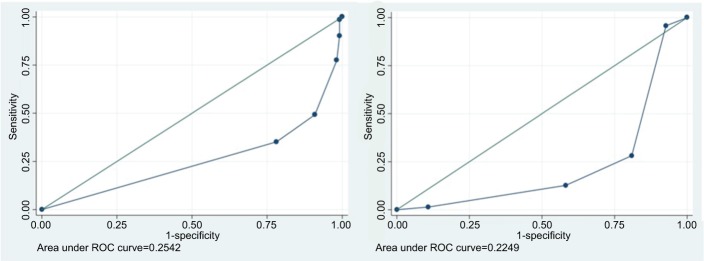
Cut-off values of RTS, Marshall classification, and the combination of both were tested to generate the highest possible Youden’s index. The best result was obtained with any of the RTS of <10 with area under receiver operating characteristic (ROC) curve of 0.2542 with risk ratio of 2.9 (95% CI=1.98–4.28; P=0.001); and MCTC ≤2 with area under ROC curve of 0.2249 with risk ratio of 3.9 (95% CI=2.52–5.89; P=0.001). The RTS–MCTC combination has a higher sensitivity with a risk ratio of 4.5 (CI 95%=2.55–8.0; P=0.001) for screening tools of unfavorable outcome (Figure 1).
Figure 1.
ROC analysis of RTS to GOS (left) and ROC analysis of Marshall score to GOS (right).
Abbreviations: GOS, Glasgow Outcome Scale; RTS, Revised Trauma Score; ROC, receiver operating characteristic.
Table 3 shows that while the combination of RTS and MCTC is superior in risk ratio, sensitivity, and negative predictive value, the MCTC alone is still superior in terms of specificity and negative predictive value (NPV). Combination of physiological and anatomical scores improve the prognostic of outcome and connect the correlation in between.
Table 3.
Sensitivity, specificity, positive predictive value, and negative predictive value of RTS, Marshall score, and RTS–Marshall combination to unfavorable GOS
| RR | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % | |
|---|---|---|---|---|---|---|
|
| ||||||
| RTS | 2.9 | 64.8 | 78.2 | 65.7 | 77.5 | 72.9 |
| Marshall score | 3.9 | 71.8 | 80.9 | 70.8 | 81.7 | 77.3 |
| Combination of both | 4.5 | 84.5 | 64.5 | 60.7 | 86.6 | 72.4 |
Abbreviations: GOS, Glasgow Outcome Scale; NPV, negative predictive value; PPV, positive predictive value; RTS, Revised Trauma Score.
The correlation between RTS and MCTC is shown in Table 4. Pearson’s correlation formula proved that RTS has a positive correlation to MCTC (R=0.464, P<0.001). In the logistic regression model of RTS and MCTC, as shown in Table 5, a formula can be generated by combining both scores into one proposed score to predict the outcome of brain injured patients. The formula stands as:
Table 4.
Pearson’s correlation of RTS to Marshall’s CT scan classification
| Variables | R | P-value |
|---|---|---|
|
| ||
| RTS CT scan Marshall |
0.464 | <0.001 |
Abbreviation: RTS, Revised Trauma Score.
Table 5.
Logistic regression model of RTS-Marshall combination and GOS
| β | 95% CI | P-value | R2 | |
|---|---|---|---|---|
|
| ||||
| RTS | 0.559 | 0.360–0.758 | <0.001 | 33.45% |
| MCTC | 0.600 | 0.383–0.817 | <0.001 | |
| Constant | –4.126 | –6.060–2.191 | <0.001 | |
Abbreviations: GOS, Glasgow Outcome Scale; MCTC, Marshall CT scan classification score; RTS, Revised Trauma Score.
Discussion
A trauma assessment tool provides information on injury severity and is important in determining the management. It attempts to quantitatively assess the severity of the injury and estimate outcome, and is also useful for research purposes. Various predictors have been used to predict the outcome of head injury, e.g. physiological and anatomical abnormalities. A single predictor has been widely used in trauma and provides an overview of the patient’s prognosis.
Previous works have demonstrated that RTS, a physiologic trauma score, is an important factor contributing to outcome and survival. Investigators studying GCS and association of hypoxia and hypotension found an association between poor outcomes. GCS can also predict functional recovery in patients with TBI outcome.7 Low GCS is a risk factor that almost always occurs in studies, indicating the significance of it to the outcome of brain-injured patients.8–15
One of the central aspects of our current understanding of the pathophysiology of TBI is that the extent of the neurological injury is not solely determined by the traumatic impact itself, but rather evolves over time. This so-called “secondary brain injury” occurs as a consequence of complicating processes initiated by the primary injury and is characterized by neuroinflammation, ischemia/reperfusion injuries, cerebral edema, intracranial hemorrhage, and intracranial hypertension.16 Those patients who survived the initial trauma are highly susceptible to secondary insults to the injured brain, mainly due to hypoxia and hypotension during the early resuscitative period.17 A myriad of data from retrospective studies and prospective clinical trials in the past three decades have unequivocally determined these two parameters as independent early predictors of adverse outcome after TBI.18
CT examination remains the investigation of choice to identify the presence and extent of structural damage in the acute phase of TBI. It is of great value in the assessment of gross pathological findings at the time of injury. This has led to a better understanding of the mechanism of head injury and has significantly improved clinical care, reducing both morbidity and mortality.19,20
A number of other CT classifications do exist,21,22 but none of these has been as extensively used as MCTC. International guidelines on prognosis include this classification as a major predictor of outcome based on class I evidence. Previous studies found that making greater use of individual CT characteristics allowed them to improve on the already sizeable predictive value of the original MCTC classification scheme.23,24 These findings need to be validated through further prospective investigations.
Predicting TBI outcome is a problem for all healthcare professionals working in this field. The most frequently asked questions by patients and their families within the first few days after a traumatic event are mostly about mortality, morbidity, and prospects for short and long term recovery. Most healthcare professionals are unable to accurately predict the prognosis since recovery is quite variable. These results may be useful to better predict the outcome and to make an appropriate plan of care.
Conclusion
The combination of RTS and MCTC as a prognostic scoring in moderate and severe TBIs is simple to use, includes minimal necessities of neurological evaluation in impaired consciousness, and can be used to calculate with improved accuracy and reliability. Further studies with a larger number of samples are needed to interpret the result of this new proposed formula.
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Faul M, Coronado V. Epidemiology of traumatic brain injury. Hand Clin Neurol. 2015;127:3–13. doi: 10.1016/B978-0-444-52892-6.00001-5. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kondo Y, Abe T, Kohshi K, Tokuda Y, Cook EF, Kukita I. Revised trauma scoring system to predict in-hospital mortality in the emergency department: Glasgow Coma Scale, Age, and Systolic Blood Pressure score. Crit Care. 2011;15(4):R191. doi: 10.1186/cc10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefering R. Development and validation of the revised injury severity classification score for severely injured patients. Eur J Trauma Emerg Surg. 2009;35(5):437–447. doi: 10.1007/s00068-009-9122-0. [DOI] [PubMed] [Google Scholar]
- 5.Smits M, Dippel DW, de Haan GG, et al. Minor head injury: guidelines for the use of CT-a multicenter validation study. Radiology. 2007;245(3):831–838. doi: 10.1148/radiol.2452061509. [DOI] [PubMed] [Google Scholar]
- 6.Stiell IG, Wells GA, Vandemheen K, et al. The Canadian CT Head Rule for patients with minor head injury. Lancet. 2001;357(9266):1391–1396. doi: 10.1016/s0140-6736(00)04561-x. [DOI] [PubMed] [Google Scholar]
- 7.Moppett IK. Traumatic brain injury: assessment, resuscitation and early management. Br J Anaesth. 2007;99(1):18–31. doi: 10.1093/bja/aem128. [DOI] [PubMed] [Google Scholar]
- 8.Hsiang JN. High-risk mild head injury. J Long Term Eff Med Implants. 2005;15(2):153–160. doi: 10.1615/jlongtermeffmedimplants.v15.i2.40. [DOI] [PubMed] [Google Scholar]
- 9.Oertel M, Kelly DF, Mcarthur D, et al. Progressive hemorrhage after head trauma: predictors and consequences of the evolving injury. J Neurosurg. 2002;96(1):109–116. doi: 10.3171/jns.2002.96.1.0109. [DOI] [PubMed] [Google Scholar]
- 10.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 11.Mizraji R, Perez-Protto S, Etchegaray A, et al. Brain death epidemiology in Uruguay and utilization of the Glasgow coma score in acute brain injured patients as a predictor of brain death. Transplant Proc. 2009;41(8):3489–3491. doi: 10.1016/j.transproceed.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Petroni G, Quaglino M, Lujan S, et al. Early prognosis of severe traumatic brain injury in an urban argentinian trauma center. J Trauma. 2010;68(3):564–570. doi: 10.1097/TA.0b013e3181ce1eed. [DOI] [PubMed] [Google Scholar]
- 13.Ting HW, Chen MS, Hsieh YC, Chan CL. Good mortality prediction by Glasgow Coma Scale for neurosurgical patients. J Chin Med Assoc. 2010;73(3):139–143. doi: 10.1016/S1726-4901(10)70028-9. [DOI] [PubMed] [Google Scholar]
- 14.Saini NS, Rampal V, Dewan Y, Grewal SS. Factors predicting outcome in patients with severe head injury: Multivariate analysis. Indian J Neurotrauma. 2012;9(1):45–48. [Google Scholar]
- 15.Golden N, Niryana W, Mahadewa TGB, Maliawan S, Chandra A. Two Different Approaches in Obtaining Head Computerized Tomography Scan in Minor Head Injuries. J Neurol Res. 2013;3(3-4):114–121. [Google Scholar]
- 16.Schmidt OI, Heyde CE, Ertel W, Stahel PF. Closed head injury--an inflammatory disease? Brain Res Brain Res Rev. 2005;48(2):388–399. doi: 10.1016/j.brainresrev.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Chesnut RM. Secondary brain insults after head injury: clinical perspectives. New Horiz. 1995;3(3):366–375. [PubMed] [Google Scholar]
- 18.Struchen MA, Hannay HJ, Contant CF, Robertson CS. The relation between acute physiological variables and outcome on the Glasgow Outcome Scale and Disability Rating Scale following severe traumatic brain injury. J Neurotrauma. 2001;18(2):115–125. doi: 10.1089/08977150150502569. [DOI] [PubMed] [Google Scholar]
- 19.Teasdale G, Galbraith S, Murray L, et al. Management of traumatic intracranial haematoma. Br Med J. 1982;285(6356):1695–1697. doi: 10.1136/bmj.285.6356.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siegel S, Castellan NJ. Nonparametric statistics of the Behavioral science. 2nd ed. New York: McGraw-Hill; 1988. [Google Scholar]
- 21.Azian AA, Nurulazman AA, Shuaib L, et al. Computed tomography of the brain in predicting outcome of traumatic intracranial haemorrhage in Malaysian patients. Acta Neurochir. 2001;143(7):711–720. doi: 10.1007/s007010170051. [DOI] [PubMed] [Google Scholar]
- 22.Teasdale G, Teasdale E, Hadley D. Computed tomographic and magnetic resonance imaging classification of head injury. J Neurotrauma. 1992;9(Suppl 1):S249–S257. [PubMed] [Google Scholar]
- 23.Maas AI, Steyerberg EW, Butcher I, et al. Prognostic value of computerized tomography scan characteristics in traumatic brain injury: results from the IMPACT study. J Neurotrauma. 2007;24(2):303–314. doi: 10.1089/neu.2006.0033. [DOI] [PubMed] [Google Scholar]
- 24.Zhu GW, Wang F, Liu WG. Classification and prediction of outcome in traumatic brain injury based on computed tomographic imaging. J Int Med Res. 2009;37(4):983–995. doi: 10.1177/147323000903700402. [DOI] [PubMed] [Google Scholar]