Abstract
Background
Women with inflammatory bowel disease (IBD) may have decreased sexual function. To understand how common this condition is in our female patients, we developed a new IBD-specific Female Sexual Dysfunction Scale (the IBD-FSDS).
Methods
We performed a prospective cross-sectional study of 454 female IBD patients ≥18 years of age attending 1 of 3 IBD clinics in the United States or Denmark. We gathered information on sexual function via a de novo 23-item scale. General sexual functioning was measured with the Female Sexual Function Index (FSFI) and the Female Sexual Distress Scale–Revised (FSDS-R). Depressive symptoms were measured with the Patient Health Questionnaire–9 (PHQ-9). Medical history and sociodemographic data were collected via chart review. Exploratory factor analyses (EFAs) of the English language version of IBD-FSDS assessed unidimensionality, factor structure, reliability, criterion validity, and construct validity.
Results
EFAs suggested retaining 15-items creating a unidimensional scale with strong internal consistency reliability (α = 0.93). Validity of the English language IBD-FSDS was measured using Spearman’s coefficient, demonstrating significant criterion validity with the FSDS-R (P < 0.05) and the FSFI (P < 0.05) and significant construct validity with the composite for cases of active IBD (P < 0.05) and PHQ-9 (P < 0.05). Sexual dysfunction in women with IBD was significantly associated with depression (P = 0.042), active IBD (P = 0.002), and no history of surgery (P = 0.044).
Conclusions
We have developed and validated an IBD-specific scale to assess the psychosexual impact of IBD in women. This novel screening questionnaire may help health care providers recognize factors contributing to impaired sexual function in their female patients.
Keywords: female sexual dysfunction, inflammatory bowel disease, quality of life, Crohn’s disease, ulcerative colitis
INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease (CD) are chronic diseases with a variable and unpredictable course. Treatment often consists of potent biological therapies with a potential for severe side effects.1 In addition, there is a significant risk for surgery with studies demonstrating overall cumulative abdominal and pelvic surgery rates at 0%–35%, 21%–59%, and 37%–61% at 1, 5, and 10 years after diagnosis, respectively.2 The symptoms, disease complications, and treatments for inflammatory bowel disease (IBD) can influence body image, intimacy, and sexual function.3 Furthermore, peak incidence and prevalence of IBD tends to occur between the ages of 15 and 40 years,4 which also coincides with the peak reproductive years.3
“Female sexual dysfunction” (FSD) is the common term for disorders of sexual function in women.5 There are 3 major categories of FSD: (1) sexual desire/arousal disorders, (2) orgasmic disorder, and (3) genito-pelvic pain/penetration disorder. In the United States, although 40% of women complain of sexual dysfunction at some time in their lives, studies investigating this topic are very limited.6 A study from 2291 sexually active women in Denmark revealed that 11% had experienced sexual dysfunction and 68% had experienced sexual difficulties in the preceding year.7 Another longitudinal study, which reported sexual functioning over a period of 4 years in a population sample of 507 women from the United Kingdom, revealed that 34.3% of women reported some sexual problem.8 Inflammatory bowel disease can affect every aspect of daily life9 and may have a negative impact on sexual function. Among women with IBD, 40%–60% report sexual dysfunction, although current data are scarce and based on small survey studies and case series.10–12 Additionally, none of the studies on FSD have targeted patients with IBD using a validated IBD-specific survey tool that specifically addresses unique problems faced by female patients.
Determinants of psychosexual functioning include relationship status, body image perception, sexual satisfaction, sexual desire, pain, guilt, and fear perceptions.10 Recent studies have used validated questionnaires such as the Female Sexual Function Index (FSFI) or the Female Sexual Distress Scale–Revised (FSDS-R), which are gold standards for measurement of FSD in general, and applied them to the field of IBD13,14. Other survey tools that have been used in previous studies focus more on quality of life and include the Sexual Function–36 (SF-36), the Brief Index of Sexual Function in Women, and the Inflammatory Bowel Disease Questionnaire (IBD-Q).15–17 As these questionnaires measure either overall quality of life or general female sexual functioning, they are not sufficient to address specific sexual concerns that may arise due to the IBD process itself.
This study details our psychometric assessment of a novel scale, specific to patients with IBD (English version), that evaluates sexual function in a population of women with IBD. We aimed to measure the psychometric properties of the IBD-FSDS, the criterion validity of the IBD-FSDS in relation to the FSFI and FSDS-R, and the construct validity of the IBD-FSDS in relation to depression and IBD activity, as measured by variables in our data set. Using the IBD-FSDS, our goal was to determine the prevalence of sexual dysfunction in our patients and to identify related factors that may be treatable and/or preventable.
METHODS
This cross-sectional multicenter study used homogenous purposive sampling. Female IBD patients were prospectively recruited during their outpatient visits to the IBD clinic at the Brigham’s and Women’s Hospital or Massachusetts General Hospital in Boston, Massachusetts, between November 1, 2013, and April 30, 2015, or at the Aarhus University Hospital in Aarhus, Denmark, from May 2015 to October 2015. Individuals who had not been sexually active within the past year were excluded. Female patients who were 18 years or older were requested to fill out the questionnaire at or before attending IBD clinic. Most patients completed the survey at their clinic appointment. Patients with time constraints were given envelopes to mail back the responses. Ten percent of patients were mailed the questionnaire before a future clinic appointment.
Development of the IBD-Specific Female Sexual Dysfunction Scale
Item Selection and Content validity
A 3-step process was followed to make certain that an exact set of items measured the content domain, which should be a subset of the universe of appropriate items. Initially, we searched the literature for existing generic surveys to classify appropriate domains of sexual function, from which a preliminary set of questions specific to IBD in females was created (such as the impact of symptoms of IBD, medical and surgical treatment on sexual functioning) in consultation with an expert in survey development. From this, a total of 43 items comprised the IBD-specific pilot scale for females.
Second, the initial battery of items was reviewed by an expert panel of IBD clinicians and a focus group of 20 female IBD patients. The study team aimed to achieve a literacy level of grade 6.5–7.0. Items were reviewed for appropriateness of the content to reflect the underlying construct of IBD-specific female sexual dysfunction, level of language sophistication, form and type, sequence of items, and how data are gathered from the responders. We then examined the outcomes of the first and second steps and developed an IBD-specific Female Sexual Dysfunction Scale that retained 23 items (Table 1). Each item was scored individually from 0 to 4 points on a Likert-type scale, with higher scores indicating greater severity of sexual dysfunction. The 23 items measure how CD or UC affect sexual functioning related to stool leakage, sexual distress, preventing sexual activity, preventing sexual relationships, delayed sexual activity, causing problems during sex, increased awareness of disease during intercourse, abdominal or pelvic or rectal pain during intercourse, worry about passing gas, stool or urine during intercourse, worry about abdominal or pelvic pain during sexual activity, worry about appliance leakage during sex, reduced desire or arousal, feeling too tired to participate in sexual activity, negative feelings toward sexual activity, feeling guilty about effect of disease on partner, feeling less attractive, feeling embarrassed during sexual activity, reduced sexual satisfaction due to diarrhea/anal bleeding or discharge, and abdominal or pelvic pain.
Table 1:
Validated IBD-Specific Female Sexual Dysfunction Scale (15 Items)
| 1. | In the past year, do you feel that your Crohn’s or UC disease contributed to distress in your sex life? |
| 2. | In the past year, has your Crohn’s or UC disease prevented you from starting a sexual relationship? |
| 3. | In the past year, has your Crohn’s or UC disease delayed your starting a sexual relationship? |
| 4. | In the past year, did your Crohn’s or UC diagnosis prevent you from having sex? |
| 5. | In the past year, did your Crohn’s or UC diagnosis cause problems during sex? |
| 6. | In the past year, have you been conscious of your Crohn’s or UC during intercourse? |
| 7. | In the past year, did you have abdominal or pelvic pain during intercourse? |
| 8. | In the past year, did you have rectal/anal pain during intercourse? |
| 9. | In the past year, do you fear experiencing abdominal/pelvic pain during sexual activity? |
| 10. | In the past year, did you have a reduced desire or interest, or did you have trouble getting aroused during intercourse due to your Crohn’s or UC? |
| 11. | In the past year, does your Crohn’s or UC make you feel too tired to participate in sexual activities? |
| 12. | In the past year, how often do you feel that your UC or Crohn’s has negatively affected your sexual life? |
| 13. | In the past year, do you feel guilty about UC and Crohn’s and its effect on your partner? |
| 14. | In the past year, how much has anal bleeding or discharge affected your satisfaction with your sex life? |
| 15. | In the past year, how much has abdominal or pelvic pain affected your satisfaction with your sex life? |
Items removed from original preliminary 23-item survey:
1. Due to your IBD, do you ever accidently have leakage of stool because you were unable to reach the bathroom in time?
2. In the past year, do you fear passing gas during sexual activity?
3. In the past year, do you fear passing stool during sexual activity?
4. In the past year, do you fear passing urine during sexual activity?
5. In the past year, have you feared your appliance leaking (ostomy bag) during sexual activity?
6. Do you feel your UC or Crohn’s diagnosis makes you less attractive to your partner?
7. In the past year, do you feel embarrassed regarding sexual intercourse due to your Crohn’s or UC diagnosis?
8. In the last year, how much has increased bowel movement frequency affected your satisfaction with your sex life?
Likert scale: 4 = always or almost always; 3 = most times (more than half the time); 2 = about half the time; 1 = a few times (less than half the time); 0 = never or very rarely.
Translation of questionnaires
Once the English version IBD-FSDS was validated, it was directly translated from English to Danish by a bilingual translator, which was critically reviewed by an expert consensus panel consisting of gastroenterologists and an associate professor in gynecology and sexology. Backwards translation was also performed by a native Danish speaker. Members of the panel had native knowledge of the cultural beliefs regarding sexual function in Danish women and were conversant with the expressions of sexual function in the Danish context based on their clinical practices. The panel reviewed differences in translation between the original English and Danish versions and achieved agreement by consensus (Supplementary Table 1). The Danish site used already existing validated Danish translations of the FSFI, FSDS-R, PHQ-9, Harvey Bradshaw Index (HBI), and Simple Clinical Colitis Activity Index (SCCAI).
Criterion validity
Criterion validity evaluated whether the new IBD-FSDS has an empirical association with the FSFI and the FSDS-R, which are considered the “gold standard” measure of sexual function in females.
The FSFI is a brief, multidimensional, self-report, 19-item questionnaire for assessing the key dimensions of sexual function in women.13 Questions ask about sexual feelings and responses during the past 4 weeks, using a Likert-type response format ranging from 0 to 5, with a greater score demonstrating no sexual dysfunction.
The FSDS-R is a 13-item questionnaire for measuring sexually related personal distress in women with female sexual dysfunction.14 Questions ask about experiences of sexual distress in the past 30 days using a Likert-type response format ranging from 0 to 4, with a greater score indicating increased sexual distress. We hypothesized that the IBD-FSDS would correlate positively with the FSFI and FSDS-R.
Construct validity
Construct validity measures the theoretical relationship of the new scale with other items in the data set that are known to be associated with IBD. After reviewing the literature, we hypothesized that sexual dysfunction in female patients with IBD would correlate positively with depression and active IBD. A composite score was generated for all active IBD cases using the HBI for CD and the SCCAI for UC.
Depressive Symptoms
Symptoms of depression were measured using the PHQ-9, which is a short self-report screening tool that concentrates on the 9 diagnostic criteria for DSM-IV depressive disorders. It queries whether a particular symptom has been present more than half the time over the past 2 weeks.18 In a meta-analysis, the PHQ-9 was found to be satisfactory for screening for depression in a range of locations and populations.19–23 The instrument uses a Likert-type response format ranging from 0 (not at all) to 3 (every day), and the total score ranges from 0 to 27, with 5 severity categories: minimal (0–4), mild (5–9), moderate (10–14), moderately severe (15–19), and severe (20–27).18 A higher score indicates increased depressive symptoms. We hypothesized that the IBD-FSDS would correlate positively with the PHQ-9.
Harvey Bradshaw Index and Simple Clinical Colitis Activity Index
We used the HBI and SCCAI, respectively, to measure disease activity in CD and UC.24, 25 A score of 5 or greater is considered to represent active IBD.24, 26 We hypothesized that the IBD-FSDS would correlate positively with the HBI in CD patients and SCCAI in patients with UC. We defined an HBI or SCCAI of 5 or greater as an active IBD score.
Data Collection
A paper questionnaire was administered to study participants. It captured information on sexual behaviors and the perceived impact of IBD symptoms and medical and/or surgical therapy on sexual function. The FSFI, FSDS-R, PHQ-9, HBI, and SCCAI were administered in the same setting. The Danish version of the IBD-FSDS was used by the Danish study center. Medical records were reviewed for each patient, and extracted information included demographics, type of IBD, medical and surgical therapies, perianal disease, disease activity score at time of survey completion, and medical comorbidities. We obtained all clinical disease activity index scores in person, directly from the patient, at the time of the clinic appointment.
Compensation
There was no patient compensation for participation in this study.
Statistical Analyses
Exploratory factor analysis
Exploratory factor analysis (EFA) is a statistical method to identify the underlying relationships between a large set of measured variables. EFA with promax rotation was suitable here because the 23 items had not been previously evaluated as a hypothetical scale that measures the underlying construct “sexual dysfunction in IBD.” EFA helped to determine whether the underlying construct is unidimensional, and the Stata default principal factor method was used, which allows each item to have its own variance, where each observed item (χ1–χ23) has a corresponding unique random error term (ε1–ε23), reflecting that there is no shared variance between the 23 items.27, 28 Factors with eigenvalue ≥1 were retained, and factors with eigenvalue <1 were eliminated.
We did not assume that the observed variables are normally distributed, therefore, the Satorra-Bentler scaled chi-square test (which is robust to violations of normality and to small sample sizes) was used to test goodness of fit by calculating the root mean square error of approximation (RMSEA) and the Comparative Fit Index (CFI).29 Stata, v14,30 was used for our analysis.
Exploratory factor analysis: English language version
EFA was undertaken to identify the underlying relationships between each individual question regarding sexual dysfunction and IBD characteristics. An examination of the English version 23-item (23-question) scale initially revealed a 3-factor structure, which suggests that the instrument is potentially measuring 3 underlying constructs, whereas this project seeks to develop an instrument measuring only 1 underlying construct—“sexual dysfunction in IBD.” On further evaluation, we dropped the third factor as it did not meet the minimum criteria for inclusion. Factors 1, 2, and 3 had eigenvalues ≥1, equal to 9.40, 1.47, and 1.04, respectively. Examining the loadings for factors 1 and 2, items 1, –10, and –11 had positive loadings ≥0.30, the minimum criterion for an item. However, for factor 3 only, item 14 loaded at 0.29, which did not meet the minimum criteria for inclusion. Consequently, item 14 was dropped, thus retaining a 2-factor structure).
Examination of the 2-factor model shows that items 10, 11, and 12 have higher loadings on factor 2, which suggests that these items measure a second underlying construct—“fear of” genito-urinary symptoms occurring during sexual activity (“fear of passing gas [item 10], fear of passing stool [item 11], or fear of passing urine during sexual activity [items 12]).
Based on the assumption that factors 1 and 2 are correlated because “fear of…” is likely to influence sexual functioning, a promax rotation was performed (this creates a basic data structure of the variables that are theoretically meaningful).31 The rotated factor structure shows that 7 items have positive loadings ≥0.30 on factor 2 (Supplementary Table 2). Items 1, 10, 11, 12, and 21 include, and are similar to, the “fear-related” items described in the above paragraph. Item 19 purports to measure the perception of the study participants’ “attractiveness to partner,” and item 20 attempts to measure the perception of “feeling embarrassed” due to IBD. Dropping these 7 items, EFA returns a 1-factor model with 15 items (questions) assessing sexual dysfunction in IBD, with the first 2 eigenvalues equal to 7.59 and 0.86, respectively, and factor loadings on the 1-factor model ranging from 0.57 to 0.89, which are statistically significant (Table 1).
Descriptive Statistics
Descriptive statistics are reported on the 23-item pool. Univariate statistics were generated, and ceiling/floor effects were considered substantial if ≥20% of subjects scored at the uppermost or bottom-most end on the IBD-FSDS.32, 33 Internal consistency reliability was assessed using Cronbach’s alpha statistic.34 Criterion and construct validity were assessed using the Spearman’s rank correlation coefficient, with significance at P < 0.05. All variables were initially checked for significance with t tests, and then statistically significant covariates were entered into the multivariate regression model. We used STATA for multivariate regression modeling.
Ethical Considerations
The Partners Institutional Review Board granted ethical approval (protocol number 2013P002317), as did the Danish Data Protection Agency (reference number 1-16-02-297-15).
RESULTS
Participant Characteristics
In total, 650 female patients were asked to participate in this study. The response rate was 73.7% (302/410) in the United States, and of 302 surveys returned, 267 (88.4%) were fully completed. In Denmark, the response rate was 78.0% (187/240), and 100% of returned surveys were fully completed. Of the 302 US surveys returned, 35 (11.6%) were removed from analysis due to very incomplete data or noneligibility. Of 454 remaining participants, there were 267 and 187 completed surveys in the United States and Denmark, respectively. Across the US and Danish study sites, statistically significant differences were observed for depression and active IBD cases. Danish participants reported greater depressive symptomatology in the moderate and moderately severe categories (P < 0.001), and the Danish site had a statistically significantly (P < 0.001) higher proportion of UC participants reporting a score of 5 or greater on the SCCAI. In addition, a significantly greater proportion of Danish participants were taking immunomodulators and narcotics (P < 0.001). Demographic and clinical details are shown in Table 2.
Table 2:
Participant Characteristics from USA and Danish Data Sets
| USA | Denmark | Significance | |
|---|---|---|---|
| Female, No. | 267 | 187 | |
| Age, mean ± SD, y | 36 ± 11.6 | 36 ± 12.6 | ns |
| Race/ethnicity, No. (%) | |||
| White | 264 (99) | 187 (100) | |
| Black | 3 (1) | ||
| Hispanic | 0 | ||
| Asian | 0 | ns | |
| Ulcerative colitis, No. (%) | 82 (34.0) | 74 (39.8) | |
| Crohn’s disease, No. (%) | 159 (66.0) | 112 (60.2) | ns |
| Montreal classification | |||
| Crohn’s disease behavior, No. (%) | |||
| B1: Inflammatory | 67 (42.2) | 59 (52.7) | |
| B2: Stricturing | 27 (16.9) | 23 (20.5) | |
| B3: Penetrating | 24 (14.9) | 11 (9.8) | |
| B4: Perianal disease | 41 (26.0) | 19 (17.0) | |
| Crohn’s disease location, No. (%) | |||
| L1: Ileal | 37 (23.2) | 14 (12.5) | |
| L2: Ileocolonic | 25 (15.5) | 34 (30.4) | |
| L3: Colonic | 91 (57.4) | 63 (56.3) | |
| L4: Upper tract | 6 (3.9) | 1 (0.8) | |
| Ulcerative colitis disease extent, No. (%) | |||
| E1: Limited to the rectum | 10 (12.0) | 19 (21.4) | |
| E2: Left sided (colorectum distal to splenic flexure) | 22 (26.7) | 23 (32.9) | |
| E3: Extensive (proximal to splenic flexure) | 50 (61.3) | 32 (45.7) | |
| Age at diagnosis, No. (%) | |||
| <40 y | 182 (68.0) | 132 (70.6) | |
| 40–59 y | 66 (24.8) | 42 (22.3) | |
| ≥60 y | 19 (7.2) | 13 (7.1) | ns |
| Presence of stoma, No. (%) | 20 (7.5) | 10 (5.4) | ns |
| Prior IBD surgery, No. (%) | 104 (39.1) | 48 (25.9) | <0.001 |
| IPAA, No. (%) | 13 (4.9) | 4 (2.1) | <0.001 |
| Current medications, No. (%) | <0.001 | ||
| Corticosteroids | 30 (11.3) | 20 (10.7) | 0.835 |
| Immunomodulators | 65 (24.2) | 97 (51.8) | <0.001 |
| Anti-TNF alpha | 124 (46.4) | 102 (54.6) | 0.089 |
| Anti IL12/23 | 19 (7.2) | 5 (2.7) | 0.036 |
| Narcotics | 22 (8.3) | 29 (15.5) | <0.001 |
| Marital status, No. (%) | |||
| Single | 69 (25.8) | 42 (22.6) | |
| Married/living together | 179 (63.5) | 122 (65.1) | |
| Divorced/separated | 28 (10.4) | 20 (10.7) | ns |
| Female scores, mean ± SD | |||
| FSFI | 46.3 ± 17.1 | 46.0 ± 18.3 | ns |
| FSDS-R | 13.0 ± 11.7 | 14.9 ± 11.5 | ns |
| PHQ-9 score, No. (%) | |||
| Minimal | 148 (55.3) | 61 (32.8) | |
| Mild | 79 (29.5) | 66 (35.5) | |
| Moderate | 31 (11.5) | 37 (19.9) | |
| Moderately severe | 6 (2.5) | 19 (10.2) | |
| Severe | 3 (1.2) | 3 (1.6) | <0.001 |
| Active IBD cases, No. (%) | |||
| Harvey-Bradshaw Index, score ≥5 | 45 (28.4) | 33 (29.7) | ns |
| SCCAI, score ≥5 | 18 (21.8) | 25 (33.3) | <0.001 |
Exploratory Factor Analysis
Please see the “Methods” section for complete exploratory factor analysis findings of the English language survey.
Goodness of Fit and Output of Modification Index
Goodness of fit is a statistical test to measure how well the observed responses match the theoretically expected responses and is measured using RMSEA. In addition, we generated an output of modification index to further calculate the goodness of fit for each individual item within the model. Results show that the final RMSEA for the 15-item English version of the survey is 0.066, which is within the recommended range of 0.05–0.08. The CFI was 0.932; this means that our model does 93.2% better than a null model in which we assume the items are all unrelated to each other (the recommended cutoff should be either 0.90 or 0.95).29
Survey Results
Survey results are detailed in Table 3. We derived a result of “yes” as a response on the Likert scale, with response choices of 4, 3, 2 being equivalent to yes (ie, 50% of the time or greater). Significant differences between the US and Danish populations include marital status—with a higher percentage of cohabitation and divorce among Danish participants and higher percentage of currently married US participants (<0.001). In the past year, 92.5% of US and 97.9% of Danish patients had sex with men (P = 0.012) and 5.3% (US) and 1.6% (Danish) had sex with women (0.043). Fear of experiencing pain was a significant concern, with 42.1% of US and 36.2% of Danish women stating this (P = 0.032). Approximately one-quarter of US (66/267) and one-third of Danish (67/187) women felt their IBD made them unattractive (P = 0.018). Body image was impaired by setons (1.9% US, 4.8% Danish), ostomy (100% of all individuals with ostomies; ie, 4.9% of total US and 6.4% of total Danish cohorts), perianal fistulae (15% US, 8.6% Danish, P = 0.038), and scars (21% US, 15.5% Danish, ns).
Table 3:
Participant Responses from USA and Danish Data Sets
| USA | Danish | P | |
|---|---|---|---|
| No. (%) | No. (%) | ||
| Sexual history and responses | |||
| Sex with men | 245 (92.5) | 183 (97.9) | 0.012 |
| Sex with women | 14 (5.3) | 3 (1.6) | 0.043 |
| Sex with men and women | 3 (1.1) | 1 (0.5) | 0.504 |
| No sex | 13 (4.9) | 4 (2.1) | 0.128 |
| Currently in a sexual relationship (Y/N) | 211 (80.8) | 142 (76.3) | 0.250 |
| Patient–provider interaction | |||
| Patient already discussed sexual function with GI physician | 33 (12.8) | 11 (6.0) | 0.019 |
| Provider initiated conversations about sexual activity/ function | 39 (15.2) | 10 (5.4) | 0.001 |
| Comfortable talking to doctor about sexual health | 185 (72.8) | 115 (63.2) | 0.032 |
| Comfortable with: | 0.005 | ||
| Male provider | — | 2 (1.1) | |
| Female provider | 173 (67.1) | 98 (53.3) | |
| No preference | 85 (32.9) | 84 (45.6) | |
| Select sexual function responses from 23-item questionnaire | |||
| Fear of passing gas | 132 (51.9) | 95 (51.4) | 0.653 |
| Fear of passing stool | 70 (27.6) | 49 (26.5) | 0.846 |
| Fear of passing urine | 30 (11.8) | 19 (10.3) | 0.808 |
| Fear of pain | 107 (42.1) | 67 (36.2) | 0.032 |
| Fear of appliance leak | 9 (69.2) | 5 (50.0) | 0.349 |
| Diarrhea affects sex life | 42 (16.2) | 33 (17.8) | 0.865 |
| Feel unattractive due to IBD | 66 (25.6) | 67 (36.0) | 0.018 |
| Due to seton | 5 (1.9) | 9 (4.8) | 0.077 |
| Due to ostomy | 13 (100.0) | 12 (100.0) | 0.99 |
| Due to perianal fistulae | 40 (15.1) | 16 (8.6) | 0.038 |
| Due to surgical scars | 56 (21.1) | 29 (15.5) | 0.132 |
| Due to skin tags | 42 (15.8) | 27 (14.4) | 0.681 |
| Select IBD-FSDS responses | |||
| IBD preventing start of sexual relationship | 55 (22.5) | 52 (32.1) | 0.065 |
| IBD causing distress in sexual relationship | 176 (67.7) | 116 (62.4) | 0.426 |
| Conscious of IBD during intercourse | 65 (25.2) | 43 (23.1) | 0.923 |
| Abdominal or pelvic pain during intercourse | 44 (17.0) | 27 (14.6) | 0.447 |
| Rectal/anal pain during intercourse | 19 (7.4) | 6 (3.3) | 0.191 |
| Anal bleeding affects sex life | 24 (9.2) | 11 (6.0) | 0.465 |
Although 73% of US and 63% of Danish participants stated that they would feel comfortable discussing sex with their physician, only 15.2% of US and 5.4% of Danish women reported that their provider had initiated a conversation about sexual health (P = 0.001). Of note, 67.1% of US (173/267) and 53.3% of Danish women preferred talking about sex with a female provider whereas the remainder were comfortable with either a male or female provider. Only 12.8% of US and 6% of Danish women had already discussed sexual function with their gastroenterologist (P = 0.019).
Although not significant between the 2 cohorts, nearly two-thirds (67.7% US, 62.4% Danish) reported that IBD caused distress in sexual relationships, and 7.4% of US and 12.8% of Danish women reported that their IBD contributed to a break up. Inflammatory bowel disease activity prevented sex in 27.2% of US and 28.6% of Danish women. Furthermore, due to their IBD, approximately one-third (26% US, 35% Danish) described feeling guilty about sex, and 33.9% of US and 38% of Danish women feared sex. Fear of passing gas and appliance leakage were prominent concerns (Table 3).
Descriptive Statistics and Reliability of the English Version IBD-FSDS
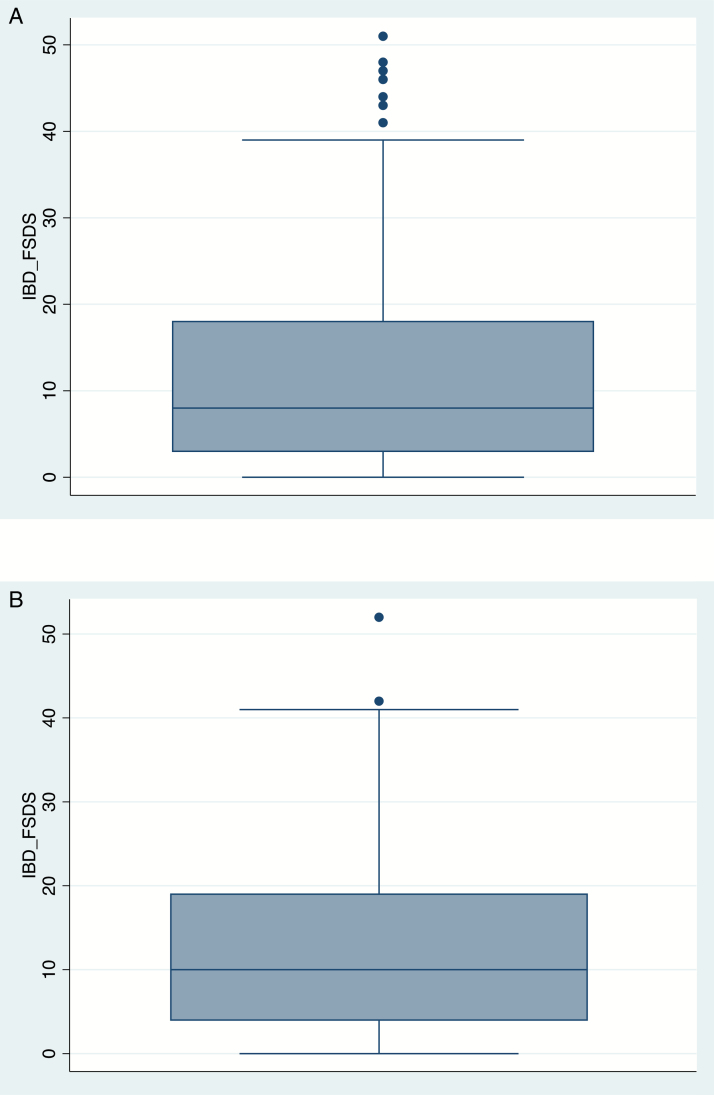
Univariate statistics demonstate a mean English version IBD-FSDS (range) of 11.6 ± 11.4 (0–51) points, with a higher score indicating greater sexual dysfunction. This is for all the subscales. There were no floor or ceiling effects observed. The Cronbach’s coefficient alpha is α = 0.93, confirming that a large proportion of the scale’s total variance is attributed to a common source. The distribution of the IBD-FSDS scores is shown in Figure 1. Mean scores for the Danish version IBD-FSDS were 12.3 ± 10.48, with a range of 0–52 points.
FIGURE 1.
Distribution of IBD-FSDS scores. Distribution of IBD-FSDS scores in US cohort (mean, 11.6; SD, 11.4; range, 0–51). Distribution of IBD-FSDS scores in Danish Cohort (mean, 12.3; SD, 10.8; range, 0–52).
Criterion and Construct Validity: English and Danish Versions of the IBD-FSDS
Spearman’s rank correlation coefficient was used to assess criterion validity. The English and Danish versions of IBD-FSDS significantly correlated with the FSDS-R (US: n = 249, R = 0.6115, P < 0.05; and Denmark: n = 186, R = 0.6929, P < 0.05) and the FSFI (US: n = 251, R = 0.1809, P < 0.05; and Denmark: n = 184, R = 0.2056, P < 0.05) (Fig. 1). Similarly, the IBD-FSDS is also significantly correlated (construct validity) with the composite score for active IBD cases (US: n = 214, R = 0.3049, P < 0.05; and Denmark: n = 178, R = 0.2495, P < 0.05), and the summary score of the PHQ-9 (US: R = 0.3478, P < 0.05; and Denmark: R = 0.5153, P < 0.05) (Supplementary Fig. 1A and B).
Regression Analysis
In the US cohort, female sexual dysfunction as measured by the IBD-FSDS was significantly associated with the presence of depression (P = 0.04), greater severity of IBD activity (P = 0.002), and no history of surgery (P = 0.04). In the Danish cohort, female sexual dysfunction as measured by the IBD-FSDS was significantly associated with the presence of depression (P = 0.02).
DISCUSSION
IBD-FSDS is the first tool to directly assess sexual dysfunction in women with IBD. Psychometric evaluation of the final 15-item instrument was assessed in 3 major ways: (1) reliability, (2) criterion validity, and (3) construct validity. Overall, the 15-item IBD-FSDS has strong internal consistency and reliability. Strong criterion validity was noted with the FSFI and FSDS-R, and positive statistically significant associations (construct validity) were shown with the measure for depression (English and Danish versions) and the composite score for active IBD cases (English version).
Our study yielded some interesting findings. One-quarter of women stated that IBD prevents them from starting a sexual relationship, with two-thirds stating that IBD causes distress in sexual relationships. Inflammatory bowel disease was attributed to causing problems during sex in one-quarter of patients, and one-third felt guilty that about how their IBD was affecting their sexual relationships. Furthermore, IBD caused fear of intercourse in more than one-third of patients. Of note, only 10% of all patients in the present study had spoken with their gastroenterologist even though two-thirds of women would be comfortable talking with the doctor about sexual health. This is in accordance with a study of 355 patients with IBD showing that patients want to discuss this issue with their doctors, with 61% of women desiring information on the effect of IBD on sexuality and intimacy at the time of IBD diagnosis 10 Despite this, only 40% of patients had asked these questions of their health care provider.10
Patients with IBD often see their gastroenterologist more frequently than their obstetrician-gynecologist, so coordination of care to screen for and treat sexual dysfunction between the gastroenterologist and gynecologist can greatly improve quality of life. Furthermore, early diagnosis of sexual dysfunction will enable providers to involve interdisciplinary specialists for referrals, such as health psychologists, and to refer patients for pelvic floor physical therapy, which has been used successfully for other somatic diseases.3
Increased inflammatory bowel disease activity, abdominal and pelvic surgery, and depression have all been linked to sexual dysfunction.35, 36 Many studies have demonstrated that disease activity in IBD is strongly linked to depression and anxiety,37–39 which in turn has been shown to increase symptoms of sexual dysfunction.38, 39 Active IBD in women is associated with reduced self-perception of attractiveness, sexual satisfaction, and sexual activity.40 However, data regarding disease activity as a reason for sexual dysfunction are conflicting. In accordance with Timmer and colleagues, we found that in the US population in the present study, disease activity significantly correlated with sexual dysfunction, whereas this was not the case in the Danish population, which is in line with previous studies.10, 41 The women in our study reported multiple symptoms that impaired their sexual function, including abdominal and pelvic pain, rectal or anal pain, rectal bleeding, diarrhea, and poor body image associated with setons, ostomies, perianal fistulae, and scars. Approximately one-quarter of US and one-third of Danish women felt that their IBD made them unattractive. Impaired sexual function in the United States and Denmark was significantly associated with depression. All 25 ostomy patients had poor body image, which is in line with previous studies in women with stomas. In a prior study, 60% of women (n = 25) with stomas stated that they felt less desirable, with 48% (n = 12) reporting difficulties in sexual intercourse psychologically and 32% (n = 8) reporting difficulties physically.42
Our study has important limitations. A limitation is that for original validation and subsequent screening, an observational cross-sectional cohort was used, without cognitive testing following new item administration. Limitations of generalizability were reduced by incorporating 2 North American centers and a European center. However, despite this, the majority of our participants were Caucasian, so the findings may not be generalizable to other races. Our white US population was multireligious. We feel, however, that religion is less likely to cause bias, as the majority of our respondents were in married or cohabiting relationships. To limit recall bias, medical charts were reviewed to establish more detailed clinical information regarding disease severity. In addition, test–retest evaluation in a longitudinal design would have been helpful to measure the reliability of our scale, and an evaluation of responsiveness when study participants are enrolled in a clinical trial protocol. Therefore, this new tool warrants further evaluation and is an adjunct to, rather than a substitute for, a detailed history of sexual dysfunction in IBD. Part of this further evaluation includes cognitive interviews with a new focus group or individual patients using the 15-item scale.
In conclusion, we have developed and validated a new IBD-specific survey that can be rapidly utilized in clinical practice to screen for sexual and psychosexual dysfunction in women, thereby helping us better understand the impact of IBD on sexual functioning and satisfaction. Hopefully, the IBD-FSDS will help improve screening, evaluation, and management for this patient population. In addition, the IBD-FSD can be used as a tool to help assess the impact, in clinical trials and in clinical practice, of psychological, medical, and physical therapy for FSD in women with IBD.
SUPPLEMENTARY DATA
Supplementary data are available at Inflammatory Bowel Diseases online.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank all the patients who participated in this study.
Conflicts of interest: P.D., A.O., L.M., C.U., M.T., T.L., D.N., A.A., J.K., A.H., and S.F. declare no competing interests. M.J. has served as a speaker, has been compensated by MSD, Ferring, UCB, and Takeda, and has been on the advisory board for Janssen. L.A.C. has served as a speaker, has been compensated by Ferring, Tillotts, Takeda, MSD, AbbVie, Janssen, and UCB, and has been on the advisory board for AbbVie and MSD.
Guarantor of the article: Punyanganie S. de Silva, MD, MPH.
Conference presentation: Preliminary data were presented at Digestive Diseases Week, San Diego USA, 2016 – Poster of Distinction.
Supported by: Analyses of data and manuscript development were financially supported, in part, by a grant awarded to M.A.T. from the Patient Centered Outcomes Research Institute (PCORI Program Award CE-1304–6756) and by a grant (L60 MD002421-02) and fellowship (R25MH083620) awarded to Linda G. Marc from the National Institutes of Health.
Author contributions: Punyanganie de Silva and Sonia Friedman designed the study. Punyanganie de Silva, Sonia Friedman, Aoibhlinn O’Toole, Deanna Ngyuen, Ashwin Ananthakrishnan, Lisbet A. Christensen, Mette Julsgaard, Tine Laursen, Astrid Højgaard, and Joshua Korzenik took part in conducting the study, interpreting the data, and drafting the manuscript. Linda G. Marc, Christine A. Ulysse, and Marcia A. Testa were responsible for analysis and drafting the manuscript. All study investigators approved the final draft of the manuscript.
REFERENCES
- 1. Van Assche G, Vermeire S, Rutgeerts P. Optimizing treatment of inflammatory bowel diseases with biologic agents. Curr Gastroenterol Rep. 2008;10:591–596. [DOI] [PubMed] [Google Scholar]
- 2. Bernstein CN, Loftus EV Jr, Ng SC, et al. ; Epidemiology and Natural History Task Force of the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) Hospitalisations and surgery in Crohn’s disease. Gut. 2012;61:622–629. [DOI] [PubMed] [Google Scholar]
- 3. Friedman S. Sexual dysfunction in inflammatory bowel disease: “Don’t ask, don’t tell” doesn’t work. Inflamm Bowel Dis. 2015;21:948–950. [DOI] [PubMed] [Google Scholar]
- 4. van der Woude CJ, Ardizzone S, Bengtson MB, et al. ; European Crohn’s and Colitis Organization The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9:107–124. [DOI] [PubMed] [Google Scholar]
- 5. Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington: American Psychiatric Association; 2013. [Google Scholar]
- 6. Palacios S, Castaño R, Grazziotin A. Epidemiology of female sexual dysfunction. Maturitas. 2009;63:119–123. [DOI] [PubMed] [Google Scholar]
- 7. Christensen BS, Grønbaek M, Osler M, et al. Sexual dysfunctions and difficulties in Denmark: prevalence and associated sociodemographic factors. Arch Sex Behav. 2011;40:121–132. [DOI] [PubMed] [Google Scholar]
- 8. Burri A, Hilpert P, Spector T. Longitudinal evaluation of sexual function in a cohort of pre- and postmenopausal women. J Sex Med. 2015;12:1427–1435. [DOI] [PubMed] [Google Scholar]
- 9. Ghosh S, Mitchell R. Impact of inflammatory bowel disease on quality of life: results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J Crohns Colitis. 2007;1:10–20. [DOI] [PubMed] [Google Scholar]
- 10. Marín L, Mañosa M, Garcia-Planella E, et al. Sexual function and patients’ perceptions in inflammatory bowel disease: a case-control survey. J Gastroenterol. 2013;48:713–720. [DOI] [PubMed] [Google Scholar]
- 11. Moody G, Probert CS, Srivastava EM, et al. Sexual dysfunction amongst women with crohn’s disease: a hidden problem. Digestion. 1992;52:179–183. [DOI] [PubMed] [Google Scholar]
- 12. Timmer A, Bauer A, Kemptner D, et al. Determinants of male sexual function in inflammatory bowel disease: a survey-based cross-sectional analysis in 280 men. Inflamm Bowel Dis. 2007;13:1236–1243. [DOI] [PubMed] [Google Scholar]
- 13. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. [DOI] [PubMed] [Google Scholar]
- 14. Derogatis L, Clayton A, Lewis-D’Agostino D, et al. Validation of the female sexual distress scale-revised for assessing distress in women with hypoactive sexual desire disorder. J Sex Med. 2008;5:357–364. [DOI] [PubMed] [Google Scholar]
- 15. Mazer NA, Leiblum SR, Rosen RC. The Brief Index of Sexual Functioning for Women (BISF-W): a new scoring algorithm and comparison of normative and surgically menopausal populations. Menopause. 2000;7:350–363. [DOI] [PubMed] [Google Scholar]
- 16. Bodger K, Ormerod C, Shackcloth D, et al. ; IBD Control Collaborative Development and validation of a rapid, generic measure of disease control from the patient’s perspective: the IBD-control questionnaire. Gut. 2014;63:1092–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Förger F, Østensen M, Schumacher A, et al. Impact of pregnancy on health related quality of life evaluated prospectively in pregnant women with rheumatic diseases by the SF-36 health survey. Ann Rheum Dis. 2005;64:1494–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilbody S, Sheldon T, House A. Screening and case-finding instruments for depression: a meta-analysis. CMAJ. 2008;178:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pignone MP, Gaynes BN, Rushton JL, et al. Screening for depression in adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:765–776. [DOI] [PubMed] [Google Scholar]
- 21. Marc LG, Henderson WR, Desrosiers A, et al. Reliability and validity of the Haitian Creole PHQ-9. J Gen Intern Med. 2014;29:1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang FY, Chung H, Kroenke K, et al. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. J Gen Intern Med. 2006;21:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin A, Rief W, Klaiberg A, et al. Validity of the brief Patient Health Questionnaire Mood Scale (PHQ-9) in the general population. Gen Hosp Psychiatry. 2006;28:71–77. [DOI] [PubMed] [Google Scholar]
- 24. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 25. Walmsley RS, Ayres RC, Pounder RE, et al. A simple clinical colitis activity index. Gut. 1998;43:29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jowett SL, Seal CJ, Phillips E, et al. Defining relapse of ulcerative colitis using a symptom-based activity index. Scand J Gastroenterol. 2003;38:164–171. [DOI] [PubMed] [Google Scholar]
- 27. Streiner DL, Norman GR.. Health Measurement Scales: A Practical Guide to their Development. New York: Oxford University Press; 1989. [Google Scholar]
- 28. Coste J, Bouée S, Ecosse E, et al. Methodological issues in determining the dimensionality of composite health measures using principal component analysis: case illustration and suggestions for practice. Qual Life Res. 2005;14:641–654. [DOI] [PubMed] [Google Scholar]
- 29. Raykov T, Marcoulides GA.. A First Course in Structural Equation Modelling. 1st ed Mahwah, New Jersey: Lawrence Erlbaum Associates; 2000. [Google Scholar]
- 30. StataCorp. Stata User’s Guide. Release 12. College Station, TX: StataCorp LP; 2011. [Google Scholar]
- 31. Brown JD. Statistics corner. Questions and answers about language testing statistics: Choosing the right number of components or factors in PCA and EFA. Shiken: JALT Testing & Evaluation SIG Newsletter; 2009;13:20–25. Available at: https://jalt.org/test/PDF/Brown31.pdf (15 December 2017, date last accessed). [Google Scholar]
- 32. Testa MA, Nackley JF. Methods for quality-of-life studies. Annu Rev Public Health. 1994;15:535–559. [DOI] [PubMed] [Google Scholar]
- 33. Marc LG, Wang MM, Testa MA. Psychometric evaluation of the HIV symptom distress scale. AIDS Care. 2012;24:1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. DeVellis RF. Scale Development: Theory and Applications. 2nd ed Applied Social Research Methods Series 26. Thousand Oaks, CA: SAGE Publications; 2003. [Google Scholar]
- 35. Ghazi LJ, Patil SA, Cross RK. Sexual dysfunction in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:939–947. [DOI] [PubMed] [Google Scholar]
- 36. Jedel S, Hood MM, Keshavarzian A. Getting personal: a review of sexual functioning, body image, and their impact on quality of life in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:923–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ananthakrishnan AN, Gainer VS, Cai T, et al. Similar risk of depression and anxiety following surgery or hospitalization for Crohn’s disease and ulcerative colitis. Am J Gastroenterol. 2013;108:594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bel LG, Vollebregt AM, Van der Meulen-de Jong AE, et al. Sexual dysfunctions in men and women with inflammatory bowel disease: the influence of IBD-related clinical factors and depression on sexual function. J Sex Med. 2015;12:1557–1567. [DOI] [PubMed] [Google Scholar]
- 39. Yanartas O, Kani HT, Bicakci E, et al. The effects of psychiatric treatment on depression, anxiety, quality of life, and sexual dysfunction in patients with inflammatory bowel disease. Neuropsychiatr Dis Treat. 2016;12:673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sanders JN, Gawron LM, Friedman S. Sexual satisfaction and inflammatory bowel diseases: an interdisciplinary clinical challenge. Am J Obstet Gynecol. 2016;215:58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rivière P, Zallot C, Desobry P, et al. Frequency of and factors associated with sexual dysfunction in patients with inflammatory bowel disease. J Crohns Colitis. 2017;11:1347–1352. [DOI] [PubMed] [Google Scholar]
- 42. Rolstad BS, Wilson G, Rothenberger DA. Sexual concerns in the patient with an ileostomy. Dis Colon Rectum. 1983;26:170–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.