miR156f modulates rice plant architecture by direct binding to the OsGH3.8 promoter through its target OsSPL7, to allow crosstalk with the auxin signaling pathway.
Keywords: Auxin, miR156f, Oryza sativa, OsGH3.8, OsSPL7, plant architecture, tiller
Abstract
Tiller number and plant height are two of the main features of plant architecture that directly influence rice yield. Auxin and miR156, an extensively studied small RNA (smRNA), are both broadly involved in plant development and physiology, suggesting a possible relationship between the two. In this study, we identified a rice T-DNA insertion cluster and dwarf (cd) mutant that has an increased tiller number and reduced plant height. The T-DNA insertion was in close proximity to the miR156f gene and was associated with its up-regulation. Plants overexpressing miR156f resembled the cd mutant. In contrast, plants overexpressing an miR156f target mimic (MIM156fOE) had a reduced tiller number and increased height. Genetic analysis showed that OsSPL7 is a target of miR156f that regulates plant architecture. Plants overexpressing OsSPL7 had a reduced tiller number, while OsSPL7 RNAi plants had an increased tiller number and a reduced height. We also found that OsSPL7 binds directly to the OsGH3.8 promoter to regulate its transcription. Overexpression of OsGH3.8 and OsGH3.8 RNAi partially complemented the MIM156fOE and cd mutant phenotypes, respectively. Our combined data show that the miR156f–OsSPL7–OsGH3.8 pathway regulates tiller number and plant height in rice, and this pathway may allow crosstalk between miR156 and auxin.
Introduction
Rice (Oryza sativa) is a staple food for nearly half of the world’s population. To address the imbalance between increasing population demands and decreasing availability of arable land, many breeding programs focus on improving grain yield and plant architecture to increase productivity. Tiller number and plant height are two important agronomic traits that directly contribute to plant architecture (Zhang, 2007).
Auxin was the first plant hormone to be identified, and is involved in an extraordinarily broad spectrum of developmental and physiological processes (Sauer et al., 2013). Examples of these include axillary bud dormancy, ectopic shoot development, and leaf angle (Yoshida et al., 2012; Zhao et al., 2013). Because of the universal role of auxin and its rapid action, auxin levels are tightly controlled by the concerted co-operation of biosynthesis, metabolism, and transport in plants. In Arabidopsis, nearly 95% of auxins exist as amino acid and protein conjugates (Ljung et al., 2002; Woodward and Bartel, 2005). Some of these molecules act as intermediates for degradation, and some provide a reusable metabolite pool for free indole-3-acetic acid (IAA) (LeClere et al., 2002; Rampey et al., 2004). Auxin rapidly and transiently induces the accumulation of at least three families of transcripts: SMALL AUXIN-UP RNAs (SAURs), GH3-related transcripts, and AUXIN/IAA (Aux/IAA) family members (Woodward and Bartel, 2005). The GH3 family of acyl-acid-amido synthetases catalyzes the ATP-dependent formation of amino acid conjugates with plant hormones, including auxins and jasmonates (Chen et al., 2010). This family is present in various plant species, and includes 19 members in Arabidopsis and 13 in rice (Staswick et al., 2002; Terol et al., 2006; Jain and Khurana, 2009). GH3 enzymes are grouped into three families according to their sequence homology and acyl-acid substrate preference. Two of these families are present in rice: class I and II, which mediate jasmonate and salicylic acid conjugation, respectively (Staswick et al., 2002; Westfall et al., 2010).
Small RNAs (smRNAs) are non-coding RNAs of ~22 nucleotides (nt) in length. There are two broad categories of smRNA, miRNAs and siRNAs (Aravin et al., 2003; Bartel and Bartel, 2003; Bartel, 2004). We have witnessed an explosion in our understanding of smRNAs across different species. Improved understanding of the mechanisms by which smRNAs interact with their target RNAs and the important roles of smRNA in various developmental and physiological processes have revealed that smRNAs are simple in their core mechanism but have profound functions (Nelson et al., 2003; Bartel, 2009). Moreover, the sequences, mechanisms, and functions of miRNAs are conserved across species (Jones-Rhoades, 2012).
Plant miRNAs target key developmental regulators, many of which are transcription factors (Rhoades et al., 2002; Jones-Rhoades et al., 2006). Among them, miR156 has a wide scope (Wang and Wang, 2015) that includes transition from vegetative to reproductive growth and flowering time (Wu et al., 2009; Huijser and Schmid, 2011; Wang, 2014; S. Yu et al., 2015), embryo patterning (Nodine and Bartel, 2010), gibberellin-mediated flowering (Yu et al., 2012), age-dependent flowering (Bergonzi et al., 2013), gynoecium differential patterning (Xing et al., 2013), leaf development (Xie et al., 2012), trichrome distribution (Xue et al., 2014), lateral root development (N. Yu et al., 2015), plant architecture (Bhogale et al., 2014; Chen et al., 2015), biosynthesis of secondary metabolites such as anthocyanin and sesquiterpene (Gou et al., 2011; Z.X. Yu et al., 2015), ovary and fruit development (Ferreira e Silva et al., 2014), and developmental timing (Cho et al., 2012). In rice, ectopic expression of miR156b and miR156h results in dwarfism, a small panicle, and delayed flowering (Xie et al., 2006). Together with miR529 and miR172, miR156 regulates vegetative and reproductive branching (Wang et al., 2015). One target of miR156, SQUAMOSA PROMOTER BINDING PROTEIN-like14 (SPL14), regulates optimal plant architecture and has great potential for improving rice yield (Jiao et al., 2010; Miura et al., 2010; Springer, 2010). Another target, OsSPL16, regulates rice seed size and quality (Wang et al., 2012). Furthermore, miR156 responds to various abiotic and nutritional stresses (Jia et al., 2010; Zhou et al., 2010; Nischal et al., 2012; Wang et al., 2013; Cui et al., 2014; Stief et al., 2014; Song et al., 2015).
The functional activity and universality of both miR156 and auxin led to the hypothesis that there is crosstalk between them in regulating plant architecture. In this study, we identified a cluster and dwarf (cd) mutant that had underlying up-regulation of miR156f. Genetic analysis of miR156f and the OsSPL7 gene showed that the miR156f/OsSPL7 module regulates plant architecture, with ectopic expression of miR156f and down-regulation of OsSPL7 leading to plants with an increased number of tillers and reduced height. Moreover, ectopic expression of an miR156f target mimic (MIM156f) and up-regulation of OsSPL7 dramatically reduced the number of tillers. Further biochemical and genetic analyses confirmed that OsSPL7 directly regulates transcription of the OsGH3.8 gene and the involvement of auxin in regulating plant architecture through the miR156f/OsSPL7 pathway. Our findings may provide a useful breeding resource for modifying rice plant architecture and enhancing rice yield.
Materials and methods
Plant species and growth conditions
The rice cd mutant was selected from a T-DNA insertion collection generated from the wild type (WT) Zhonghua 11 (Orayza sativa L. subsp. japonica cv. Zhonghua No. 11, ZH11) (Wang et al., 2004). All the rice plants were grown in a greenhouse, with 10 h light and 14 h dark, or in the field under natural conditions in summer, Shanghai, China.
Tiller outgrowth analysis
Four-week-old rice seedlings were used for analysis; plants were peeled carefully to expose the young tiller buds in the basal region where tillers begin to develop.
Treatment of rice plants with naphthaleneacetic acid (NAA)
Four-week-old WT rice seedlings were watered with 10 μM NAA, and samples were collected at the indicated time after treatment.
Expression profile analysis
For expression profile analysis of miR156f, and some OsSPL genes, 1-month-old WT plants were used to collect leaf, leaf sheath, roots, and stems; and young panicles were collected at the reproductive stage from the WT.
Anatomical analysis and in situ hybridization
Anatomical analysis and in situ hybridization were carried out as previously described by Wang et al. (2010) and Dai et al. (2016), respectively.
Plasmid construction and transformation of rice
The full-length IPS gene of Arabidopsis was amplified by KOD-plus DNA polymerase (TOYOBO) using primers IPSF and IPSR, cloned into the PBSK vector using BamHI and KpnI, and the mature miRNA region was substituted into the MIM156f sequence using overlapping PCR (Higuchi et al., 1988); IPSF was paired with MIM156R and IPSR with MIM156F to get the substituted sequence into two separate but overlapping PCR fragments, and then used as template. IPSF and IPSR were used as primers to amplify the full-length IPS-MIM156f fragment. The IPS-MIM156f fragment was re-cloned into p1301-35SNos vector to form the MIM156fOE vector.
OsSPL7, OsSPL2, OsSPL13, and OsGH3.8 overexpression (OE) vectors were constructed by cloning full-length cDNA of respective genes into the p1301-35SNos vector using BamHI and KpnI double digestion.
RNAi of OsSPL7 (SPL7RNAi) and OsGH3.8 (GH3.8R) were constructed by cloning a 466 bp and a 273 bp cDNA fragment, respectively, into p1301RNAi vector in the sense orientation using BamHI and KpnI, and in the antisense orientation using SacI and SpeI.
OsSPL7::SPL7Flag (SPL7Flag) vector was constructed by cloning the full-length cDNA of OsSPL7 in-frame into the p1305 vector using KpnI and BamHI, and the OsSPL7 promoter was further cloned using SacI and KpnI.
SPL7::GUS plasmid was constructed by amplifying the OsSPL7 promoter using primers SPL7PF2 and SPL7PR and cloned into the promoter::GUS plasmid derived from pCAMBIA1301.
MIM156fOE, SPL2OE, SPL7OE, SPL13OE, SPL7RNAi, GH3.8R, SPL7::GUS, and OsSPL7::SPL7Flag plasmid were transformed into WT rice using the Agrobacterium-mediated method with minor modification (Hiei et al., 1994).
RT–PCR analysis and quantitative real-time RT-PCR (qRT-PCR)
Total RNA was extracted from different tissues using TRIzol (Invitrogen), followed by DNase I digestion. For reverse transcription–PCR (RT–PCR) analysis, 1 μg of total RNA was reverse transcribed using oligo(dT) primer and M-MLV reverse transcriptase (TOYOBO) according to the manufacturer’s instructions. RT–PCR analyses were performed at least three times with the actin gene as internal control.
For quantitative real-time PCR (qRT-PCR), cDNA was synthesized from 1 μg of total RNA using the One Step SYBR PrimeScript RT-PCR Kit (TaKaRa), and 1 μl of cDNA was used as template for real-time analysis. Sampling and expression measurement were repeated three times with the actin gene as internal reference.
Yeast-one-hybrid assays
The full-length cDNAs of OsSPL2, OsSPL7, and OsSPL13 were amplified with gene-specific primers (see Supplementary Table S1 at JXB online), and then fused into the activation domain (AD) in vector pPC86. Fragments containing ‘GTAC’ in the OsGH3.8 promoter were amplified with gene-specific primers and fused into the vector p178 at the XhoI site. The p178 and pPC86 constructs were co-transformed into the yeast strain EGY48. The yeast was growth on SD selective medium (SD-His-Leu) and observed in blue on Chromogenic medium. The transformants containing void plasmid pPC86 and p178 constructs were used as negative control. Yeast one-hybrid assay was carried out as described (Matchmaker One-hybrid System; Clontech).
miRNA northern blot analysis
Approximately 30 μg of total RNA was separated on 15% polyacrylamide denaturing gels. RNAs were transferred to Amersham Hybond™-N+ membranes and cross-linked by UV irradiation; the membranes were hybridized with biotin-labeled DNA probes complementary to the miRNA sequences at 42 °C overnight. The membranes were then washed and incubated with stabilized streptavidin–horseradish peroxidase at 42 °C. After washing with substrate equilibration buffer and adding stable peroxide solution and enhancer solution, the membranes were imaged using an FLA-5000 Phosphorimager. The DNA probes were synthesized and biotin labeled using a 3′ end DNA labeling method.
ChIP analysis
Immunoprecipitation of DNA associated with modified histones was carried out according to the instructions of the EpiQuik™ Plant ChIP Kit. Rice young panicles were cross-linked, and Flag antibody was used for ChIP, with normal mouse IgG as the negative control. The immunoprecipitated sample and whole-cell extract (input) were incubated at 65 °C to reverse cross-linked DNA, and ethanol precipitation to elute purified DNA. ChIP DNA and whole-cell extract (input) were subjected to 35 cycles of qRT-PCR with the primers designed to amplify a sequence in the promoter, with a sequence in the coding region as the control.
Dual luciferase (LUC) analysis
The plasmid pHB::SPL7-GFP was transformed into Agrobacterium tumefaciens strain GV3101 to act as an effector. The reporter construct was constructed by inserting the OsGH3.8 promoter into the pGreenII 0800-LUC vector (Hellens et al., 2005) and subsequently co-transformed with the helper plasmid pSoup19 into GV3101 to act as the reporter. pHB::GFP plasmid was used as negative control. Overnight A. tumefaciens cultures were collected by centrifugation, re-suspended in Murashige and Skoog (MS) medium to OD600=0.6, and incubated at room temperature for 3 h. The reporter strain was mixed with the effectors strain harboring pHB::SPL7-GFP or pHB::GFP at a ratio of 1:1. The mixture of A. tumefaciens suspension was infiltrated into tobacco (Nicotiana benthamiana) leaves, with the experimental group and the control group infiltrated into the opposite position on the same leaves. The leaves were collected after 3 d under long-day white light conditions and infiltrated with 150 μg ml–1 luciferin solution; images were captured using a CCD camera 5 min later, and quantification was performed using a Dual-Luciferase Reporter Assay System according to the instructions (Promega, Madison, WI, USA). Three biological repeats were measured for each sample.
Histochemical GUS staining
β-Glucuronidase (GUS) staining was carried out by staining different tissues of the SPL7::GUS transgenic plants in GUS reaction solution which contained 100 mM sodium phosphate, 10 mM EDTA, 0.1% (v/v) Triton X-100, and 1 mM 5-bromo-4-chloro-3-indolyl-β-glucuronic acid (Sigma. USA) with pH 7.0, overnight at 37 °C, and then clearing in 75% ethanol.
Microarray analysis
Seedlings of the 4-month old cd mutant and WT were used for microarray analysis. Ten plants for each sample were collected to extract total RNA and sent to the Beijing Genomics Institute for the following tests and analysis. RNA quality was checked using a NanoPhotometer® spectrophotometer (IMPLEN, CA, USA). A 3 μg aliquot of total RNA per sample was used as input material for the RNA library construction with NEBNext® Multiplex RNA Library Prep Set for Illumina® (NEB, USA) following the manufacturer’s recommendations. After cluster generation, the library preparations were sequenced on an Illumina Hiseq2000 Platform and the clean reads were mapped to the O. sativa genome (RGAP, version 7.0) using cufflink software after filtering the dirty raw reads.
Subcellular localization of OsSPL7 protein in rice protoplasts
The pA7-EGFP vector was used to construct green fluorescent protein (GFP) fusion proteins or as a negative control. The coding sequence of OsSPL7 was cloned into pA7-EGFP to generate OsSPL7–GFP. The mCherry–VirD2NLS (mCherry) vector was used as nuclear control. The fusion constructs or control plasmids were transformed into rice protoplasts. Fluorescence was visualized under a Zeiss Axioimager Z2 fluorescence microscope. Experiments were biologically repeated three times.
Accession numbers
OsSPL2 (LOC_Os01g69830), OsSPL3 (LOC_Os02g04680), OsSPL4 (LOC_Os02g07780), OsSPL7 (LOC_Os04g46580), OsSPL11 (LOC_Os06g45310), OsSPL12 (LOC_Os06g49010), OsSPL13 (LOC_Os07g32170), OsSPL14 (LOC_Os08g39890), OsSPL16 (LOC_Os08g41940), OsSPL17 (LOC_Os09g31438), OsSPL18 (LOC_Os09g32944), OsSPL19 (LOC_Os11g30370), OsGH3.1 (AK063368), OsGH3.2 (LOC-Os01g55940), OsGH3.3 (AK072125), OsGH3.4 (AK101932), OsGH3.5 (AK071721), OsGH3.6 (AK106538), OsGH3.7 (AK099376), OsGH3.8 (AK101193), OsGH3.9 (AK106839), OsGH3.10 (LOC-Os07g38860), OsGH3.11 (LOC-Os07g47490), OsGH3.12 (LOC-Os11g08340), OsGH3.13 (LOC_Os11g32510), OsGH3.14 (LOC_Os11g32520).
Results
A T-DNA insertion mutant with a cluster and dwarf phenotype is associated with overexpression of miR156f
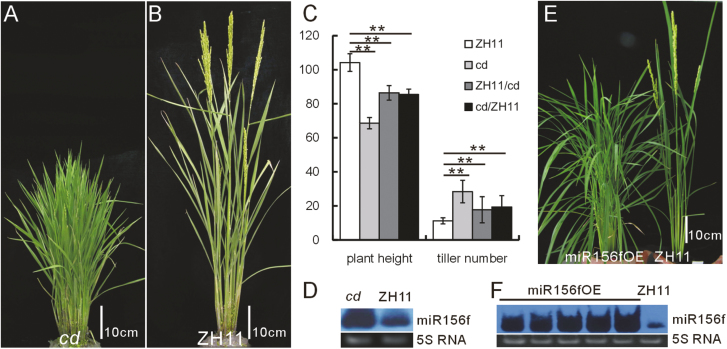
We identified a T-DNA insertion mutant from our mutant population that showed an obvious cluster (increased number of tillers) and a dwarf (decreased plant height) phenotype (Wang et al., 2004), and named it cd (cd is shown in Fig. 1A and compared with the WT in Fig. 1B). Under natural field conditions in summer in Shanghai, the cd mutant had an average plant height of 62.7 cm, in comparison with the WT plants which were on average 94.9 cm tall. The cd mutant had an average of 42.1 effective tillers, while the WT plants had 14.1 (Fig. 1C). The cd mutant also flowered ~5–6 d later than the WT plants (data not shown).
Fig. 1.
Characterization of the cd mutant phenotype and association of miR156f expression with the cluster and dwarf phenotype. (A and B) Representative cd mutant (A) and WT (B) plants. (C) Plant heights and tiller numbers of the cd mutant, and the crosses between the cd mutant and WT. The asterisks represent a significant difference, as determined by a Student’s t-test at *P<0.05 and **P<0.01, respectively (n=20). (D) miR156f expression in the cd mutant. (E) Phenotype of the miR156fOE transgenic plants (left) as compared with the WT (right). (F) miR156f expression in the miR156fOE lines.
We next carried out genetic analysis by making genetic reciprocal crosses of the cd mutant with the WT. The F1 plants from both the reciprocal cross and backcross had semi-cluster and semi-dwarf phenotypes (Fig. 1C). This suggests that the phenotype of the cd mutant is semi-dominant. Among the 691 F2 population of the cd/ZH11 cross, there were 170 taller plants, 352 average height plants, and 169 dwarf plants. The average height plants and dwarf plants were resistant to hygromycin. Among the F2 population of the ZH11/cd cross, the ratio of taller to average height to dwarf plants was 211:392:189. Therefore, the segregation ratios of both the reciprocal cross and the backcross were ~1:2:1, which is in accordance with Mendelian segregation. Furthermore, in the F2 population, both the cluster and dwarf phenotypes showed tight co-segregation with the T-DNA insert (Supplementary Table S2). From our results, we concluded that the cluster and dwarf phenotype in the cd mutant was controlled by a single semi-dominant genetic locus.
Next, we isolated the T-DNA flanking sequence in the cd mutant using thermal asymmetric interlaced PCR (TAIL-PCR) (Liu et al., 1995). This revealed that the T-DNA was integrated at position 21575719 of NC-008401.2 (http://www.ncbi.nlm.nih.gov/, accessed 27 July 2018), and that the miR156f gene is positioned ~9 kb upstream of the insertion. miRNA northern blot analysis showed that miR156f was markedly up-regulated in the cd mutant (Fig. 1D).
To test whether up-regulation of miR156 caused the cluster and dwarf phenotype, we cloned the miR156f gene and genetically overexpressed it. More than 90% of the transgenic plants (miR156fOE) had a cluster and dwarf phenotype that was similar to that of the cd mutant (Fig. 1E). We sampled five T0 plants and confirmed miR156f overexpression in all of them (Fig. 1F). Together, these results confirm that the T-DNA insertion in the cd mutant causes up-regulation of miR156f, which leads to the cluster and dwarf phenotype.
Plants overexpressing an miR156f target mimic (MIM156fOE) have fewer tillers and increased plant height
To better understand the function of miR156f in rice, we constructed a target mimic of miR156f (MIM156f) and genetically overexpressed it in WT plants (Franco-Zorrilla et al., 2007).
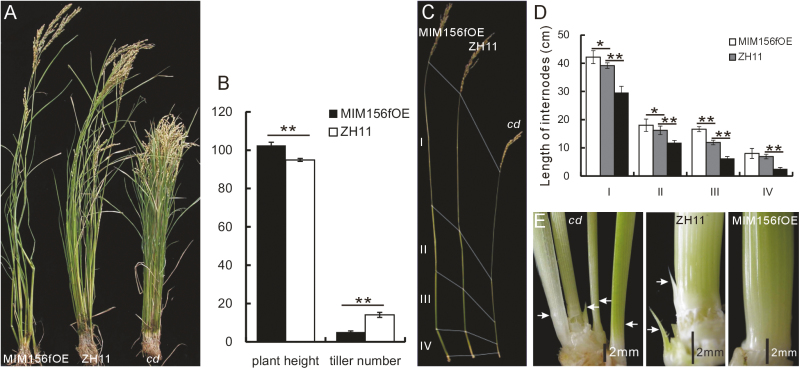
Approximately 91.2% of the transgenic plants (MIM156fOE) had a reduced number of tillers and an increased plant height (Fig. 2A). MIM156f overexpression was confirmed in the transgenic plants, while endogenous miR156f was down-regulated (Supplementary Fig. S1A). Expression of OsSPL13, a candidate target of miR156, was up-regulated (Supplementary Fig. S1A). These results suggest that MIM156f expression interferes with the expression of endogenous miR156 and its targets.
Fig. 2.
Phenotype of the MIM156f OE plants. (A) The appearances of the MIM156fOE, WT, and cd mutant plants (left to right). (B) The plant heights and tiller numbers of the MIM156fOE plants in comparison with the WT plants (n=20). (C) Appearance of the stems of the MIM156fOE, WT, and cd mutant plants, showing the numbered internodes I, II, III, IV. (D) Lengths of each internode in the MIM156fOE, WT, and cd mutant plants (n=10). The asterisks in (B) and (D) represent a significant difference, as determined by a Student’s t-test at *P<0.05 and **P<0.01, respectively. (E) The basal region of the stems of the cd mutant, WT, and MIM156fOE plants (left to right).
The T1 generation of MIM156fOE plants were grown in the field and analyzed. The MIM156fOE plants had an average of 5.1 tillers, in comparison with 14.1 tillers in the WT plants. The MIM156fOE plants had an average height of 102.5 cm, while the average height of the WT plants was 94.9 cm (Fig. 2B). Further analysis revealed that miR156 expression influenced the elongation of the internodes in rice plants. The length of almost every internode was increased in the MIM156fOE plants, while it was reduced in the cd mutant. These changes in internode length influenced the overall plant height accordingly (Fig. 2C, D).
Development of tillers in rice involves two successive processes: the formation of an axillary bud at the basal region of the stem, and subsequent outgrowth. A defect in either process can influence the number of tillers (Li et al., 2003). We examined the role of miR156f at the tiller initiation stage, and found that there were fewer axillary buds at the basal region of the stem in young MIM156fOE plants, in comparison with the WT (Fig. 2E). In contrast, there were more axillary buds in the cd mutant in comparison with the WT (Fig. 2E). These data suggest that miR156f regulates the initiation of the axillary buds. Our combined data show that miR156f regulates tiller number and plant height in rice.
Transgenic plants overexpressing OsSPL7 have a single tiller and OsSPL7 RNAi plants have a cluster and dwarf phenotype
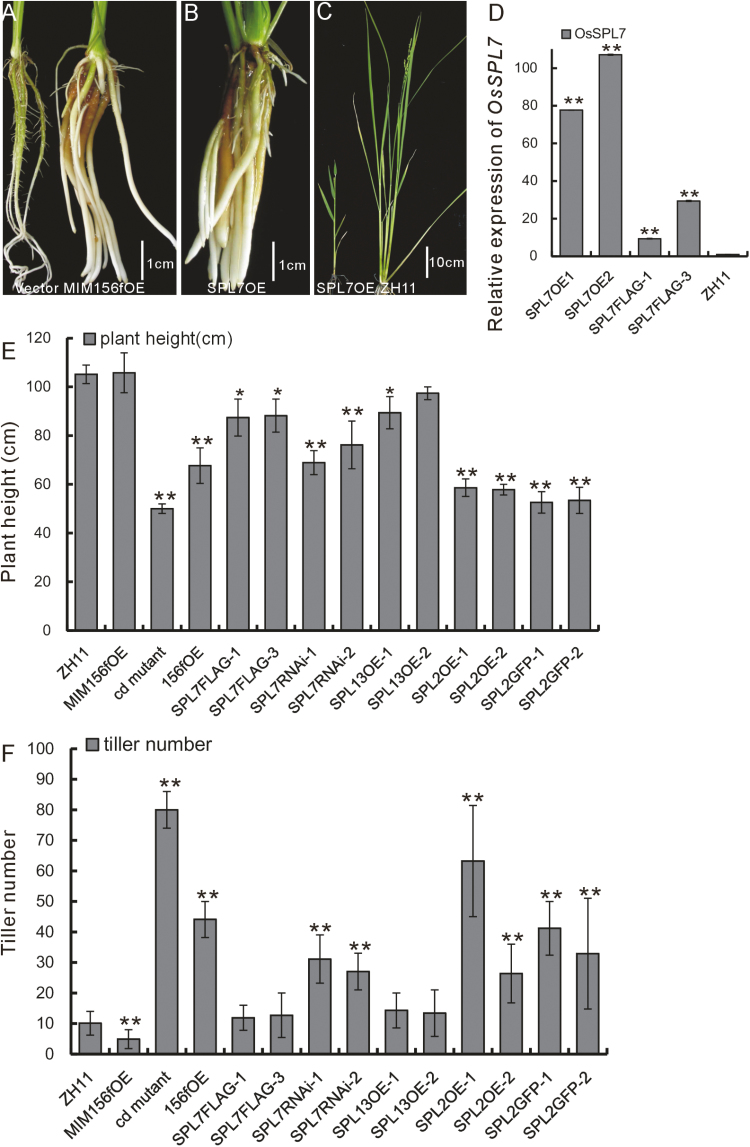
miRNAs function mainly through negative regulation on their targets. In rice, there are 12 and 18 genes coding for miR156 and its target OsSPL genes, respectively (http://www.mirbase.org, accessed 27 July 2018; Xie et al., 2006). In the microarray analysis of the cd mutant, most of the OsSPL genes were down-regulated. Among these genes, OsSPL2, OsSPL7, and OsSPL13 were the most down-regulated, according to the fold change (Supplementary Table S3). We examined OsSPL expression in the cd mutant, miR156fOE, and MIM156fOE plants using qRT-PCR, and further confirmed the regulatory effect of miR156f on the expression of most OsSPL genes (Supplementary Fig. S1B). To examine miR156f function further, we carried out genetic analysis of OsSPL2, OsSPL7, and OsSPL13.
First, we individually overexpressed each of these three genes in the WT background. Transgenic plants overexpressing OsSPL7 (SPL7OE) had thickened roots with fewer lateral roots (Fig. 3B), and resembled the MIM156fOE phenotype (Fig. 3A). Cellular analysis revealed that the thickened roots in the MIM56fOE plants were associated with an increased number of parenchymal cells (Supplementary Fig. S2A–C). Furthermore, most SPL7OE plants had single tillers and were short and infertile (Fig. 3C). To rule out the possibility that the infertility of the SPL7OE plants might be caused by high-level expression of OsSPL7 under the 35S promoter, we generated SPL7Flag transgenic lines with OsSPL7 under its native promoter and fused to a Flag tag. In these SPL7Flag plants, the OsSPL7 gene was still up-regulated, but to a lesser degree than in the SPL7OE plants (Fig. 3D). Moreover, the phenotype of the SPL7Flag plants was not as severe as that of the SPL7OE plants. The SPL7Flag plants had a reduced height in comparison with the WT (Fig. 3E, F), but a similar tiller number. We also constructed OsSPL7 RNAi transgenic plants (SPL7RNAi), which had reduced plant heights and increased tiller numbers, and resembled the cd mutant and the miR156fOE plants (Fig. 3E, F).
Fig. 3.
Expression of OsSPL genes and functional activity. (A) Root phenotype of a representative T0 generation MIM156fOE plant in a tissue culture tube (control phenotype of the transgenic plant from the empty vector is shown on the left for comparison). (B) Root phenotype of a representative T0 generation SPL7OE plant in a tissue culture tube. (C) Appearance of a representative SPL7OE plant in comparison with a WT plant. (D) Relative expression levels of OsSPL7 in SPL7OE and SPL7Flag plants (n=3). (E) Heights of the miR156- and OsSPL -related mutant and transgenic plants (n=20). (F) Tiller numbers of the miR156- and OsSPL-related mutant and transgenic plants (n=20). The asterisks in (D–F) represent a significant difference, as determined by a Student’s t-test at *P<0.05 and **P<0.01, respectively.
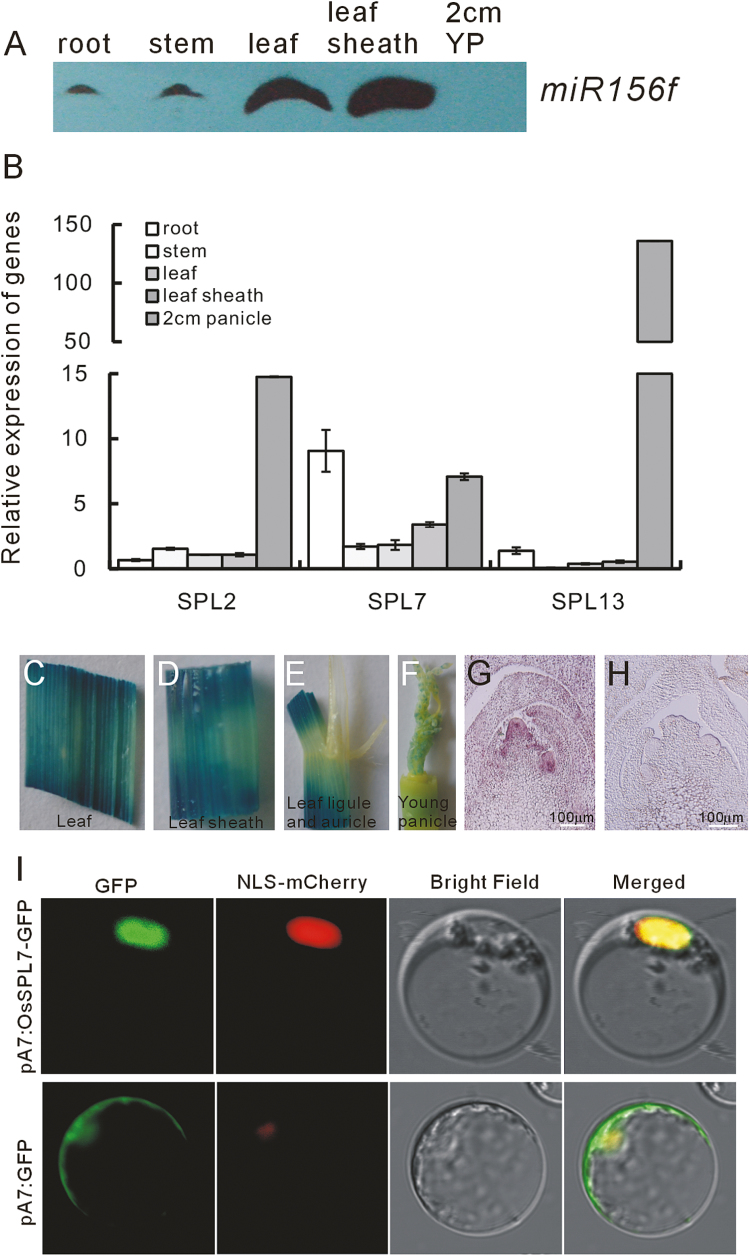
To characterize OsSPL7 further, we analyzed its expression profile together with that of miR156f (Fig. 4A). miR156f was expressed at low levels in the roots and very young panicles (2 cm in length). However, there was clear OsSPL7 expression in the root (Fig. 4B), in accordance with its function in root development (Fig. 3B). In young panicles, OsSPL7 showed a relatively high expression level (Fig. 4B). In comparison, expression of OsSPL2 and OsSPL13 was not as high in the roots, and, similarly, both OsSPL2 and OsSPL13 showed high expression in the 2 cm panicle whereas miR156f was undetectable (Fig. 4B). We also constructed SPL7::GUS transgenic plants in which the OsSPL7 promoter was used to drive GUS gene expression. The GUS signal was detected in the leaf and leaf sheath (Fig. 4C, D), and the young 1.5 cm panicle (Fig. 4F), but not the leaf ligule or the leaf auricle (Fig. 4E). In situ hybridization confirmed the OsSPL7 expression at the early stage of panicle development (Fig. 4G). Together, these results suggest that OsSPL7 is a ubiquitous protein.
Fig. 4.
miR156f and OsSPL7 expression profiles and localization of the OsSPL7–GFP fusion protein. (A) Northern blot analysis of miR156f in different tissues (30 μg of total RNA loaded for each). YP, young panicle. (B) Expression of OsSPL2, OsSPL7, and OsSPL13 in different tissues (the same samples as in A) as revealed by qRT-PCR (n=3). (C–F) GUS staining of the SPL7::GUS transgenic plant tissues, namely the leaf (C), the leaf sheath (D), the leaf ligule and auticle (E), and the 1.5 cm panicle (F). (G) In situ hybridization of OsSPL7 mRNA at the panicle initiation stage. (H) Sense probe signal from the in situ hybridization. (I) Localization of the OsSPL7–GFP fusion protein in the rice protoplast.
We also examined OsSPL7–GFP fusion protein expression in rice protoplasts and found that the florescence signal was exclusively concentrated in the nucleus (Fig. 4I).
Plants overexpressing OsSPL13 (SPL13OE) had a similar phenotype to that of SPL7Flag plants, while plants overexpressing OsSPL2 alone (SPL2OE) or tagged with GFP (SPL2GFP) both had reduced plant height and increased tiller numbers, and resembled the cd mutant (Fig. 3E, F).
These genetic analyses suggest that several OsSPL genes might be involved in the regulation of plant architecture, but with differing degrees of activity and function. In this study, we chose to focus on OsSPL7 as the downstream target of miR156 due to its phenotypic similarity to miR156f genetic plants.
We also found that there was up-regulation of miR156f in SPL2OE, SPL7OE, and SPL13OE plants, indicating that increased OsSPL expression has feedback regulation effects on miR156f (Supplementary Fig. S1C).
OsGH3.8 is directly regulated by OsSPL7
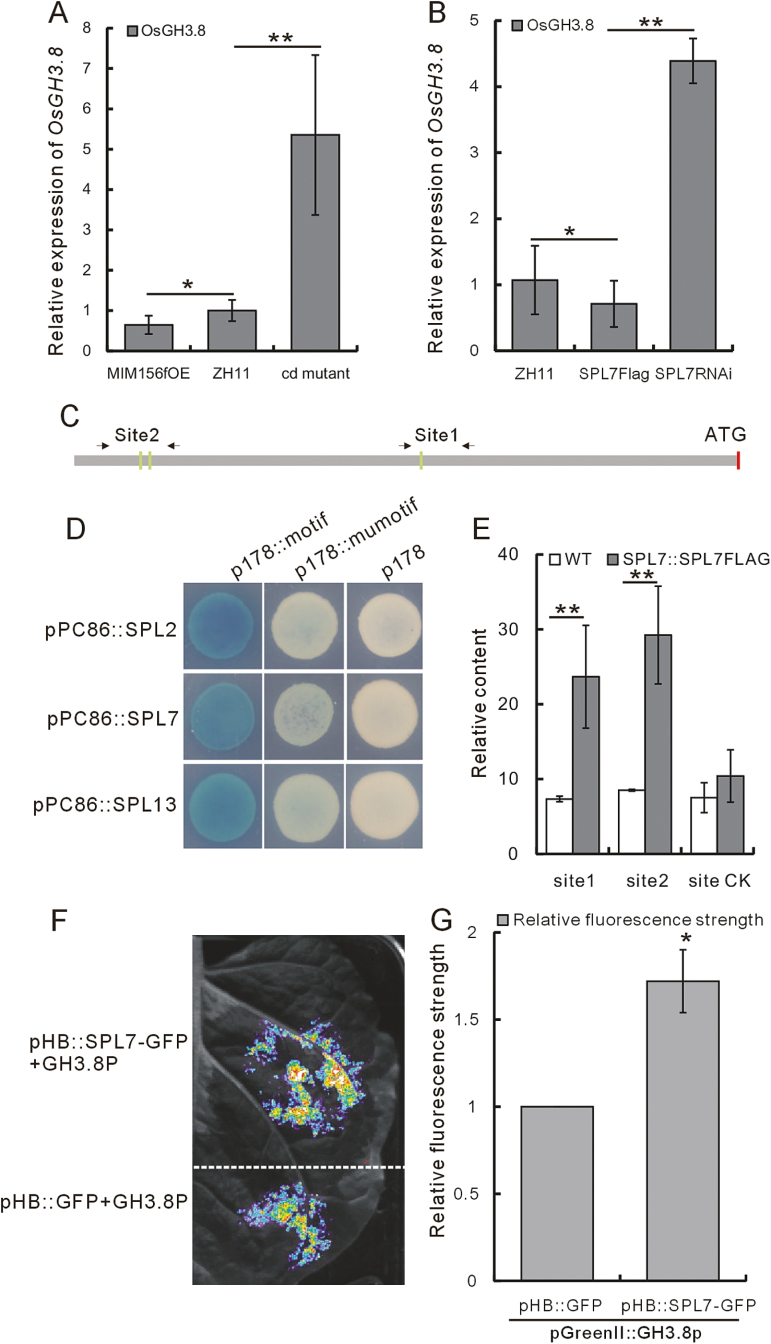
The role of auxin in maintaining apical dominance by suppressing axillary development is important. The cd mutant developed more ectopic shoots, while there were fewer in the MIM156fOE plants. Moreover, in the cd mutant, the dormancy of axillary buds was disrupted, especially in the upper nodes (Supplementary Fig. S2D). The disturbed axillary bud dormancy and ectopic shoots in the cd mutant suggested the possible involvement of auxin in miR156f function. To investigate this, we examined the cd mutant microarray data for auxin factors and found that OsGH3.8 was up-regulated among the OsGH3 family genes (Supplementary Table S3). Further, qRT-PCR analysis confirmed that OsGH3.8 was the most significantly up-regulated of these genes in the cd mutant (Supplementary Fig. S1D).
We further measured OsGH3.8 expression in the cd mutant, the WT, and MIM156fOE plants, and found that OsGH3.8 was up-regulated in the cd mutant and down-regulated in MIM156fOE plants (Fig. 5A; Supplementary Fig. S3A). Moreover, OsGH3.8 was down-regulated in SPL7Flag plants and up-regulated in SPL7RNAi plants (Fig. 5B; Supplementary Fig. S3B). These data suggest that miR156f/OsSPL7 might modulate plant architecture by regulating OsGH3.8.
Fig. 5.
Confirmation of the transcriptional regulation of the OsGH3.8 gene by OsSPL7 protein. (A) OsGH3.8 expression in the cd mutant and the MIM156fOE plants (n=3). (B) OsGH3.8 expression in the SPL7Flag and SPL7RNAi plants (n=3). (C) Schematic of the SPL-binding motifs in the 2 kb OsGH3.8 promoter. Green bars indicate the positions of the GTAC motifs at 1022 bp (site 1), 1932 bp, and 1936 bp motifs (site 2). The regions amplified by the correspondingly named primers (see the Materials and methods) at sites 1 and 2 are indicated by arrows. (D) Yeast one-hybrid analysis of the OsSPL2, OsSPL7, and OsSPL13 proteins binding to the 1022 bp motif. (E) ChIP analysis of SPL7Flag binding to sites 1 and 2 (n=3). (F) Image of the dual-LUC assay. (G) The LUC/REN ratio in the dual-LUC assay indicating relative luciferase activity. The empty vector pHB::GFP was used as control. Values are given as the mean±SD (n=3). The asterisks in (A), (B), (E), and (G) represent a significant difference, as determined by a Student’s t-test at *P<0.05 and **P<0.01, respectively.
Next, we examined whether there were any SPL-binding motifs in the 2 kb OsGH3.8 promoter (http://www.dna.affrc.go.jp/PLACE/signalscan.html). We identified three GTAC motifs, located 1022, 1932, and 1936 bp upstream of the ATG initiation codon (Fig. 5C). Using a yeast one-hybrid approach, we found that OsSPL7 could bind to the 1022 bp motif. When the GTAC motif was mutated to GAAC, OsSPL7 could no longer bind to it (Fig. 5D), indicating that OsSPL7 specifically binds to the OsGH3.8 promoter through the GTAC motif. Moreover, our yeast one-hybrid analysis also showed that OsSPL2 and OsSPL13 could bind to the 1022 bp GTAC motif (Fig. 5D). These data suggest that at least some SPL proteins can bind to the OsGH3.8 promoter.
ChIP analysis of the SPL7Flag plants using a Flag antibody showed obvious binding to the sites corresponding to the 1022 bp motif (site1; Fig. 5E) and the combined 1932 bp and 2426 bp motifs (site2; Fig. 5E). Furthermore, to validate the activation of the OsGH3.8 gene by OsSPL7, we carried out a dual-LUC assay in tobacco leaf; it was revealed that OsSPL7 activated the expression of the OsGH3.8 gene promoter which showed a higher value of LUC/REN than the GFP control (Fig. 5F, G).
From our combined analyses, we conclude that OsSPL7 can directly bind to the OsGH3.8 promoter and regulate its expression.
Overexpression of OsGH3.8 partially complements the MIM156fOE phenotype
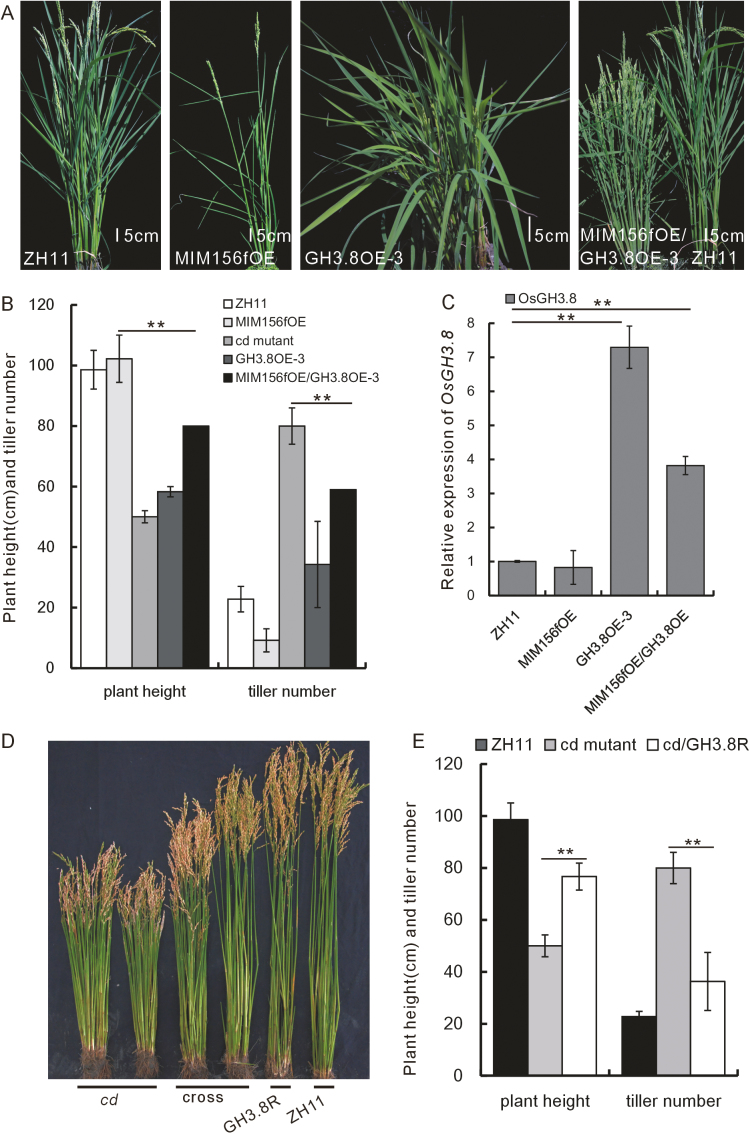
To characterize further the genetic relationship between OsGH3.8 and miR156f, we cloned the OsGH3.8 gene and overexpressed it in the WT background. The transgenic plants (OsGH3.8OE) showed up-regulation of OsGH3.8, an increased number of tillers, and a reduced plant height in comparison with the WT (Fig. 6A).
Fig. 6.
Characterization of MIM156fOE/GH3.8OE and cd/GH3.8R hybrid plants. (A) Appearance of representative WT, MIM156fOE, GH3.8OE-3, and hybrid MIM156fOE/GH3.8OE-3 plants (left to right). (B) Plant height and tiller number of the MIM156fOE/GH3.8OE-3 hybrid and genetically related plants (n=20). (C) OsGH3.8 expression in the MIM156fOE/GH3.8OE-3 plant and its parent lines (n=3). (D) Plant height and tiller number phenotypes of the cd mutant, the cd/GH3.8R hybrid, and the GH3.8R and WT plants (left to right). (E) Plant height and tiller number of the cd/GH3.8R hybrid as compared with the cd mutant and WT (n=10). The asterisks in (B), (C), and (E) represent a significant difference, as determined by a Student’s t-test at *P<0.05 and **P<0.01, respectively.
We next crossed the OsGH3.8OE-3 and OsGH3.8OE-4 transgenic plant lines with homozygous MIM156fOE plants. We obtained a single MIM156f/GH3.8OE-3 hybrid plant and two MIM156f/GH3.8OE-4 hybrid plants. We molecularly confirmed these genotypes by the existence of the plasmids used for transformation (Supplementary Fig. S3C). The hybrid plants had an increased number of tillers and a reduced plant height, in comparison with MIM156fOE, with phenotypes intermediate between those of MIM156fOE and cd mutant plants (Fig. 6A, B). OsGH3.8 was also up-regulated in the hybrid plants (Fig. 6C).
Next, we constructed OsGH3.8RNAi transgenic plants (OsGH3.8R). Although they did not show obvious phenotypic changes in tiller number or plant height (Fig. 6D), when we crossed the OsGH3.8R plants with the cd mutant, the hybrid had obvious changes in both features that were intermediate between those of the WT and the cd mutant (Fig. 6D, E).
From these hybridizations, we found that OsGH3.8 overexpression complemented the architectural defects of MIM156fOE plants by increasing the tiller number and reducing the plant height; whereas RNAi of OsGH3.8 in the cd mutant complemented the cd mutant phenotype. The direct binding of OsSPL7 to the OsGH3.8 promoter and the genetic relationship between miR156f and OsGH3.8 suggest that miR156f/OsSPL7 could directly regulate OsGH3.8 to modulate plant architecture.
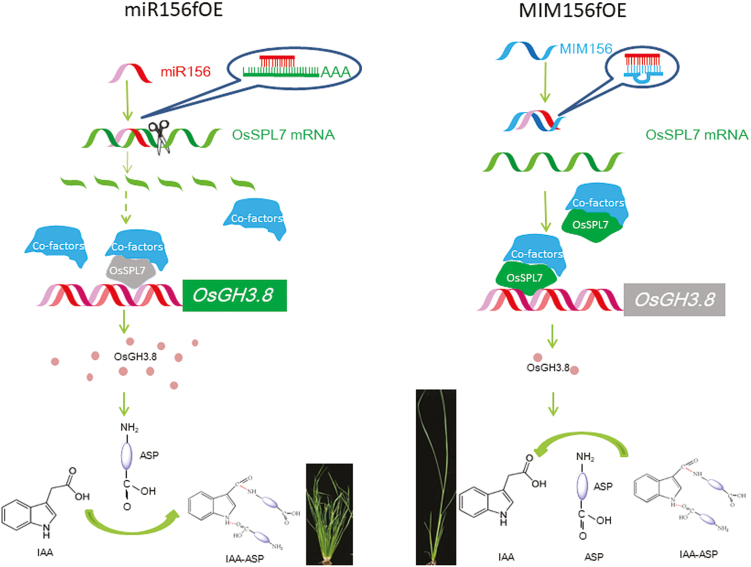
Together, we propose the following model (Fig. 7). First, miR156f negatively regulates its target OsSPL7, which turns out to be a negative regulator of OsGH3.8. OsGH3.8 promotes the combination of IAA with aspartic acid, and the consequent change in IAA content influences plant architecture to alter the plant height and tiller number in rice. Since OsGH3.8 is a fast-acting auxin-responsive gene, treatment of rice plants with NAA could rapidly induce the expression of OsGH3.8. This might have a feedback effect to reduce miR156f expression (Supplementary Fig. S4A, B).
Fig. 7.
Schematic of the proposed miR156–OsSPL7–OsGH3.8 pathway. In miR156fOE plants (left), excessive miR156 cleaves OsSPL7 transcripts and represses its expression. This releases OsSPL7 transcriptional control of OsGH3.8 expression. The plentiful OsGH3.8 facilitates the combination of IAA with aspartic acid (ASP), leading to cluster and dwarf plant phenotypes. In MIM156fOE plants, miR156f is sequestered by MIM156f, so that there is enough OsSPL7 available to suppress the expression of OsGH3.8. This then promotes the dissociation of ASP from IAA, leading to a phenotype of reduced tiller number and increased plant height.
The involvement of auxin in MIM156fOE plants might favor the grain-filling process in dense-panicle plants
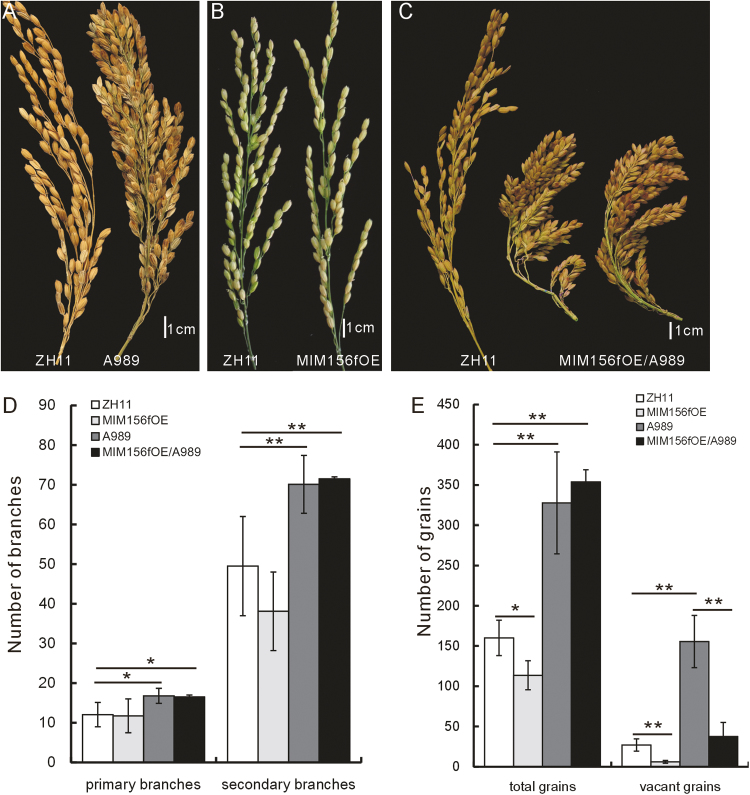
There is a great challenge to improve crop plant yield to provide enough food for the increasing world population. In a previous study, we isolated a dense-panicle T-DNA insertion mutant, A989, in which the RCN2 gene was up-regulated (Nakagawa et al., 2002; Li et al., 2010). The seed setting per panicle in this mutant is approximately double that of the WT; however, approximately half of the seeds are empty. Therefore, the enhanced seed set in A989 is offset by the high ratio of vacant seeds (Fig. 8A). To improve the rice yield, the filling efficiency in the A989 mutant would need to be improved.
Fig. 8.
MIM156fOE favored the grain-filling process in dense-panicle plants. (A–C) Panicles of A989 (A), MIM156fOE (B), and MIM156fOE/A989(C) plants (the WT is shown to the left in each image for comparison). (D) Numbers of primary and secondary branches in the MIM156f/A989 hybrid, its parent lines, and the WT (n=10). (E) Numbers of total and vacant grains in the MIM156fOE/A989 hybrid, its parent lines, and the WT (n=20). The asterisks in (D) and (E) represent a significant difference, as determined by a Student’s t-test at *P<0.05 and **P<0.01, respectively.
Because auxin signaling participates in the grain-filling process in rice (Liu et al., 2015), we investigated whether the miR156–OsSPL7–OsGH3.8 pathway might influence grain filing. To this end, we crossed the MIM156fOE plants with the A989 mutant. The hybrid had a similar number of tillers to that of the MIM156fOE plants, but the panicle was shortened and the seeds were densely set (Fig. 8A–C). However, the hybrid panicle had similar numbers of first and secondary branches as that of the A989 mutant (Fig. 8D). More importantly, the seed-filling efficiency of the hybrid was dramatically improved (Fig. 8E). These results show that the MIM156fOE genetic background improves the grain-filling limitation of A989.
Discussion
It has been extensively reported that the function of miR156 is far ranging and conserved among different species (Huijser and Schmid, 2011; Wang and Wang, 2015). In this study, we showed that miR156f is a positive regulator of tiller number and a negative regulator of plant height. Consistent with its conserved function, several of the 12 miR156 genes in rice have been reported as influencing tiller number and plant height (Xie et al., 2006; Chen et al., 2015; Hayashi-Tsugane et al., 2015; Wang et al., 2015). There are also 18 miR156 target SPL genes in rice, and the functions of some of these have already been determined (Jiao et al., 2010; Miura et al., 2010; Springer, 2010). We found that SPL7OE plants had a single tiller phenotype that has not previously been reported for other SPL genes (Fig. 3C). We also found that SPL7RNAi plants had a cluster and dwarf phenotype (Fig. 3E, F). Based on the concept that miRNAs negatively regulate expression of their targets, we determined that OsSPL7 is a negative regulator of tiller number and OsSPL7 functions as a downstream target of miR156f in regulating plant architecture (Vaucheret, 2006; Liu, 2008). At the same time, OsSPL2 functions as a positive regulator of tiller number and a negative regulator of plant height, as confirmed by the cluster and dwarf phenotype of OsSPL2OE and OsSPL2GFP plants (Fig. 3E, F; Supplementary Fig. S5A). Therefore, it appears that the targets of miR156 might function in the same processes, but the direction of regulation might differ. This might be a buffering mechanism to offset the cumulative effects of there being several targets of miR156. Supporting this hypothesis, we found that SPL7OE plants had a single tiller and were infertile (Fig. 3C), which is a phenotype that is more severe than that of the MIM156fOE plants (Fig. 2A).
In this study, we showed that there was direct binding of OsSPL7 to the OsGH3.8 promoter. From these data, together with genetic analysis of the interaction between miR156f and OsGH3.8, we demonstrated that the miR156f–OsSPL7–OsGH3.8 pathway is important in regulating rice plant architecture (Fig. 7). We also found that miR156f overexpression caused a cluster and dwarf phenotype (Fig. 1), the development of ectopic shoots, and disturbed axillary bud dormancy (Supplementary Fig. S2D). These features are associated with alterations in auxin signaling. However, our analysis did not rule out the possible involvement of other targets (such as OsSPL2) and additional factors that could regulate plant architecture. Based on the following observations, we propose that there might also be additional pathways through which miR156f is involved in auxin signaling in addition to the miR156f–OsSPL7–OsGH3.8 pathway. First, the tiller number and plant height of the cd mutant were partially complemented by OsGH3.8R plants (Fig. 6D, E). Secondly, although the SPL7RNAi and GH3.8OE plants both had increased numbers of tillers and reduced plant heights, neither of these plant lines had phenotypes that were as pronounced as those in the cd mutant (Figs 3E, F, 6B; Supplementary Fig. S5B).
In this study, the OsSPL7 activity had obvious dosage effects. These were associated not only with the tiller number but also with fertility, with high OsSPL7 expression in SPL7OE plants resulting in infertility (Fig. 3C, D). In the SPL7Flag plants and the MIM156fOE plants where the OsSPL7 levels were not as highly up-regulated (Fig. 3D; Supplementary Fig. S1B), the grain-filling efficiency and fertility were restored. Furthermore, the low grain-filling efficiency of the A989 mutant was greatly enhanced by hybridization with the MIM156fOE plant line. Therefore, both miR156f and OsSPL7 might have roles in regulating fertility. This hypothesis is supported by previous discussions of the possible roles of the miR156/SPL in fertility regulation in Arabidopsis (Huijser and Schmid, 2011) and rice (Wang and Wang, 2015), and further extends the possible roles of miR156. Auxin is also a multifunctional factor in plant development. The combined actions of miR156 and auxin might also mediate changes in fertility such as that demonstrated by the crossing of MIM156fOE and A989 (Fig. 8). Therefore, crosstalk between important developmental and physiological factors such as miR156f and auxin might be important for modulating plant development and physiology in more than one aspect of plant growth.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Primer sequences used in this study.
Table S2. Co-segregation analysis of the T-DNA insert and the cluster and dwarf phenotype in the F2 population obtained from the reciprocal cross and backcross between WT and the cd mutant.
Table S3. Microarray analysis of the cd mutant as compared with the WT.
Fig S1. Gene expression in the miR156f-related plant lines.
Fig S2. Further phenotypes of MIM156fOE and the cd mutant.
Fig S3. OsGH3.8 expression in miR156f/OsSPL7-related genetic lines and confirmation of the cross between the GH3.8OE and MIM156fOE plants.
Fig S4. miR156f and OsGH3.8 expression in response to NAA treatment.
Fig S5. qRT-PCR analysis of OsSPL2 expression in the OsSPL2OE and OsSPL2GFP plants (A) and statistical analysis of the plant height and tiller number in the cd mutant, SPL7RNAi and GH3.8OE plants (B).
Acknowledgements
We would like to thank Jiawei Wang from our institute for helpful suggestions on this work. This work was supported by the National Key R&D Program of China (2016YFD0100600), the National Special Program on Research and Commercialization of Transgenic Plant (2016ZX08009-003-001, 2014ZX08009-003-003), Scholarship Foundation from Shanghai Institutes for Biological Sciences (2007KIP206), and the SA-SIBS 2009 Young Faculty Award. We thank Shelley Robison, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac, accessed 27 July 2018), for editing the English text of a draft of this manuscript. The authors declare no conflict of interest.
Glossary
Abbreviations:
- MIM156f
mimicry miR156f
- MIM156fOE
mimicry miR156f overexpression
- miR156fOE
miR156f overexpression
- OsGH3.8OE
OsGH3.8 overexpression
- OsGH3.8RNAi
OsGH3.8 RNA inference
- OsGH3.8R
OsGH3.8 RNA inference transgenic plants
- SPL7Flag
OsSPL7 fused with the Flag tag driven by its own promoter
- SPL2GFP
OsSPL2 fused with GFP driven by its own promoter
- SPL7OE
OsSPL7 overexpression
- SPL2OE
OsSPL2 overexpression
- SPL13OE
OsSPL13 overexpression
- SPL7RNAi
OsSPL7 RNA interference
- smRNA
small RNA
- SPL
SQUAMOSA PROMOTER BINDING PROTEIN-like
References
- Aravin AA, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, Snyder B, Gaasterland T, Meyer J, Tuschl T. 2003. The small RNA profile during Drosophila melanogaster development. Developmental Cell 5, 337–350. [DOI] [PubMed] [Google Scholar]
- Bartel B, Bartel DP. 2003. MicroRNAs: at the root of plant development?Plant Physiology 132, 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. 2004. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297. [DOI] [PubMed] [Google Scholar]
- Bartel DP. 2009. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergonzi S, Albani MC, Ver Loren van Themaat E, Nordström KJ, Wang R, Schneeberger K, Moerland PD, Coupland G. 2013. Mechanisms of age-dependent response to winter temperature in perennial flowering of Arabis alpina. Science 340, 1094–1097. [DOI] [PubMed] [Google Scholar]
- Bhogale S, Mahajan AS, Natarajan B, Rajabhoj M, Thulasiram HV, Banerjee AK. 2014. MicroRNA156: a potential graft-transmissible microRNA that modulates plant architecture and tuberization in Solanum tuberosum ssp. andigena. Plant Physiology 164, 1011–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Westfall CS, Hicks LM, Wang S, Jez JM. 2010. Kinetic basis for the conjugation of auxin by a GH3 family indole-acetic acid-amido synthetase. Journal of Biological Chemistry 285, 29780–29786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Gao X, Zhang J. 2015. Alteration of osa-miR156e expression affects rice plant architecture and strigolactones (SLs) pathway. Plant Cell Reports 34, 767–781. [DOI] [PubMed] [Google Scholar]
- Cho SH, Coruh C, Axtell MJ. 2012. miR156 and miR390 regulate tasiRNA accumulation and developmental timing in Physcomitrella patens. The Plant Cell 24, 4837–4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui LG, Shan JX, Shi M, Gao JP, Lin HX. 2014. The miR156–SPL9–DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. The Plant Journal 80, 1108–1117. [DOI] [PubMed] [Google Scholar]
- Dai Z, Wang J, Zhu M, Miao X, Shi Z. 2016. OsMADS1 represses microRNA172 in elongation of palea/lemma development in rice. Frontiers in Plant Science 7, 1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira e Silva GF, Silva EM, Azevedo Mda S, Guivin MA, Ramiro DA, Figueiredo CR, Carrer H, Peres LE, Nogueira FT. 2014. microRNA156-targeted SPL/SBP box transcription factors regulate tomato ovary and fruit development. The Plant Journal 78, 604–618. [DOI] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Valli A, Todesco M, et al. 2007. Target mimicry provides a new mechanism for regulation of microRNA activity. Nature Genetics 39, 1033–1037. [DOI] [PubMed] [Google Scholar]
- Gou JY, Felippes FF, Liu CJ, Weigel D, Wang JW. 2011. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. The Plant Cell 23, 1512–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi-Tsugane M, Maekawa M, Tsugane K. 2015. A gain-of-function Bushy dwarf tiller 1 mutation in rice microRNA gene miR156d caused by insertion of the DNA transposon nDart1. Scientific Reports 5, 14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellens RP, Allan AC, Friel EN, Bolitho K, Grafton K, Templeton MD, Karunairetnam S, Gleave AP, Laing WA. 2005. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 1, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiei Y, Ohta S, Komari T, Kumashiro T. 1994. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. The Plant Journal 6, 271–282. [DOI] [PubMed] [Google Scholar]
- Higuchi R, Krummel B, Saiki RK. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Research 16, 7351–7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijser P, Schmid M. 2011. The control of developmental phase transitions in plants. Development 138, 4117–4129. [DOI] [PubMed] [Google Scholar]
- Jain M, Khurana JP. 2009. Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice. FEBS Journal 276, 3148–3162. [DOI] [PubMed] [Google Scholar]
- Jia X, Mendu V, Tang G. 2010. An array platform for identification of stress-responsive microRNAs in plants. Methods in Molecular Biology 639, 253–269. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wang Y, Xue D, et al. 2010. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nature Genetics 42, 541–544. [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades MW. 2012. Conservation and divergence in plant microRNAs. Plant Molecular Biology 80, 3–16. [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades MW, Bartel DP, Bartel B. 2006. MicroRNAs and their regulatory roles in plants. Annual Review of Plant Biology 57, 19–53. [DOI] [PubMed] [Google Scholar]
- LeClere S, Tellez R, Rampey RA, Matsuda SP, Bartel B. 2002. Characterization of a family of IAA-amino acid conjugate hydrolases from Arabidopsis. Journal of Biological Chemistry 277, 20446–20452. [DOI] [PubMed] [Google Scholar]
- Li L, Shi Z, Shen G, Wang X, An L, Zhang L. 2010. Dense-panicle-related gene cloning from rice mutant A989 and transgenic plant analysis (in Chinese). Acta Agronomica Sinica 36, 887–894. [Google Scholar]
- Li X, Qian Q, Fu Z, et al. 2003. Control of tillering in rice. Nature 422, 618–621. [DOI] [PubMed] [Google Scholar]
- Liu J. 2008. Control of protein synthesis and mRNA degradation by microRNAs. Current Opinion in Cell Biology 20, 214–221. [DOI] [PubMed] [Google Scholar]
- Liu L, Tong H, Xiao Y, Che R, Xu F, Hu B, Liang C, Chu J, Li J, Chu C. 2015. Activation of Big Grain1 significantly improves grain size by regulating auxin transport in rice. Proceedings of the National Academy of Sciences, USA 112, 11102–11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YG, Mitsukawa N, Oosumi T, Whittier RF. 1995. Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. The Plant Journal 8, 457–463. [DOI] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Kowalczyk M, Marchant A, Celenza J, Cohen JD, Sandberg G. 2002. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Plant Molecular Biology 49, 249–272. [PubMed] [Google Scholar]
- Miura K, Ikeda M, Matsubara A, Song XJ, Ito M, Asano K, Matsuoka M, Kitano H, Ashikari M. 2010. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nature Genetics 42, 545–549. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Shimamoto K, Kyozuka J. 2002. Overexpression of RCN1 and RCN2, rice TERMINAL FLOWER 1/CENTRORADIALIS homologs, confers delay of phase transition and altered panicle morphology in rice. The Plant Journal 29, 743–750. [DOI] [PubMed] [Google Scholar]
- Nelson P, Kiriakidou M, Sharma A, Maniataki E, Mourelatos Z. 2003. The microRNA world: small is mighty. Trends in Biochemical Sciences 28, 534–540. [DOI] [PubMed] [Google Scholar]
- Nischal L, Mohsin M, Khan I, Kardam H, Wadhwa A, Abrol YP, Iqbal M, Ahmad A. 2012. Identification and comparative analysis of microRNAs associated with low-N tolerance in rice genotypes. PLoS One 7, e50261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodine MD, Bartel DP. 2010. MicroRNAs prevent precocious gene expression and enable pattern formation during plant embryogenesis. Genes & Development 24, 2678–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampey RA, LeClere S, Kowalczyk M, Ljung K, Sandberg G, Bartel B. 2004. A family of auxin-conjugate hydrolases that contributes to free indole-3-acetic acid levels during Arabidopsis germination. Plant Physiology 135, 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades MW, Reinhart BJ, Lim LP, Burge CB, Bartel B, Bartel DP. 2002. Prediction of plant microRNA targets. Cell 110, 513–520. [DOI] [PubMed] [Google Scholar]
- Sauer M, Robert S, Kleine-Vehn J. 2013. Auxin: simply complicated. Journal of Experimental Botany 64, 2565–2577. [DOI] [PubMed] [Google Scholar]
- Song A, Wang L, Chen S, Jiang J, Guan Z, Li P, Chen F. 2015. Identification of nitrogen starvation-responsive microRNAs in Chrysanthemum nankingense. Plant Physiology and Biochemistry 91, 41–48. [DOI] [PubMed] [Google Scholar]
- Springer N. 2010. Shaping a better rice plant. Nature Genetics 42, 475–476. [DOI] [PubMed] [Google Scholar]
- Staswick PE, Tiryaki I, Rowe ML. 2002. Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. The Plant Cell 14, 1405–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stief A, Altmann S, Hoffmann K, Pant BD, Scheible WR, Bäurle I. 2014. Arabidopsis miR156 regulates tolerance to recurring environmental stress through SPL transcription factors. The Plant Cell 26, 1792–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terol J, Domingo C, Talón M. 2006. The GH3 family in plants: genome wide analysis in rice and evolutionary history based on EST analysis. Gene 371, 279–290. [DOI] [PubMed] [Google Scholar]
- Vaucheret H. 2006. Post-transcriptional small RNA pathways in plants: mechanisms and regulations. Genes & Development 20, 759–771. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang H. 2015. The miR156/SPL module, a regulatory hub and versatile toolbox, gears up crops for enhanced agronomic traits. Molecular Plant 8, 677–688. [DOI] [PubMed] [Google Scholar]
- Wang J, Gao X, Li L, Shi X, Zhang J, Shi Z. 2010. Overexpression of Osta-siR2141 caused abnormal polarity establishment and retarded growth in rice. Journal of Experimental Botany 61, 1885–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Li L, Wan X, An L, Zhang J. 2004. Distribution of T-DNA carrying a Ds element on rice chromosomes. Science in China. Series C, Life Sciences 47, 322–331. [DOI] [PubMed] [Google Scholar]
- Wang JW. 2014. Regulation of flowering time by the miR156-mediated age pathway. Journal of Experimental Botany 65, 4723–4730. [DOI] [PubMed] [Google Scholar]
- Wang L, Sun S, Jin J, et al. 2015. Coordinated regulation of vegetative and reproductive branching in rice. Proceedings of the National Academy of Sciences, USA 112, 15504–15509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Wang Q, Zhang B. 2013. Response of miRNAs and their targets to salt and drought stresses in cotton (Gossypium hirsutum L.). Gene 530, 26–32. [DOI] [PubMed] [Google Scholar]
- Wang S, Wu K, Yuan Q, et al. 2012. Control of grain size, shape and quality by OsSPL16 in rice. Nature Genetics 44, 950–954. [DOI] [PubMed] [Google Scholar]
- Westfall CS, Herrmann J, Chen Q, Wang S, Jez JM. 2010. Modulating plant hormones by enzyme action: the GH3 family of acyl acid amido synthetases. Plant Signaling & Behavior 5, 1607–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. 2005. Auxin: regulation, action, and interaction. Annals of Botany 95, 707–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Park MY, Conway SR, Wang JW, Weigel D, Poethig RS. 2009. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Shen J, Hou X, Yao J, Li X, Xiao J, Xiong L. 2012. Gradual increase of miR156 regulates temporal expression changes of numerous genes during leaf development in rice. Plant Physiology 158, 1382–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Wu C, Xiong L. 2006. Genomic organization, differential expression, and interaction of SQUAMOSA promoter-binding-like transcription factors and microRNA156 in rice. Plant Physiology 142, 280–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S, Salinas M, Garcia-Molina A, Höhmann S, Berndtgen R, Huijser P. 2013. SPL8 and miR156-targeted SPL genes redundantly regulate Arabidopsis gynoecium differential patterning. The Plant Journal 75, 566–577. [DOI] [PubMed] [Google Scholar]
- Xue XY, Zhao B, Chao LM, Chen DY, Cui WR, Mao YB, Wang LJ, Chen XY. 2014. Interaction between two timing microRNAs controls trichome distribution in Arabidopsis. PLoS Genetics 10, e1004266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Ohmori Y, Kitano H, Taguchi-Shiobara F, Hirano HY. 2012. Aberrant spikelet and panicle1, encoding a TOPLESS-related transcriptional co-repressor, is involved in the regulation of meristem fate in rice. The Plant Journal 70, 327–339. [DOI] [PubMed] [Google Scholar]
- Yu N, Niu QW, Ng KH, Chua NH. 2015. The role of miR156/SPLs modules in Arabidopsis lateral root development. The Plant Journal 83, 673–685. [DOI] [PubMed] [Google Scholar]
- Yu S, Galvão VC, Zhang YC, et al. 2012. Gibberellin regulates the Arabidopsis floral transition through miR156-targeted SQUAMOSA promoter binding-like transcription factors. The Plant Cell 24, 3320–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Lian H, Wang JW. 2015. Plant developmental transitions: the role of microRNAs and sugars. Current Opinion in Plant Biology 27, 1–7. [DOI] [PubMed] [Google Scholar]
- Yu ZX, Wang LJ, Zhao B, Shan CM, Zhang YH, Chen DF, Chen XY. 2015. Progressive regulation of sesquiterpene biosynthesis in Arabidopsis and Patchouli (Pogostemon cablin) by the miR156-targeted SPL transcription factors. Molecular Plant 8, 98–110. [DOI] [PubMed] [Google Scholar]
- Zhang Q. 2007. Strategies for developing Green Super Rice. Proceedings of the National Academy of Sciences, USA 104, 16402–16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao SQ, Xiang JJ, Xue HW. 2013. Studies on the rice LEAF INCLINATION1 (LC1), an IAA-amido synthetase, reveal the effects of auxin in leaf inclination control. Molecular Plant 6, 174–187. [DOI] [PubMed] [Google Scholar]
- Zhou L, Liu Y, Liu Z, Kong D, Duan M, Luo L. 2010. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. Journal of Experimental Botany 61, 4157–4168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.