Investigations reveal that Cd can enter into rice cells via Ca-permeable channels, and the uptake varies longitudinally along the rice root.
Keywords: Calcium-permeable channels, Cd influx, Cd uptake, longitudinal variation, rice
Abstract
To develop elite crops with low cadmium (Cd), a fundamental understanding of the mechanism of Cd uptake by crop roots is necessary. Here, a new mechanism for Cd2+ entry into rice root cells was investigated. The results showed that Cd2+ influx in rice roots exhibited spatially and temporally dynamic patterns. There was a clear longitudinal variation in Cd uptake along rice roots, with the root tip showing much higher Cd2+ influx and concentration than the root mature zone, which might be due to the much higher expression of the well-known Cd transporter genes OsIRT1, OsNRAMP1, OsNRAMP5, and OsZIP1 in the root tip. Both the net Cd2+ influx and the uptake of Cd in rice roots were highly inhibited by ion channel blockers Gd3+ and TEA+, supplementation of Ca2+ and K+, and the plasma membrane H+-ATPase inhibitor vanadate, with Gd3+ and Ca2+ showing the most inhibitory effects. Furthermore, Ca2+- or Gd3+-induced reduction in Cd2+ influx and Cd uptake did not coincide with the expression of Cd transporter genes, but with that of two Ca channel genes, OsAAN4 and OsGLR3.4. These results indicate that Cd transporters are in part responsible for Cd2+ entry into rice root, and provide a new perspective that the Ca channels OsAAN4 and OsGLR3.4 might play an important role in rice root Cd uptake.
Introduction
As a result of atmospheric deposition, wastewater irrigation, use of metal-containing fertilizers and pesticides, and many other industrial processes, cadmium (Cd) has become one of the most toxic and widespread environmental pollutants in agricultural soil (Rizwan et al., 2016). Being a non-essential element for plants, excess Cd in soils interferes with plant growth and development, reduces crop yield, and accumulates to a high level in plant tissues, thus posing a threat to human and animal health through the food chain (Ahmad et al., 2015). To ensure food safety, breeding ‘low-Cd’ crops has become one of the most important strategies to reduce Cd in crops, for which a fundamental understanding of the Cd uptake mechanism in plant roots would be a critical issue.
Although great progress has been achieved over the last decades, the complex pathways of Cd entry into root cells are still not fully understood. Since Cd is a non-essential element for plants and interferes with the uptake of other ions, it was assumed that Cd could be absorbed into cells by the transporters for essential elements such as Fe3+, Zn2+, and Mn2+, due to the lack of specificity of these transporters (Clemens 2001; Ishimaru et al., 2006; Sasaki et al., 2012). It has been well documented that Cd could enter into root cells through various NRAMP (natural resistance-associated macrophage protein) family members, such as OsNRAMP1, OsNRAMP5, and AtNRAMP6; ZIP (zinc/iron-regulated transporter-like protein) transporters, such as AtIRT1 and TcZNT1/TcZIP4; the low affinity calcium transporters such as TaLCT1; or through YSL (Yellow-Stripe 1-Like) proteins such as ZmYSL1 as Cd chelates (Schaaf et al., 2004; Cailliatte et al., 2009; Lux et al., 2011, and references therein; Sasaki et al., 2012;). Of all the transporters above, OsNRAMP5, which is a major contributor to constitutive Mn2+ uptake, was considered to be a major route of Cd uptake from the external environment and entry into cells in rice (Ishimaru et al., 2012; Sasaki et al., 2012). However, a recent study by Takahashi et al. (2014) revealed that the knock-down of OsNRAMP5 triggered only ~20% reduction in root Cd content but a significant increase in shoot Cd content in both hydroponic and field trials. These results indicated that there might be other pathways for Cd entry into root cells, apart from the poor selectivity of transition ion transporters. Indeed, some studies suggested that Cd could also possibly be taken up via cation channels, such as K+ and Ca2+ channels, which are relatively non-selective between cations (White and Broadley, 2003; Lindberg et al., 2004; L.Z. Li et al., 2012). However, the contribution of this pathway to root Cd uptake remains to be further verified. Therefore, more effort is still needed to elucidate thoroughly the mechanism by which Cd is taken up into plant roots and cells.
With the innovation of non-invasive microelectrode measurements such as the microelectrode ion flux estimation (MIFE) technique (Newman, 2001; Shabala, 2006) and Cd-selective microelectrodes (Piñeros et al. 1998), Cd2+ fluxes have been characterized in various Cd-hyperaccumulating plants (He et al., 2011, L.Z. Li et al., 2012; Sun et al., 2013). However, little is known about the kinetics of Cd2+ flux across root cells in the rice plant, which is considered to be a model among monocots for biological research. Furthermore, as rice is a staple food crop for half of the world’s population, Cd in rice grain has become a major source of dietary Cd intake for this part of the world (Wang et al., 2016). Therefore, the characteristics of the transmembrane Cd2+ transport and Cd uptake in different root zones were investigated in this study in the absence and presence of a series of treatments by measuring Cd2+ flux, Cd concentration, Cd fluorescence localization, and gene expression. The aim of this study was (i) to reveal the longitudinal variation in Cd2+ influx and Cd distribution along rice root and explain how such variation is generated; and (ii) to elucidate whether Cd2+ enters into rice root cells through ion channels permeable to Ca2+ or K+ and explore the candidate transporter genes involved in this process.
Materials and methods
Plant materials, growth conditions, and treatments
Rice cultivar IR8, which accumulates high amounts of Cd (Yan et al., 2010), was used in the present study. Seeds were surface sterilized with 12.5% NaClO solution, thoroughly rinsed with tap water, and then soaked in distilled water at 25 °C. Two days later, the seeds were germinated with limited water at 35 °C for another 1 d, and sown on the mesh screen in a container with nutrient solution. All the rice seedlings were grown in a controlled-environmental chamber with a photoperiod of 16 h light/8 h dark, light intensity of 225 ± 25 µmol m–2 s–1, temperature of 30 °C light/25 °C dark, and relative humidity of 85%. The nutrient solution was made according to Zeng et al. (2008). Uniform 10-day-old seedlings were selected for electrophysiological measurements or to be treated with 20 µM CdCl2 with/without different blockers for further investigations.
Microelectrode Cd2+ flux measurements
Net Cd2+ fluxes were measured from the root epidermis of rice seedlings using non-invasive ion-selective vibrating microelectrodes (the MIFE technique, University of Tasmania, Hobart, Australia). The Cd ion-selective microelectrodes with tip diameter of 2–3 µm were manufactured and silanized with tributylchlorosilane (Cat. No. 90796; Sigma-Aldrich, Steinem, Switzerland), and then back-filled with an electrolyte buffer (0.1 mM KCl plus 10 mM CdCl2) and front-filled with an ion-selective Cd2+ cocktail made up with Cd2+ ionophore I, potassium tetrakis, and 2-nitrophenyl octyl ether (Cat. Nos 20909, 60588, and 73732; Sigma-Aldrich) according to Piñeros et al. (1998). The well-filled microelectrodes were equilibrated in basic salt medium solution (BSM; 0.5 mM KCl plus 0.10 mM CaCl2, buffered with 5 mM MES and 2 mM Tris at pH 5.6) for 1 h and calibrated in 5, 10, and 20 µM Cd in the absence and presence of either pharmaceutical prior to the Cd2+ flux measurement. Only electrodes with Nernstian slopes ≥25 mV and correlation >0.9990 were used. Details on measuring ion flux have been described previously (Newman, 2001; Shabala, 2006).
Experimental protocols for MIFE measurements
Measurement of Cd2+ flux along the rice root
Cd2+ flux profiles along rice root were measured after 1 h incubation in BSM containing 20 µM CdCl2, Root scanning started from the root cap and was carried out with 0.1 mm increments between 0 mm and 1 mm, 0.5 mm increments between 1 mm and 10 mm, and 1.0 mm increments between 10 mm and 20 mm, with net ion fluxes measured for 1 min at each point. Five individual seedlings were measured for each treatment.
Transient ion flux kinetics
Roots (≥5 cm) of intact seedlings were mounted in a horizontal chamber filled with 30 ml of BSM 1 h prior to measurements. Net ion fluxes were measured for 5 min under the control condition (BSM) to record the steady control flux values. Subsequently, 2 ml or 10 ml of CdCl2 stock (80 µM, made up in the background of BSM) was gently added to the chamber to yield the final Cd2+concentration of 5 µM or 20 µM, and the transient ion flux responses were measured for another 30 min. The period of time for mixing the solution (~2 min) was omitted from the data analysis and figures. Net ion fluxes were measured in either the elongation zone (EZ, ~2 mm from the root cap, without root hair) or the mature zone (MZ, ~10 mm from the root cap, with root hair). Six individual seedlings were measured for each treatment.
Pharmacological measurements
In pharmacological experiments, plants roots were pre-treated for 1 h prior to measurements with 30 ml of one of the following solutions: 100 µM sodium orthovanadate (vanadate); 20 mM tetraethylammonium chloride hydrate (TEA+); 100 µM GdCl3 (Gd3+); 5 mM CaCl2; or 10 mM KCl. Net Cd2+ fluxes were first measured for 5 min under the condition with either pharmacological treatment to record the steady control flux values. Subsequently, 10 ml of CdCl2 stock (80 µM, made up in the background of BSM with each corresponding pharmaceutical) was gently added to the chamber to yield the final Cd concentration of 20 µM, and the transient ion flux responses were measured for another 30 min. All the above pharmacological solutions were made up in the background of BSM, and buffered with MES–Tris (5 mM MES, 2 mM Tris base) at pH 5.6. Five individual seedlings were measured for each treatment.
Fluorescence labeling of Cd in root
Rice roots were pre-treated with different pharmaceuticals for 1 h, and then given the appropriate volume of Cd stock to yield the final Cd concentration of 20 µM. Rice roots were subsequently treated for 24 h prior to measurements. Cadmium stock was made up in the background of BSM with each corresponding pharmaceutical, and the one made up in the background of BSM only was used as the control. The localization of Cd in rice roots was investigated using the Cd Probe Leadmium Green AM dye (Molecular Probes, Invitrogen, Calsbad, CA, USA) according to L.Z. Li et al. (2012) with some modifications. Briefly, a stock solution of fluorescent dye was made by adding 50 ml of DMSO to one vial of Leadmium Green AM. The stock dye solution was then diluted 1:10 with 0.85% NaCl prior to being used. Root segments of 5 mm long from the root cap were thoroughly immersed in diluted dye solution for 1.5 h in the dark. The root segments were rinsed sequentially with 0.85% NaCl, 1 mM Na2EDTA, and distilled water, and subsequently slowly shaken in 0.85% NaCl solution for 24 h to get rid of all Cd ions from the root surface. Thereafter, the thoroughly washed root segments were observed under a florescence microscope (ECLIPSE, Nikon, Japan) with excitation at 488 nm and emission at 500–550 nm. Images were taken with a megapixel digital color camera (Leica DFC425C, Leica Microsystems) and images were acquired using ACDsee software (ACDSee Pro 8, ACD Systems International Inc., Canada). All the features of the camera were set to constant values for each image as follows: exposure time 1.3 s for fluorescence, gain 1.5×, saturation 1.5×, and gamma 1.0×. Each test was repeated at least eight times. The fluorescence intensity was calculated with Image J software (version 1.8.0, National Institutes of Health, USA).
Determination of root Cd concentration by ICP-MS
The concentration of Cd in rice roots pre-treated with different pharmaceuticals plus 20 µM Cd (see the details in the section on Cd fluorescence labeling) for 3 d was investigated by the inductively coupled plasma mass spectrometry (ICP-MS) technique. Prior to the determination, the roots from each treatment were immersed with 1 mM Na2EDTA for 15 min to remove the metal ions from the root surface, and washed thoroughly with double-distilled water. Thereafter, root segments 0–5, 5–10, 10–15, and 15–20 mm from the root cap and the bulk roots were separately collected and oven dried at 70 °C. The weighted dry samples were wet-digested with HNO3 plus HClO4 (HNO3:HClO4 =4:1). The resulting clear solutions were diluted with Mili Q water with a ratio of 1:4. Cd concentration was determined using the NexION300X (PerkinElmer, USA) with radial configuration.
RNA extraction and qRT-PCR
The transcript levels of genes involved in Cd transmembrane transport, such as OsIRT1, OsNRAMP1, OsNRAMP5, and OsZIP1, were determined in both the root tip (0–5 mm from the root cap) and the MZ (15–20 mm from the root cap) of rice root. At 0 h, 3 h, and 3 d of 20 µM Cd treatment, root segments from the root tip and the MZ of IR8 were collected and immediately frozen in liquid nitrogen for total RNA extraction. Three biological replicates were measured for each treatment.
To investigate the impact of pharmaceuticals on the expression of genes involved in Cd, Ca, and K transmembrane transport, the seedlings of IR8 were treated with 20 µM Cd and with or without different pharmaceuticals as described in the Cd fluorescence labeling experiment. At 3 h and 3 d of Cd treatment, the bulk of roots from two seedlings were collected and immediately frozen in liquid nitrogen for total RNA extraction. Three biological replicates were measured for each treatment.
Total RNA was isolated using the MiniBEST Plant RNA Extraction Kit (TaKaRa, Japan), and quantitative real-time PCR (qRT-PCR) was performed using Light Cycler 480 II (Roche, Swiss Confederation) with the iTaq™ Universal SYBR Green Spermix (Bio-Rad Laboratories, USA). Primer sequences for qRT-PCR are listed in Supplementary Table S1 at JXB online. Three technical replicates were performed for each biological replicate. The relative gene expression was calculated based on the 2-△△Ct method using OsActin as the internal standard (Livak and Schmittgen, 2001).
Data analysis
Statistical analysis was performed by a statistical package IBM SPSS Statistics 20 (IBM, New York, USA). All data in the figures and table are given as means ±SE. The significant difference between means was evaluated by ANOVA test. Significant differences among the means were compared using Tukey’s multiple range tests.
Results
Profiles of net Cd2+ flux along rice roots
To verify whether different root zones would show different Cd2+ uptake ability, transient Cd2+ flux was measured from different regions along the root axis (0–20 mm) after 1 h exposure to 20 µM CdCl2 (Fig. 1). Net Cd2+ influx was observed at all positions examined along the rice root, which was saturated at 0.6 mm and started to stabilize ~6 mm from the root cap. The root tip (including the meristem and EZs) showed much stronger Cd2+ influx than the MZ, with the largest Cd2+ influx of ~6 nmol m–2 s–1 measured at 0.6 mm from root cap. This is ~4-fold greater than average Cd2+ influx in the MZ (10–20 mm).
Fig. 1.
Net Cd2+ flux profiles along the root axis of cultivar IR8. Net Cd2+ fluxes were measured after 1 h exposure to 20 µM CdCl2 with 0.1 mm increments between 0 mm and 1 mm, 0.5 mm increments between 1 mm and 10 mm, and 1.0 mm increments between 10 mm and 20 mm, starting from the root cap. The insert displays the close-up view of net Cd2+ flux along the root segment between 0 mm and 1 mm. At each position, an average Cd2+ flux was measured for 1 min before the electrode was repositioned. Data are means ±SE (n=5). For all MIFE data, the sign convention is ‘influx positive’.
Net Cd2+ fluxes of rice root epidermal cells under different Cd concentrations
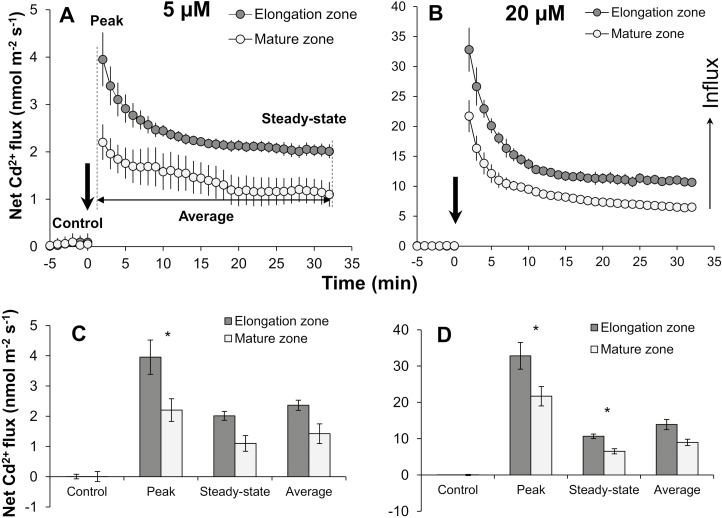
Prior to adding CdCl2 treatments, Cd2+ fluxes were kept at ~0 nmol m–2 s–1 (Fig. 2), indicating that no Cd uptake occurred under the control condition. However, external CdCl2 treatments resulted in immediate Cd2+ influx from the rice root surface (Fig. 2A, B). Also, a clear dose–response relationship was found between the external Cd concentration and magnitude of Cd2+ influx, with a >8-fold larger magnitude of Cd2+ flux observed under 20 µM CdCl2 (32.8 nmol m–2 s–1 at the EZ and 21.7 nmol m–2 s–1 at the MZ) than under 5 µM CdCl2 (3.9 nmol m–2 s–1 at the EZ and 2.2 nmol m–2 s–1 at the MZ) (Fig. 2C, D). A similar difference was also found for the mean Cd2+ influx over the total measurement time of 30 min. In addition, the sharp increase in net Cd2+ influx after Cd addition was very short lived and declined to the steady-state value in 20–25 min.
Fig. 2.
Kinetics of net Cd2+ fluxes measured from the elongation zone (EZ; ~2 mm from the root cap, without root hair) and mature zone (MZ; ~10 mm from the root cap, with root hair). (A and B) Transient Cd2+ fluxes in response to 5 µM or 20 µM CdCl2; Cd treatments were applied at time 0 as indicated by arrows. (C and D) Initial Cd2+ flux (under control conditions before CdCl2 treatments), magnitude of Cd2+ influx (immediately after CdCl2 treatments), steady-state Cd2+ influx (at the end of the measurements), and average Cd2+ influx (measured over the 30 min after CdCl2 application) as shown in (A). Data are means ±SE (n=6). An asterisk shows the significant difference between two root zones at P<0.05.
As expected, a significant difference in the net Cd2+ influx was found between the EZ and MZ, regardless of the external Cd concentration. The EZ showed a >1.5-fold higher magnitude, mean, or steady-state value of Cd2+ influx than the MZ, in agreement with the results of the Cd2+ flux profile along the root (Fig. 1).
Net Cd2+ fluxes from rice root in the presence of a proton pump inhibitor and ion channel blockers
The effects of a proton pump inhibitor and channel blockers on Cd2+ flux kinetics were studied to reveal the possible pathways mediating root Cd uptake. None of the inhibitors used in the present study significantly affected the control ion flux after 1 h of incubation (Fig. 3; Table 1).
Fig. 3.
Transient Cd2+ fluxes in response to 20 µM CdCl2 treatment (added at time zero) from the elongation EZ; ~2 mm from the root cap, without root hair) and mature zone (MZ; ~10 mm from the root cap, with root hair) of rice roots pre-treated with 100 µM vanadate (A), 20 mM TEA+ (B), or 100 µM Gd3+ (C). Roots were pre-treated with various inhibitors for 1 h before the CdCl2 solution (prepared in the background of each inhibitor) was added. Data are means ±SE (n=5).
Table 1.
Mean control, peak, steady-state, and average Cd2+ flux of rice roots pre-treated with different pharmacological agents
| Root zone | Cd fluxes under different pharmacological treatments (nmol m–2 s–1) | |||||
|---|---|---|---|---|---|---|
| Cd+vanadate | Cd+TEA | Cd+Gd | Cd+K | Cd+Ca | ||
| Control | EZ | 0.005 ± 0.009 | 0.006 ± 0.002 | 0.006 ± 0.038 | 0.008 ± 0.006 | 0.005 ± 0.000 |
| (–0.5) | (–0.4) | (–0.4) | (0.0) | (–0.8) | ||
| MZ | 0.003 ± 0.098 | 0.004 ± 0.063 | 0.003 ± 0.136 | 0.003 ± 0.086 | 0.003 ± 0.149 | |
| (–0.2) | (–0.1) | (–0.5) | (–0.2) | (–0.6) | ||
| Peak | EZ | 3.009 ± 0.449 | 5.106 ± 3.156 | 0.781 ± 0.883 | 5.717 ± 1.005 | 1.151 ± 0.443 |
| (–3.4**) | (–2.7*) | (–5.4***) | (–2.5*) | (–4.8***) | ||
| MZ | 1.138 ± 0.856 | 1.541 ± 0.275 | 0.341 ± 0.438 | 3.438 ± 1.493 | 0.019 ± 0.283 | |
| (–4.2**) | (–3.8**) | (–6.0***) | (–2.6*) | (–10.2***) | ||
| Steady-state | EZ | 2.129 ± 0.544 | 0.591 ± 0.137 | 0.639 ± 0.216 | 1.389 ± 0.427 | 0.230 ± 0.013 |
| (–2.4*) | (–4.2**) | (–4.1**) | (–3.0**) | (–5.6***) | ||
| MZ | 1.734 ± 0.662 | 0.239 ± 0.108 | 0.059 ± 0.279 | 1.212 ± 0.090 | 0.727 ± 0.263 | |
| (–1.8*) | (–4.7***) | (–6.7***) | (–2.3*) | (–3.1**) | ||
| Average | EZ | 1.375 ± 0.250 | 1.199 ± 0.410 | 0.596 ± 0.240 | 1.842 ± 0.536 | 0.395 ± 0.097 |
| (–3.4**) | (–3.6**) | (–4.6***) | (–2.9*) | (–5.2***) | ||
| MZ | 0.832 ± 0.548 | 0.482 ± 0.100 | 0.073 ± 0.292 | 1.287 ± 0.337 | 0.511 ± 0.180 | |
| (–3.4**) | (–4.2**) | (–6.9***) | (–2.8*) | (–4.1**) | ||
Control, Cd2+ flux under control conditions before CdCl2 treatments; Peak, Cd2+ influx immediately after CdCl2 treatments; Steady-state, Cd2+ influx at the end of the measurements; Average, Cd2+ influx measured over the 30 min after CdCl2 application. Cd+vanadate, 20 µM CdCl2 with pre-treatment with 100 µM vanadate; Cd+TEA, 20 µM CdCl2 with pre-treatment with 20 mM TEA+; Cd+Gd, 20 µM CdCl2 with pre-treatment with 100 µM Gd3+; Cd+K, 20 µM CdCl2 with pre-treatment with 10 mM K+; Cd+Ca, 20 µM CdCl2 with pre-treatment with 5 mM Ca2+. Cd2+ flux data are the mean ±SE (n=5). Data in parentheses are the fold changes of each pharmacological treatment relative to Cd treatment only, fold change=log2[(Cd2+ flux)pharmacological treatment/(Cd2+ flux)Cd only]. *, **, or *** represents the significance between the treatment with CdCl2 plus various pharmaceuticals and the treatment with CdCl2 only at P <0.05, 0.01, or 0.001.
Root pre-treatment in 100 µM vanadate, a well-known inhibitor of plasma membrane (PM) H+-ATPase, caused reduction of the net Cd2+ influx response to 20 µM CdCl2 treatment, leading to a reduction in the magnitude of Cd2+ influx by 3.4-fold for the EZ and 4.2-fold for the MZ, the steady-state Cd2+ influx by 2.4-fold for the EZ and 1.8-fold for the MZ, and the mean Cd2+ influx by 3.4-fold for both the EZ and MZ (Fig. 3A; Table 1). However, such an inhibitory effect of vanadate was short lived and started to recover ~20 min after adding Cd treatment for the EZ and only 5 min for the MZ.
TEA+ is a known blocker of K+-selective channels (Maathuis and Sanders, 1996). Pre-treatment with 20 mM TEA+ caused a significant decrease of the Cd2+ influx, with the magnitude of Cd2+ influx reduced by 2.7-fold for the EZ and 3.8-fold for the MZ, the steady-state Cd2+ influx reduced by 4.2-fold for the EZ and 4.7-fold for the MZ, and the mean Cd2+ influx reduced by 3.6-fold for the EZ and 4.2-fold for the MZ (Fig. 3B; Table 1).
Gd3+ is a known blocker of non-selective cation channels (NSCCs), which are known to be permeable to cations such as Ca2+, K+, and Na+ (Demidchik et al., 2002). In comparison with vanadate and TEA+, pre-treatment with 100 µM Gd3+ induced the largest inhibitory effect on the response of Cd2+ flux (Fig. 3C; Table 1). It totally blocked the Cd2+ influx to <1 nmol m–2 s–1 in both the EZ and MZ. The magnitude, steady-state, and mean Cd2+ influx were reduced by 4.1- to 5.4-fold for the EZ and by 6.0- to 6.9-fold for the MZ.
Moreover, like the performance under the treatment with Cd only, the EZ also showed much higher net Cd2+ influxes than the EZ under treatments with all three inhibitors, with a 1.2- to 10.8-fold difference in the magnitude, steady-state, or mean value of the Cd2+ influx.
Net Cd2+ flux changes under elevated external K and Ca concentration
It has been well documented that Cd significantly interferes with the uptake and accumulation of various nutrients in plant tissues, including K+ and Ca2+ (Chang et al., 2003; S. Li et al., 2012; Farooq et al., 2016). Conversely, the supplementation of K+ and Ca2+ was also reported to reduce Cd accumulation efficiently in plant tissues (Ahmad et al., 2015, 2016). To examine whether the supplementation of K+ and Ca2+ interfered with the kinetics of net Cd2+ influx, rice roots were pre-treated with 10 mM K+ or 5 mM Ca2+ for 1 h before measuring the net Cd2+ flux. Pre-treatment with 10 mM K+ strongly reduced the net Cd2+ influx into the rice root, causing an ~2.5-fold reduction in the magnitude of Cd2+ influx and an ~3.0-fold reduction in the mean Cd2+ influx for both the EZ and MZ in comparison with the basic K+ level of 0.5 mM (Fig. 4A; Table 1). Surprisingly, the effect of 5 mM Ca2+ on Cd2+ influx was different between the EZ and MZ (Fig. 4B; Table 1). The pre-treatment with 5 mM Ca2+ reduced the Cd2+ influx in the EZ by ~5.0-fold. However, the Cd2+ flux response to 20 µM CdCl2 in the MZ was altered by the pre-treatment with 5 mM Ca2+. At the beginning of Cd application, the net Cd2+ influx was completely inhibited, whereas it was slowly recovered with extension of the measuring time.
Fig. 4.
Transient Cd2+ fluxes in response to 20 µM CdCl2 treatment (added at time zero) from the elongation zone (EZ; ~2 mm from the root cap, without root hair) and mature zone (MZ; ~10 mm from the root cap, with root hair) of rice roots pre-treated with 10 mM K+ (A) or 5 mM Ca2+ (B). Roots were pre-treated with solution containing high K+ or Ca2+ concentration for 1 h before the CdCl2 solution (prepared in the background of high K+ or Ca2+ concentration). Data are means ±SE (n=5).
Total Cd concentration
Total Cd concentration was determined in either root segments or the bulk roots of rice seedlings pre-treated with different pharmacological agents after 3 d of Cd exposure to verify the results of MIFE measurements (Fig. 5). Results from Cd determination were mostly in agreement with MIFE data. The root tip (0–5 mm from root cap) had a significantly higher Cd concentration than those segments far away from it (5–10, 10–15, and 15–20 mm from the root cap) (P<0.05; Fig. 5A). Pre-treatment with vanadate, TEA+, Gd3+, K+, and Ca2+ significantly restricted the accumulation of Cd in rice roots, both in each root segment and in the bulk roots (Fig. 5). Their inhibitory effects on Cd accumulation were in the order of Gd3+ (58.1%) ~Ca2+ (55.6%) >K+ (32.2%) ~TEA+ (31.1%) >vanadate (16.5%) (Fig. 5B).
Fig. 5.
Total Cd concentration in rice root segments (0–5, 5–10, 10–15, and 15–20 mm from the root cap (A) and bulk roots (B) after 3 d of 20 µM CdCl2 treatment without or with 100 µM vanadate, 20 mM TEA+, 100 µM Gd3+, 10 mM K+, or 5 mM Ca2+. Roots were pre-treated with various pharmaceuticals for 1 h before the CdCl2 solution (prepared in the background of each pharmacological reagent) was added. Data are means ±SE (n=5). Different lower case letters represent a significant difference between root segments at P<0.05; different upper case letters represent the significant difference between treatments at P<0.05.
The distribution of Cd along the rice root
The effect of inhibitors and nutrients on Cd2+ distribution along rice roots (~5 mm long from the root cap) was further investigated using Leadmium Green AM dye after 24 h of Cd exposure (Fig. 6). One hour after the incubation with the fluorescent dye, a clear and bright green fluorescence was observed in the roots treated with 20 µM Cd only (Fig. 6). Pre-treatment with ion channel blockers TEA+ and Gd3+ suppressed Cd green fluorescence in rice roots (Fig. 6), with the NSCC blocker Gd3+ showing a much stronger inhibitory effect than the potassium-selective channel blocker TEA+. Supplementation of K+ and Ca2+ also caused significant reduction in the Cd green fluorescence in rice roots (Fig. 6), but much greater inhibition was seen for Ca2+ than for K+. Pre-treatment with vanadate showed much less impact on Cd green fluorescence in rice roots compared with the other pharmacological treatments (Fig. 6), in agreement with the results of the Cd concentration (Fig. 5). These results suggest that Cd exhibits high affinity for Ca2+-binding sites over K+-binding sites during transport across the PM. In addition, a stronger intensity of Cd green fluorescence was observed close to the root cap, regardless of pre-treatments, in accordance with the results of the Cd2+ flux profile along the root in our study Fig. 1 or in previous studies (Piñeros et al., 1998; Sun et al., 2013).
Fig. 6.
Cd2+ accumulation in rice root, visualized by fluorescent imaging using Leadamium Green AM dye. Control, root without CdCl2 treatment; Cd, roots treated with 20 µM CdCl2 only for 24 h; Cd + Van, Cd + TEA, Cd + Gd, Cd + K or Cd + Ca: roots pre-treated with 100 µM vanadate, 20 mM TEA+, 100 µM Gd3+, 10 mM K+, or 5 mM Ca2+, respectively for 1 h before the CdCl2 solution (prepared in the background of each pharmaceutical) was added. Green fluorescence in the image represents the binding of the dye to Cd. One (of 8–12) representative images is shown for each treatment. Scale bar=500 µm. Data are means ±SE (n=8–12). Different lower case letters represent a significant difference between pharmacological treatments at P<0.05.
Gene expression at different root zones
The measurements of MIFE (Figs 1, 2), ICP-MS (Fig. 5), and Leadmium Green AM dying (Fig. 6) indicated that there was a significant difference in Cd influx and accumulation between the root tip and the MZ. To reveal the reason for such a difference, the expression level of genes for Cd transport was investigated in the root tip (0–5 mm from the root cap) and the MZ (15–20 mm from the root cap). As expected, the expression of OsIRT1, OsNRAMP1, OsNRAMP5, and OsZIP1 was induced by 20 µM Cd (Fig. 7), regardless of root zones. Surprisingly, the transcript levels of all these genes were much higher in the root tip than in the MZ prior to or at 3 h after Cd treatment (Fig. 7). With the exposure time of Cd treatment increasing to 3 d, although the difference in gene expression between the root tip and the MZ was greatly reduced, the transcript levels of OsIRT1 and OsZIP1 were still significantly higher in the root tip than in the MZ. These results indicated that the root tip had much greater capacity for Cd transport than the MZ, which consequently led to more Cd influx and accumulation in the root tip.
Fig. 7.
Gene expression of Cd transporters in rice root tip (0–5 mm from the root cap) and mature (15–20 mm from the root cap) zones prior to (0 h) or after onset of 20 µM CdCl2 for 3 h and 3 d. Gene expression in the mature zone at 0 h was normalized to 1, and gene expression in the other root tissues or at the other time were all compared relative to it. Data are means ±SE (n=3 biological replicates). *, **, or *** represent a significant difference between two root zones at P<0.05, 0.01, or 0.001.
Gene expression under pharmacological treatments
As mentioned above, Cd2+ influx and uptake were significantly inhibited by the treatments with different pharmaceuticals (100 µM vanadate, 20 mM TEA+, and 100 µM Gd3+) and with elevation of Ca and K levels in the medium (5 mM CaCl2 and 10 mM KCl) (Figs 3–6). One possible reason for such an inhibitory impact of these pharmacological treatments on Cd2+ influx could be attributed to the significant reduction in Cd transport activity by the application of these pharmacological treatments. Therefore, the gene expression of four major plasma transporters for Cd, namely OsIRT1, OsNRAMP5, OsNRAMP1, and OsZIP1, was determined by qRT-PCR under treatment with 20 µM Cd with or without the pharmaceuticals. Under treatment with 20 µM Cd for 3 h and 3 d, the expression of OsIRT1, OsNRAMP5, OsNRAMP1, and OsZIP1 was significantly induced (Fig. 8A). With the pharmaceuticals under the Cd condition, the expression of OsIRT1 and OsZIP1 was inhibited by the application of vanadate and the elevation of Ca and K in the medium, but such inhibition was nearly extinguished when the exposure time increased to 3 d (Fig. 8B). The expression of OsNRAMP1 was significantly inhibited by the application of vanadate at both exposure times of Cd treatment (Fig. 8B). Surprisingly, however, the expression of OsNRAMP5 was not inhibited but induced by the all of the above pharmacological treatments (Fig. 8B). These results indicated that the pharmacological treatment-induced (especially Gd3+-induced) inhibition in Cd2+ influx had little to do with the gene expression of the above four major plasma Cd transporters.
Fig. 8.
Gene expression of Cd transporters, Ca or K channels, and transporters under 20 µM CdCl2 without (A) or with (B) pharmacological treatments: 100 µM vanadate, 20 mM TEA+, 100 µM Gd3+, 10 mM K+, or 5 mM Ca2+ in the bulk roots. (A) Gene expression under Cd was compared relative to the control condition without Cd; (B) gene expression under Cd treatment plus pharmaceuticals was compared relative to the treatment with Cd only (H2O). Data are means ±SE (n=3 biological replicates). # in (A) represents the genes induced by 20 µM CdCl2; *, **, or *** in (B) represent the significance between the treatment with CdCl2 plus various pharmaceuticals and the treatment with CdCl2 only at P<0.05, 0.01, or 0.001.
To examine the role of genes relevant to the pharmacological treatment-induced inhibition in Cd2+ influx, the transcript levels of 10 genes involved in transmembrane transport of Ca (OsANN1, OsANN4, OsCNGC1, OsGLR3.4, OsTPC1, OsACA3, OsACA7, and OsCAX2) and K (OsAKT1 and OsHAK5) were also determined under treatment with 20 µM Cd with or without the pharmaceuticals. Results showed that three genes encoding Ca channels and transporters, namely OsANN4, OsGLR3.4 and OsCAX2, were induced by the treatment with 20 µM Cd (Fig. 8A). The expression of OsANN4 was inhibited by all the pharmacological treatments at 3 h and 3 d of Cd treatment (Fig. 8B). The expression of OsGLR3.4 was significantly inhibited by the application of Gd3+ and the elevation of Ca and K in the medium at both exposure times of Cd treatment (Fig. 8B). On the other hand, the expression of OsCAX2 was significantly inhibited by the application of vanadate and TEA+ at both exposure times of Cd treatment and the elevation of K at 3 d of Cd treatment (Fig. 8B). The expression of these three genes under the pharmacological treatments partially coincided with the results of Cd2+ influx and uptake (Figs 3–6), indicating a possible function for these proteins in transmembrane Cd transport.
Discussion
Cd uptake varies longitudinally along the rice root
In this study, the spatial kinetics of net Cd2+ flux across rice root cells was examined using the MIFE technique, which has a high spatial and temporal resolution and sensitivity to ion movement. In agreement with the previous microelectrode measurements in other plant species (Piñeros et al., 1998; He et al., 2011; Sun et al., 2013), the present study found that the Cd2+ influx in roots of rice exposed to 20 µM CdCl2 was much greater in the root tip (0–2 mm from the root cap) than in the MZ (10–20 mm from root cap) (Figs 1, 2), which was further evidenced by the measurements of Cd concentration in root segments and the fluorescent labeling of Cd ions in the root tip, whether with or without pre-treatments (Figs 5, 6). Similar longitudinal variation in Cd2+ influx was also observed in sunflower roots using radioactive tracer techniques (Laporte et al., 2013).
There may be several reasons to explain such longitudinal variation in Cd2+ influx. First, it may result from the alteration of root anatomy; that is, the development of Casparian bands and suberin lamellae in the endodermis and exodermis and cell lignification (Schreiber et al., 1999; White, 2001; Laporte et al., 2013). It has been documented that the presence of Cd produced Casparian bands, suberin lamellae, and lignification, which could restrict the apoplastic diffusion of Cd in root cells and consequently the whole-plant Cd accumulation (Enstone et al., 2002; Lux et al., 2004, 2011; Redjala et al., 2011; Laporte et al., 2013). In the present study, however, no significant difference in the development of Casparian bands and suberin lamellae and cell lignification was observed between the root tip (0–2 mm from root cap) and the MZ (10–15 mm from root cap) after 3 d of 20 µM CdCl2 (data not shown), indicating an inability of the apoplastic barrier to explain the occurrence of longitudinal variation in Cd2+ influx in this study. Another plausible reason for the longitudinal variation in Cd2+ influx may be attributed to the higher Cd transport activity of the root tip than the MZ, which could be reflected by the expression level of transporters for Cd. As expected, much higher expression of four well-known Cd transport genes, namely OsIRT1, OsNRAMP1, OsNRAMP5, and OsZIP1 (Uraguchi and Fujiwara, 2012), was observed in the root tip than in the MZ, both prior to and after onset of Cd treatment (Fig. 7). These findings suggest that the cells of the meristem and EZs have stronger absorbing capacity for Cd uptake than the mature root cells, which might be attributed to the greater energy in that part of roots (Jones, 1998).
Cd can enter into rice root cells via the pathways for Ca and K
A fundamental understanding of how Cd is taken up by rice roots would be a critical issue for revealing the mechanisms of Cd accumulation in rice grains. However, the non-essentiality and toxicity of Cd to plants make its entry into root cells a deep mystery. With years of effort, many transporters for essential elements, such as OsNRAMPs, OsZIPs, and OsIRTs, have been demonstrated to have an influx activity of Cd2+ in rice roots (reviewed by Uraguchi and Fujiwara, 2012, 2013). In the present study, our results revealed that the transcript levels of OsIRT1, OsNRAMP1, OsNRAMP5, and OsZIP1 were indeed significantly induced by Cd treatment (Fig. 8A), proving their contribution to Cd uptake by rice roots. With the pharmacological treatments (100 µM vanadate, 20 mM TEA+, 100 µM Gd3+, 5 mM CaCl2, or 10 mM KCl) under Cd conditions, the expression of these four genes was either not changed or even up-regulated (Fig. 8B), especially for OsNRAMP5 which is considered to be a major route of Cd uptake from the external environment and entry into cells in rice (Ishimaru et al., 2012; Sasaki et al., 2012). However, our MIFE and ICP-MS measurements revealed that the Cd2+ influx and uptake were significantly inhibited by the pharmacological treatments mentioned above (Figs 3–5), suggesting that these transporters only contribute in part to root Cd2+ influx and uptake, at least in the case where the external Cd concentration is as high as 20 µM in the present study (Lee and An, 2009; Takahashi et al., 2014). Therefore, it can be assumed that there might be other pathways for Cd entry into rice root cells.
It is well known that there is a competition between Cd2+ and Ca2+, because of their similarities in charge and ionic radius (Lindberg et al., 2004; L.Z. Li et al., 2012; S. Li et al., 2012; Ahmad et al., 2016). Many studies have reported that Cd may compete with Ca for uptake through ion channels in insects (Craig et al., 1999), humans (Souza et al., 1997), and plant guard cells (Perfus-Barbeoch et al., 2002). The previous studies on Cd hyperaccumulators have also suggested that Cd could possibly enter the PM of root cells via Ca2+ channels (Lindberg et al., 2004; Lu et al., 2010; L.Z. Li et al., 2012; Zhang et al., 2017). To obtain an insight into the role of the Ca pathway in rice root Cd uptake, the present study investigated the competitive interactions of Cd and Ca with different techniques. The results of pharmacological measurements clearly showed that the elevation of the Ca level (from 0.1 mM to 5 mM) in the medium significantly inhibited root Cd uptake, in terms of both transient Cd2+ influx (Fig. 4; Table 1) and long-term total Cd uptake in rice roots (Figs 5, 6). These results suggested that Cd entry into rice roots was probably through Ca channels or transporters. Actually, several kinds of calcium-permeable channels, such as depolarization-activated calcium channels (DACCs), hyperpolarization-activated calcium channels (HACCs), and voltage-insensitive cation channels (VICCs), were reported to mediate Cd transport in plant roots (Lux et al., 2011, and references therein). All of these channels are relatively non-selective between cations, and can be blocked by the NSCCs blocker Gd3+ (Lux et al., 2011, and references therein). Indeed, the transient net Cd2+ influx of rice root in this study was completely blocked by Gd3+ (Fig. 3; Table 1) and the total Cd concentration in rice root was reduced by 58.1% (Fig. 5), implying that these non-selective channels may mediate Cd2+ transmembrane transport as it does for Ca2+ (S. Li et al., 2012).
Potassium is the most abundant element in the plant cytosol and plays a vital role in nullifying the adverse impacts of stress on plants (Cakmak, 2005). Ahmad et al. (2016) reported that K supplementation could minimize the uptake of Cd. In the present study, the elevation of the K level (from 0.5 mM to 10 mM) in the medium was also observed to reduce the transient Cd2+ influx (Fig. 4; Table 1) and long-term total Cd uptake in rice roots (Figs 5, 6). These results indicated that the uptake of Cd into the cytosol of rice roots proceeds through channels permeable to K+. This was confirmed by the >95% reduction in the net Cd2+ influx (Fig. 3; Table 1) and 31.1% reduction in total Cd concentration (Fig. 5) of rice roots pre-treated with 20 mM TEA+, which is a well-known blocker of K+-selective channels (Maathuis and Sanders, 1996). A similar result was also found in the pharmacological measurements with fluorescent imaging in this study (Fig. 6) and in previous studies (Lindberg et al., 2004). Unfortunately, no K channel or transporter has been found in plants yet to have the capacity to transport Cd2+, and the transcript levels of two well-known genes for transporting K to the cytosol (i.e. OsAKT1 and OsHAK5) were not significantly induced by Cd treatment (Fig. 8A). However, one prokaryotic potassium channel isolated from Methanobacterium thermoautotrophicum named MthK, which contains a region called the regulator of the conductance of K+ (RCK) domain, has been found to bind divalent cations, such as Cd2+ and Ca2+ (Jiang et al., 2002; Dvir et al., 2010). It is also activated by Cd2+ more effectively than by Ca2+ (Kuo et al., 2007). So, it could be hypothesized that some K channels, which have structural homology to the RCK domain of MthK, might have an ability to binding to Cd2+ similar to that of MthK. Indeed, several channels from higher plants, such as CASTOR and POLLUX in Lotus japonicas (Charpentier et al., 2008), DMI1 (DOES NOT MAKE INFECTIONS1) in Medicago truncatula (Ané et al., 2004), and SYM8 in Pisum sativum, have already been reported to share a short stretch of predicted structural homology with the pore region of MthK (Edwards et al., 2007). All these channels are identified to bind Ca2+ and have the biological function of ion transmembrane transport. Yet there is no information reported yet on the possibility of these channels binding to and transporting Cd2+.
Annexins and GLRs seems to be an important pool to explore the candidate channels participating in Cd absorption into rice root
It has been speculated that in Arabidopsis the gene families annotated as cyclic-nucleotide gated channels (CNGCs) and/or glutamate receptors (GLRs) are the most likely sources of genes encoding VICCs (Demidchik et al., 2002; White, 2004; Swarbreck et al., 2013). Moreover, it has also been suggested that the AtTPC1 gene encodes a DACC (Furuichi et al., 2001), and the annexin genes encode HACCs (White et al., 2002). Thus, the expression level of several genes in these families and the other Ca transporter genes, namely OsGLR3.4 (an ortholog of AtGLR3.4; Vincill et al., 2012; Ni et al., 2016), OsCNGC1 (an ortholog of AtCNGC1; Nawaz et al., 2014), OsTPC1 (Kurusu et al., 2004), OsANN1 and OsANN4 (LOC_Os01g31270 and LOC_Os05g31760; Jami et al., 2012), OsACA3 and OsACA7, and OsCAX2, which are involved in the transmembrance transport of Ca2+ (Singh et al., 2014), were determined after onset of 20 µM Cd for 3 h and 3 d with or without pharmacological treatments. The results showed that three of them, OsANN4, OsGLR3.4, and OsCAX2, were induced by the treatment with 20 µM Cd (Fig. 8A). However, the expression of these genes was reduced by the application of pharmacological treatments to a different extent (Fig. 8B), partially coinciding with the results of Cd2+ influx and uptake (Figs 3–6), suggesting a possible function of these channels or transporters in transmembrane Cd transport. The response of annexin genes to Cd has been reported previously. Orthologs of OsANN4, such as ANNAh3 in peanut (He et al., 2015) and ZmAnx9 in maize (Zhou et al., 2013), were found to be induced by short (2–24 h) or long (14 d) Cd treatments. Furthermore, the ortholog of OsCAX2 in Arabidopsis, AtCAX2, has been identified as a PM Cd transporter into root cells (Hirschi et al., 2000). Therefore, the contribution of these channels or transporters to Cd absorption into rice root should not be ignored.
Plasma membrane potential plays an important role in controlling transmembrane Cd transport
It is surprising that much greater inhibition of rice root Cd uptake was seen for Ca2+ and Gd3+ than for K+ and TEA+ (Figs 3–6; Table 1). Furthermore, a patch-clamp experiment on guard cells of fava bean demonstrated that Cd could permeate through Ca channels rather than K channels in guard cells (Perfus-Barbeoch et al., 2002). These results suggest that Cd exhibits high affinity for Ca2+-binding sites over K+-binding sites during transport across the PM. In addition, pre-treatment with vanadate (a well-known inhibitor of PM H+-ATPase) also showed a significant impact on net influx (Fig. 3; Table 1) and the total uptake (Figs 5, 6) of Cd2+ in rice roots, although its inhibitory effect was much less compared with the other pharmacological agents used in the present study, indicating that the uptake of Cd into root cells is partly dependent on membrane potential. So, it cannot be excluded that the pharmaceuticals in this study may inhibit the uptake of cadmium by depolarizing the PM, as they all can trigger membrane potential depolarization (Huddart and Hill, 1996). It has been reported that high K+ in the medium was likely to depolarize the membrane by opening high conductance K+ channels (Beilby, 1985), and high Ca2+ in the medium could reduce background conductance which was thought to be mediated by the NSCCs (Shepherd et al., 2008). Therefore, it would be expected that Cd may enter root cells via hyperpolarized ion channels, for example the HACCs, such as OsANN4 in the present study, like those described in previous studies (Perfus-Barbeoch et al., 2002; Lux et al., 2011; L.Z. Li et al., 2012).
In conclusion, Cd uptake exhibited a clear longitudinal variation along rice roots, with the root tip (including the meristem and EZs) showing much higher Cd2+ influx and Cd concentration than the MZ, which might be attributed to the much higher gene expression of OsIRT1, OsNRAMP1, OsNRAMP5, and OsZIP1 in comparison with the MZ. The Cd2+ uptake by rice root cells was restricted by the blockage of ion channels and the elevated external levels of K+ and Ca2+, regardless of Cd exposure time, suggesting an important role for ion channels permeable to cations such as K+ and Ca2+ in transmembrane Cd transport. The Cd transporters OsIRT1, OsNRAMP1, OsNRAMP5, and OsZIP1 are in part responsible for the Cd2+ entry into rice root, and the Ca channels OsAAN4 and OsGLR3.4 might play an important role in rice root Cd uptake. However, the permeability and selectivity of these channels to Cd2+ and their value in ‘low-Cd’ rice innovation need to be further elucidated using electrophysiological and molecular techniques.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Primers for qRT-PCR.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos 31371559 and 31571599), Public Benefit Technology Applied Research Project of Zhejiang Province, China (2014C32035), the Chinese Academy of Agricultural Science Innovation Project (grant no. CAAS-ASTIP-2013-CNRRI), and Jiangsu Collaborative Innovation Center for Modern Crop Production (JCIC-MCP). We are grateful to the China National Rice Seed Bank for supplying the rice seed.
Abbreviations
Abbreviations
- Ca
calcium
- Cd
cadmium
- CNGC
cyclic-nucleotide gated channel
- DACC
depolarization-activated calcium channel
- EZ
elongation zone
- GLR
glutamate receptor
- HACC
hyperpolarization-activated calcium channel;
- K
potassium
- LCT
low affinity calcium transporter
- MIFE
microelectrode ion flux estimation
- MZ
mature zone
- NRAMP
natural resistance-associated macrophage protein
- NSCC
non-selective cation channel
- PM
plasma membrane
- TEA+
tetraethylammonium
- VICC
voltage-insensitive cation channel
- YSL
Yellow-Stripe 1-Like
- ZIP
zinc/iron-regulated transporter-like protein
References
- Ahmad P, Abdel LAA, Abd AEF, Hashem A, Sarwat M, Anjum NA, Gucel S. 2016. Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.). Frontiers in Plant Science 7, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran LS. 2015. Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS One 10, e0114571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ané JM, Kiss GB, Riely BK, et al. 2004. Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303, 1364–1367. [DOI] [PubMed] [Google Scholar]
- Beilby MJ. 1985. Potassium channels at Chara plasmalemma. Journal of Experimental Botany 36, 228–239. [Google Scholar]
- Berkelaar E, Hale B. 2000. The relationship between root morphology and cadmium accumulation in seedlings of two durum wheat cultivars. Canadian Journal of Botany 78, 381–387. [Google Scholar]
- Cailliatte R, Lapeyre B, Briat JF, Mari S, Curie C. 2009. The NRAMP6 metal transporter contributes to cadmium toxicity. Biochemical Journal 422, 217–228. [DOI] [PubMed] [Google Scholar]
- Cakmak I. 2005. The role of potassium in alleviating detrimental effects of abiotic stresses in plants. Journal of Plant Nutrition and Soil Science 168, 521–530. [Google Scholar]
- Chang Y, Zouari M, Gogorcena Y, Lucena JJ, Abadía J. 2003. Effects of cadmium and lead on ferric chelate reductase activities in sugar beet roots. Plant Physiology and Biochemistry 41, 999–1005. [Google Scholar]
- Charpentier M, Bredemeier R, Wanner G, Takeda N, Schleiff E, Parniske M. 2008. Lotus japonicus CASTOR and POLLUX are ion channels essential for perinuclear calcium spiking in legume root endosymbiosis. The Plant Cell 20, 3467–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens S. 2001. Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212, 475–486. [DOI] [PubMed] [Google Scholar]
- Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI. 1998. The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proceedings of the National Academy of Sciences, USA 95, 1243–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A, Hare L, Tessier A. 1999. Experimental evidence for cadmium uptake via calcium channels in the aquatic insect Chironomus staegeri. Aquatic Toxicology 44, 255–262. [Google Scholar]
- Demidchik V, Davenport RJ, Tester M. 2002. Nonselective cation channels in plants. Annual Review of Plant Biology 53, 67–107. [DOI] [PubMed] [Google Scholar]
- Dvir H, Valera E, Choe S. 2010. Structure of the MthK RCK in complex with cadmium. Journal of Structural Biology 171, 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards A, Heckmann AB, Yousafzai F, Duc G, Downie JA. 2007. Structural implications of mutations in the pea SYM8 symbiosis gene, the DMI1 ortholog, encoding a predicted ion channel. Molecular Plant-Microbe Interactions 20, 1183–1191. [DOI] [PubMed] [Google Scholar]
- Enstone DE, Peterson CA, Ma F. 2002. Root endodermis and exodermis: structure, function, and responses to the environment. Journal of Plant Growth Regulation 21, 335–351. [Google Scholar]
- Farooq MA, Detterbeck A, Clemens S, Dietz KJ. 2016. Silicon-induced reversibility of cadmium toxicity in rice. Journal of Experimental Botany 67, 3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T, Cunningham KW, Muto S. 2001. A putative two pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant & Cell Physiology 42, 900–905. [DOI] [PubMed] [Google Scholar]
- He J, Qin J, Long L, et al. 2011. Net cadmium flux and accumulation reveal tissue-specific oxidative stress and detoxification in Populus × canescens. Physiologia Plantarum 143, 50–63. [DOI] [PubMed] [Google Scholar]
- He M, Yang X, Cui S, Mu G, Hou M, Chen H, Liu L. 2015. Molecular cloning and characterization of annexin genes in peanut (Arachis hypogaea L.). Gene 568, 40–49. [DOI] [PubMed] [Google Scholar]
- Hirschi KD, Korenkov VD, Wilganowski NL, Wagner GJ. 2000. Expression of Arabidopsis CAX2 in tobacco. Altered metal accumulation and increased manganese tolerance. Plant Physiology 124, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huddart H, Hill RB. 1996. Ionic dependency of membrane potential and autorhythmicity in the atrium of the whelk Busycon canaliculatum. General Pharmacology 27, 819–825. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Tsukamoto T, et al. 2006. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. The Plant Journal 45, 335–346. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Takahashi R, Bashir K, et al. 2012. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Scientific Reports 2, 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jami SK, Clark GB, Ayele BT, Roux SJ, Kirti PB. 2012. Identification and characterization of annexin gene family in rice. Plant Cell Reports 31, 813–825. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature 417, 515–522. [DOI] [PubMed] [Google Scholar]
- Jones DL. 1998. Organic acids in the rhizosphere—a critical review. Plant and Soil 205, 25–44. [Google Scholar]
- Kuo MM, Baker KA, Wong L, Choe S. 2007. Dynamic oligomeric conversions of the cytoplasmic RCK domains mediate MthK potassium channel activity. Proceedings of the National Academy of Sciences, USA 104, 2151–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T, Sakurai Y, Miyao A, Hirochika H, Kuchitsu K. 2004. Identification of a putative voltage-gated Ca2+-permeable channel (OsTPC1) involved in Ca2+ influx and regulation of growth and development in rice. Plant & Cell Physiology 45, 693–702. [DOI] [PubMed] [Google Scholar]
- Laporte MA, Denaix L, Pagès L, Sterckeman T, Flénet F, Dauguet S, Nguyen C. 2013. Longitudinal variation in cadmium influx in intact first order lateral roots of sunflower (Helianthus annuus. L). Plant and Soil 372, 581–595. [Google Scholar]
- Lee S, An G. 2009. Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant, Cell & Environment 32, 408–416. [DOI] [PubMed] [Google Scholar]
- Li LZ, Liu XL, Peijnenburg WJG, Zhao JM, Chen XB, Yu JB, Wu HF. 2012. Pathways of cadmium fluxes in the root of the halophyte Suaeda salsa. Ecotoxicology and Environmental Safety 75, 1–7. [DOI] [PubMed] [Google Scholar]
- Li S, Yu J, Zhu M, Zhao F, Luan S. 2012. Cadmium impairs ion homeostasis by altering K+ and Ca2+ channel activities in rice root hair cells. Plant, Cell & Environment 35, 1998–2013. [DOI] [PubMed] [Google Scholar]
- Lindberg S, Landberg T, Greger M. 2004. A new method to detect cadmium uptake in protoplasts. Planta 219, 526–532. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lu L, Tian S, Zhang M, Zhang J, Yang X, Jiang H. 2010. The role of Ca pathway in Cd uptake and translocation by the hyperaccumulator Sedum alfredii. Journal of Hazardous Materials 183, 22–28. [DOI] [PubMed] [Google Scholar]
- Lux A, Luxová M, Abe J, Morita S. 2004. Root cortex: structural and functional variability and responses to environmental stress. Root Research 13, 117–131. [Google Scholar]
- Lux A, Martinka M, Vaculík M, White PJ. 2011. Root responses to cadmium in the rhizosphere: a review. Journal of Experimental Botany 62, 21–37. [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. 1996. Mechanisms of potassium absorption by higher plant roots. Physiologia Plantarum 96, 158–168. [Google Scholar]
- Nawaz Z, Kakar KU, Saand MA, Shu QY. 2014. Cyclic nucleotide-gated ion channel gene family in rice, identification, characterization and experimental analysis of expression response to plant hormones, biotic and abiotic stresses. BMC Genomics 15, 853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman IA. 2001. Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant, Cell & Environment 24, 1–14. [DOI] [PubMed] [Google Scholar]
- Ni J, Yu Z, Du G, Zhang Y, Taylor JL, Shen C, Xu J, Liu X, Wang Y, Wu Y. 2016. Heterologous expression and functional analysis of rice GLUTAMATE RECEPTOR-LIKE family indicates its role in glutamate triggered calcium flux in rice roots. Rice 9, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C. 2002. Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. The Plant Journal 32, 539–548. [DOI] [PubMed] [Google Scholar]
- Piñeros MA, Shaff JE, Kochian LV. 1998. Development, characterization, and application of a cadmium-selective microelectrode for the measurement of cadmium fluxes in roots of Thlaspi species and wheat. Plant Physiology 116, 1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redjala T, Zelko I, Sterckeman T, Legue V, Lux A. 2011. Relationship between root structure and root cadmium uptake in maize. Environmental and Experimental Botany 71, 241–248. [Google Scholar]
- Rizwan M, Ali S, Adrees M, Rizvi H, Zia-Ur-Rehman M, Hannan F, Qayyum MF, Hafeez F, Ok YS. 2016. Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environmental Science and Pollution Research International 23, 17859–17879. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Yamaji N, Yokosho K, Ma JF. 2012. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. The Plant Cell 24, 2155–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wirén N. 2004. ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. Journal of Biological Chemistry 279, 9091–9096. [DOI] [PubMed] [Google Scholar]
- Schreiber L, Hartmann K, Skrabs M, Zeier J. 1999. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. Journal of Experimental Botany 50, 1267–1280. [Google Scholar]
- Shabala S. 2006. Non-invasive microelectrode ion flux measurements in plant stress physiology. In: Volkov A, ed. Plant electrophysiology—theory and methods. Heidelberg: Springer, 35–72. [Google Scholar]
- Shepherd VA, Beilby MJ, Al Khazaaly SA, Shimmen T. 2008. Mechano-perception in Chara cells: the influence of salinity and calcium on touch-activated receptor potentials, action potentials and ion transport. Plant, Cell & Environment 31, 1575–1591. [DOI] [PubMed] [Google Scholar]
- Singh A, Kanwar P, Yadav AK, Mishra M, Jha SK, Baranwal V, Pandey A, Kapoor S, Tyagi AK, Pandey GK. 2014. Genome-wide expressional and functional analysis of calcium transport elements during abiotic stress and development in rice. FEBS Journal 281, 894–915. [DOI] [PubMed] [Google Scholar]
- Souza V, Bucio L, Gutiérrez-Ruiz MC. 1997. Cadmium uptake by a human hepatic cell line (WRL-68 cells). Toxicology 120, 215–220. [DOI] [PubMed] [Google Scholar]
- Sun J, Wang R, Liu Z, Ding Y, Li T. 2013. Non-invasive microelectrode cadmium flux measurements reveal the spatial characteristics and real-time kinetics of cadmium transport in hyperaccumulator and nonhyperaccumulator ecotypes of Sedum alfredii. Journal of Plant Physiology 170, 355–359. [DOI] [PubMed] [Google Scholar]
- Swarbreck SM, Colaço R, Davies JM. 2013. Plant calcium-permeable channels. Plant Physiology 163, 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Ishimaru Y, Shimo H, et al. 2014. From laboratory to field: OsNRAMP5-knockdown rice is a promising candidate for Cd phytoremediation in paddy fields. PLoS One 9, e98816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uraguchi S, Fujiwara T. 2012. Cadmium transport and tolerance in rice: perspectives for reducing grain cadmium accumulation. Rice 5, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uraguchi S, Fujiwara T. 2013. Rice breaks ground for cadmium-free cereals. Current Opinion in Plant Biology 16, 328–334. [DOI] [PubMed] [Google Scholar]
- Vincill ED, Bieck AM, Spalding EP. 2012. Ca2+ conduction by an amino acid-gated ion channel related to glutamate receptors. Plant Physiology 159, 40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Guo W, Ye S, Wei P, Ow DW. 2016. Reduction of Cd in rice through expression of OXS3-like gene fragments. Molecular Plant 9, 301–304. [DOI] [PubMed] [Google Scholar]
- White PJ. 2001. The pathways of calcium movement to the xylem. Journal of Experimental Botany 52, 891–899. [DOI] [PubMed] [Google Scholar]
- White PJ. 2004. Calcium signals in root cells: the roles of plasma membrane calcium channels. Biologia 59/S13, 77–83. [Google Scholar]
- White PJ, Bowen HC, Demidchik V, Nichols C, Davies JM. 2002. Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochimica et Biophysica Acta 1564, 299–309. [DOI] [PubMed] [Google Scholar]
- White PJ, Broadley MR. 2003. Calcium in plants. Annals of Botany 92, 487–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Choi D, Kim D, Lee B. 2010. Genotypic variation of cadmium accumulation and distribution in rice. Journal of Crop Science and Biotechnology 13, 69–73. [Google Scholar]
- Zeng F, Chen S, Miao Y, Wu F, Zhang G. 2008. Changes of organic acid exudation and rhizosphere pH in rice plants under chromium stress. Environmental Pollution 155, 284–289. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sa G, Zhang Y, et al. 2016. Paxillus involutus-facilitated Cd2+ influx through plasma membrane Ca2+-permeable channels is stimulated by H2O2 and H+-ATPase in ectomycorrhizal Populus × canescens under cadmium stress. Frontiers in Plant Science 7, 1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ML, Yang XB, Zhang Q, Zhou M, Zhao EZ, Tang YX, Zhu XM, Shao JR, Wu YM. 2013. Induction of annexin by heavy metals and jasmonic acid in Zea mays. Functional & Integrative Genomics 13, 241–251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.