Abstract
Background
Vedolizumab (ENTYVIO) is a humanized α4β7 integrin antagonist approved for the treatment of inflammatory bowel disease, which selectively blocks gut-specific lymphocyte trafficking. We evaluated the risk of opportunistic infections of interest in patients treated with vedolizumab.
Methods
We determined the frequency of opportunistic infections and tuberculosis in patients receiving vedolizumab in phase 3 clinical trials and post-marketing settings. We also evaluated adverse events reported in the post-marketing setting in patients with a history of or concurrent hepatitis B/C virus infection.
Results
The incidence of opportunistic infections in patients receiving vedolizumab was 0.7 (GEMINI 1 and 2 clinical trials) and 1.0 (long-term safety study) per 100 patient-years, with 217 events reported in approximately 114,071 patient-years of exposure (post-marketing setting). Most opportunistic infections were nonserious and the majority of patients continued treatment with vedolizumab. Clostridium difficile was the most commonly reported infection, with an incidence rate of 0.5 per 100 patient-years (clinical trials). Tuberculosis was reported at 0.1 per 100 patient-years (clinical trials), with 7 events in the post-marketing setting. No tuberculosis-related deaths were reported in either setting. No cases of progressive multifocal leukoencephalopathy were reported. In 29 patients with a history of or concurrent hepatitis B/C infection in the post-marketing setting, no viral reactivation was observed.
Conclusions
Clinical trials and post-marketing data showed that the rate of serious opportunistic infections in patients receiving vedolizumab was low and most patients could continue vedolizumab treatment. The frequency of tuberculosis infection was also low and no hepatitis B/C viral reactivation was reported.
Keywords: opportunistic infections, tuberculosis, vedolizumab, hepatitis
INTRODUCTION
The treatment goals for patients with inflammatory bowel disease (IBD) include inducing and maintaining clinical remission and avoiding complications, hospitalization, and surgery. Biological agents used for the treatment of IBD, such as tumor necrosis factor (TNF) antagonists, are associated with systemic immunosuppression.1, 2 The risk of opportunistic infections in IBD increases with the use of biologics (odds ratio 1.9),3 including TNF antagonists.4–6 Opportunistic infections have been reported among patients with IBD receiving TNF antagonists (with incidence rates [IR] per 100 patient-years [PY] of exposure) and include candidiasis (IR 1.7), herpes simplex (IR 1.1), herpes zoster (IR 1.1), abdominal abscess (IR 0.9), sepsis (IR 0.8), Clostridium difficile (C. difficile) colitis/diarrhea (IR 0.4), histoplasmosis (IR 0.1), and cytomegalovirus (CMV) colitis (IR <0.1).7 In particular, TNF antagonist use is associated with an increased risk of new tuberculosis infection and reactivation of latent tuberculosis.4 In TNF antagonist-treated patients with IBD, 1.7% and 3.1% of patients in studies conducted in Spain8 and the Republic of Korea,9 respectively, developed tuberculosis (general population incidence in 2015: 12/100,000 population [Spain]; 80/100,000 population [Korea]).10 TNF antagonists could also predispose to reactivation of viral hepatitis, especially hepatitis B virus (HBV) infection.11 The frequency of HBV reactivation reported in patients with IBD receiving immunosuppressive therapy varied from 17% (1 in 6 patients) to 36% (9 in 25 patients); HBV reactivation was significantly associated with treatment with more than one immunosuppressive therapy, particularly when one of the therapies was a TNF antagonist.12, 13
Vedolizumab (ENTYVIO; Takeda Pharmaceuticals, Deerfield, IL) is a humanized monoclonal antibody that targets α4β7 integrin and selectively blocks the interaction of α4β7 with mucosal addressin cell adhesion molecule-1, thereby inhibiting the migration of memory T lymphocytes across the endothelium into inflamed gastrointestinal tissue.14, 15 Vedolizumab is approved for the treatment of adults with moderately to severely active ulcerative colitis or Crohn’s disease.16 The gut-selective mode of action of vedolizumab should theoretically be associated with a lower risk of infections. An integrated analysis of safety data from 6 vedolizumab clinical trials reported that vedolizumab was not associated with an increased risk of opportunistic infections compared with placebo, and tuberculosis was also reported infrequently (0.1/100 PY, 95% confidence interval [CI] 0.0–0.2).17 The clinical trials had excluded patients with chronic HBV or hepatitis C virus (HCV) infection. Thus, clinical evidence on the risk of reactivation of HBV or HCV infection with vedolizumab therapy has so far been lacking.
Safety data have been collected from an ongoing long-term safety study of vedolizumab in IBD and, since the market approval of vedolizumab in May 2014, additional safety data have also been collected from post-marketing surveillance. Taken together, these data provide further insights on the long-term safety of vedolizumab.
Here, we report the frequency of opportunistic infections and tuberculosis in patients treated with vedolizumab in clinical trials and the post-marketing setting. The adverse event profile observed in patients with a history of or concurrent HBV or HCV infection who had received vedolizumab in the post-marketing setting was also characterized.
MATERIALS AND METHODS
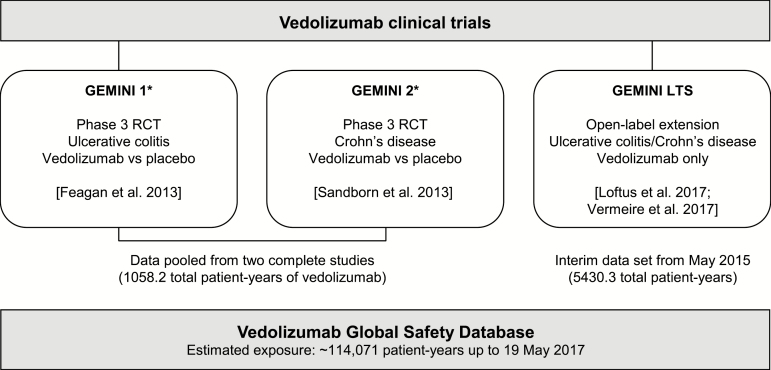
Safety data were obtained from 2 randomized, placebo-controlled trials of vedolizumab for ulcerative colitis and Crohn’s disease (GEMINI 1 and GEMINI 2, respectively),18, 19 an ongoing long-term safety (LTS) study,20, 21 and the vedolizumab Global Safety Database (Figure 1). Data from the GEMINI 1 and GEMINI 2 clinical trials were pooled. In the clinical trials, patients were excluded if they had active or latent tuberculosis at screening, evidenced by a history of tuberculosis, a positive diagnostic tuberculosis test within 1 month prior to enrollment, or a chest radiograph within 3 months of enrollment in which active or latent tuberculosis could not be excluded. The clinical trials excluded patients with chronic HBV or HCV infection, so safety data from patients with a history of HBV or HCV infection reflect the post-marketing setting only.
FIGURE 1.
Analysis populations. *Excludes those patients receiving vedolizumab in the induction phase who were randomized to placebo in the maintenance phase. Clinical trials registered at clinicaltrials.gov with numbers NCT00783718 (GEMINI 1), NCT00783692 (GEMINI 2), and NCT00790933 (GEMINI LTS). LTS indicates long-term safety; RCT, randomized controlled trial.
Ethical Considerations
Patients enrolled in clinical trials, postauthorization safety studies, and patient support and market research programs provided informed consent for participation in the studies, including the collection of adverse event data. Institutional Review Board review and approvals were obtained for these studies.
Data Sets
GEMINI 1 and GEMINI 2
These phase 3 clinical trials were designed to assess the efficacy and safety of vedolizumab 300 mg IV vs placebo as induction (at Week 0 and Week 2) and maintenance therapy (4-weekly or 8-weekly IV injections from Week 6 up to Week 52). The eligibility criteria for these studies have been reported previously.18, 19 In brief, patients had moderately to severely active ulcerative colitis or Crohn’s disease and a history of unsuccessful treatment with one or more of immunomodulators, TNF antagonists, or corticosteroids.
GEMINI LTS study
The GEMINI LTS study was designed to evaluate the long-term safety of vedolizumab 300 mg IV every 4 weeks and enrolled patients with ulcerative colitis or Crohn’s disease who had previously participated in vedolizumab clinical trials (GEMINI 1, GEMINI 2, GEMINI 3 [a phase 3, 10-week, placebo-controlled, induction trial in patients with Crohn’s disease],22 or a phase 2 extension study23) as well as patients who were vedolizumab-naïve (de novo patients). The eligibility criteria for this study have been reported elsewhere.20, 21 The LTS study was ongoing when this interim analysis, which includes data up to May 21, 2015, was performed.
Vedolizumab Global Safety Database
All individual case safety reports of adverse events experienced in patients treated with vedolizumab that are reported to Takeda are held in the vedolizumab Global Safety Database. Sources of data held in the vedolizumab Global Safety Database include spontaneous reports received directly from patients, healthcare professionals, or regulatory authorities; reports in the scientific and medical literature; and solicited reports from patient support programs and market research programs. Upon receipt of a report, Takeda reviews the data and requests additional information required to evaluate the adverse event(s).24 The vedolizumab Global Safety Database was searched to identify post-marketing safety reports received up to May 19, 2017.
Outcomes
Opportunistic infections and tuberculosis
Opportunistic infections were identified using a search query (see Table, Supplemental Digital Content 1) based on a comprehensive list of preferred terms commonly regarded as opportunistic infections from the Medical Dictionary for Regulatory Activities (MedDRA; MedDRA version 14.0 for clinical trial data and MedDRA version 20.0 for data from the vedolizumab Global Safety Database [the opportunistic infections queried were the same for both sets of data]). A serious adverse event was defined in line with the FDA Code of Federal Regulations.25 In the GEMINI clinical trials, any diagnosis of progressive multifocal leukoencephalopathy would have been considered a serious adverse event.
Tuberculosis infections were classified according to MedDRA version 14.0 in the clinical trials and version 20.0 in the post-marketing setting (using the high-level term group tuberculous infections). The distinction between primary tuberculosis infection and reactivation of latent tuberculosis was based on patients’ medical history and concurrent conditions, diagnostic tests (QuantiFERON or tuberculin skin test), and chest radiography. These assessments were made by the investigator for patients enrolled in clinical trials and by the reporter in the post-marketing setting.
Viral hepatitis reactivation
The clinical trials excluded patients with chronic HBV or HCV infection, but in the post-marketing setting, there were patients with a medical history of hepatitis infection who were treated with vedolizumab. Patients with a history of or concurrent HBV or HCV infection who had an adverse event reported to Takeda after receiving vedolizumab were identified in the Global Safety Database using the MedDRA version 20.0 high-level term group hepatitis viral infections and the MedDRA preferred term viral hepatitis carrier. Only patients with a reported history of or concurrent HBV or HCV infection or both were included in this analysis. Patients with a history of or concurrent hepatitis A, with hepatitis B vaccination, or unspecified hepatitis were excluded.
Analysis
The clinical trial population for the GEMINI 1 and GEMINI 2 trials included patients who had received the same treatment (ie, placebo or vedolizumab only) in both induction and maintenance phases of the respective trials. Patients in the GEMINI LTS study included rollover patients from a previous qualifying study (patients with ulcerative colitis or Crohn’s disease who participated in the phase 2, open-label, long-term safety study [C13004], patients who withdrew early from GEMINI 1 or GEMINI 2 due to sustained nonresponse, disease worsening, or the need for rescue medications, or patients who completed GEMINI 1, GEMINI 2, or GEMINI 3 trials) and de novo patients (ie, those with ulcerative colitis or Crohn’s disease who met the inclusion/exclusion criteria).
Rates of infections were calculated as exposure-adjusted incidence rates (rate per 100 PY = [number of patients experiencing an adverse event of interest / total patient exposure time in years] × 100). Additionally, the incidence of infections in percentages was provided for completeness.
Details of adverse events reported in clinical trials were summarized, including their intensity (mild, moderate, or severe), seriousness (serious or nonserious), and relationship to the study drug (as determined by the investigator).
In the post-marketing setting, adverse events were summarized in tables by the preferred term and seriousness.
RESULTS
Patient Population and Follow-Up
For the 52-week GEMINI 1 and GEMINI 2 clinical trials, 1434 patients who received vedolizumab (reflecting 1058.2 total patient-years [TPY] of vedolizumab exposure) and 297 patients who received placebo (171.3 TPY of placebo exposure) were included in the analysis. The mean (standard deviation [SD]) duration of exposure to vedolizumab was 258.5 ± 118.0 days and 246.8 ± 112.4 days in GEMINI 1 and GEMINI 2, respectively. Placebo was received for 181.4 ± 118.4 days and 203.0 ± 127.0 days in GEMINI 1 and GEMINI 2, respectively. A total of 59.0% of vedolizumab-treated patients from GEMINI 1 and GEMINI 2 had prior exposure to TNF antagonists compared with 48.8% of patients who received placebo.
A total 2243 patients in the LTS study received at least one dose of vedolizumab (5430.3 TPY of exposure). The patient population consisted of 894 patients with ulcerative colitis and 1349 patients with Crohn’s disease. At the data cut-off for the analysis, the mean (SD) duration of exposure to vedolizumab was 1046.2 ± 688.4 days for patients with ulcerative colitis and 914.7 ± 655.9 days for those with Crohn’s disease. From the LTS study, 46.4% of patients with ulcerative colitis and 66.6% with Crohn’s disease had prior exposure to TNF antagonists.
The Global Safety Database reflected an estimated 114,071 PY of vedolizumab exposure at the data cut-off.
Opportunistic Infections
GEMINI 1 and GEMINI 2
In the randomized, controlled phase 3 trials, opportunistic infections were reported in 7 of 1434 patients (0.5%) exposed to vedolizumab, with over 1058.2 TPY of vedolizumab exposure (incidence 0.7/100 PY; Table 1). The infections reported were C. difficile colitis/infection (n = 5), CMV colitis (n = 1), and CMV infection (n = 1); 0.5, 0.1, and 0.1 per 100 PY, respectively. No opportunistic infections were reported in patients receiving placebo (in 171.3 TPY of exposure).
Table 1:
Opportunistic Infections in Vedolizumab Clinical Trials
| GEMINI 1 and GEMINI 2 | GEMINI LTS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vedolizumab, n = 1434, PY = 1058 | Placebo, n = 297, PY = 171 | Ulcerative colitis, n = 894, PY = 2285 | Crohn’s disease, n = 1349, PY = 3145 | Total in LTS, n = 2243, PY = 5430.3 | |||||||||||
| n* | % | IR† | n* | % | IR† | n* | % | IR† | n* | % | IR† | n* | % | IR† | |
| All opportunistic infections | 7 | 0.5 | 0.7 | 0 | – | – | 27 | 3.0 | 1.2 | 24 | 1.8 | 0.8 | 51 | 2.3 | 1.0 |
| Drug-related | 2 | 0.1 | 0.2 | 0 | – | – | 10 | 1.1 | 0.4 | 8 | 0.6 | 0.3 | 18 | 0.8 | 0.3 |
| Resulted in study discontinuation | 1 | <0.1 | 0.1 | 0 | – | – | 4 | 0.4 | 0.2 | 0 | – | – | 4 | 0.2 | <0.1 |
| Serious | 1‡ | <0.1 | 0.1 | 0 | – | – | 9§ | 1.0 | 0.4 | 9║ | 0.7 | 0.3 | 18¶ | 0.8 | 0.3 |
| Serious and drug-related | 1‡ | <0.1 | 0.1 | 0 | – | – | 5 | 0.6 | 0.2 | 3 | 0.2 | 0.1 | 8 | 0.4 | 0.2 |
| Serious and resulted in discontinuation | 0 | – | – | 0 | – | – | 3 | 0.3 | 0.1 | 0 | – | – | 3 | 0.1 | <0.1 |
| Fatal | 0 | – | – | 0 | – | – | 0 | – | – | 0 | – | – | 0 | – | – |
| Type of opportunistic infection | |||||||||||||||
| C. difficile colitis | 5 | 0.3 | 0.5 | 0 | – | – | 14 | 1.6 | 0.6 | 13 | 1.0 | 0.4 | 27 | 1.2 | 0.5 |
| Clostridial infection | – | – | – | – | – | – | 11 | 1.2 | 0.5 | 4 | 0.3 | 0.1 | 15 | 0.7 | 0.3 |
| Esophageal candidiasis | – | – | – | – | – | – | 2 | 0.2 | 0.1 | 5 | 0.4 | 0.2 | 7 | 0.3 | 0.1 |
| CMV colitis | 1 | <0.1 | 0.1 | 0 | – | – | 3 | 0.3 | 0.1 | 2 | 0.1 | 0.1 | 5 | 0.2 | 0.1 |
| CMV infection | 1 | <0.1 | 0.1 | 0 | – | – | |||||||||
| CMV gastrointestinal infection | – | – | – | – | – | – | 1 | 0.1 | <0.1 | 1 | <0.1 | <0.1 | 2 | <0.1 | <0.1 |
*Number of patients who each experienced at least one occurrence of that type of adverse event.
†Exposure-adjusted incidence rate per 100 PY.
‡A patient with C. difficile colitis.
§C. difficile colitis (7 patients), clostridial infection (1 patient), and CMV colitis (2 patients).
║C. difficile colitis (5 patients), clostridial infection (3 patients), CMV colitis (1 patient), and esophageal candidiasis (1 patient).
¶Two of the 18 patients reported 2 serious events each: C. difficile colitis and CMV colitis in one patient, and C. difficile colitis and clostridial infection in the second patient.
CMV indicates cytomegalovirus; IR, incidence rate; LTS, long-term safety; PY, patient-years.
Seven patients reporting an opportunistic infection had a median age of 39 years (range 31–45 years); of these, 4 were female and 6 had prior exposure to TNF antagonists. The median time from the first vedolizumab infusion to the occurrence of an opportunistic infection was 85 days (range 29–449 days) and the most recent vedolizumab infusion was administered a median of 15 days (range 10–101 days) before an opportunistic infection was reported.
All reported opportunistic infections were mild (n = 2) to moderate (n = 5) in intensity, none were fatal, and in all but one patient, they were considered nonserious events. A 39-year-old female patient with Crohn’s disease who was previously treated with TNF antagonist therapy had a serious event of C. difficile colitis that involved hospitalization and treatment with metronidazole (during the event, the patient also received ciprofloxacin, amoxicillin/clavulanate, levomepromazine, tramadol hydrochloride, and glycerol). The infection was resolved within 7 days and vedolizumab treatment was continued. One patient discontinued the study due to a C. difficile infection, although the infection was considered nonserious.
The opportunistic infections described were reported from France and the United States (2 patients each), and from Canada, Austria, and Hungary (1 patient each).
GEMINI LTS study
In the GEMINI LTS study, opportunistic infections were reported in 2.3% of patients (51 of 2243; 27 ulcerative colitis, 24 Crohn’s disease), with over 5430.3 TPY of vedolizumab exposure. The 51 patients with IBD reporting opportunistic infections had a median age of 41.1 years (range 19–72), 34 were female, and 40 had prior exposure to a TNF antagonist. The incidence rates in ulcerative colitis and Crohn’s disease were 1.2 per 100 PY and 0.8 per 100 PY, respectively. C. difficile colitis was the most frequently reported opportunistic infection, reported in 27 patients (incidence 0.5 per 100 PY; Table 1).
None of the reported opportunistic infections were fatal. There were 20 serious opportunistic infections among 18 patients with IBD, including C. difficile colitis (12 patients), CMV colitis (3 patients), clostridial infection (4 patients), and esophageal candidiasis (1 patient). Two of the 18 patients reported 2 serious events each (C. difficile colitis and CMV colitis in one patient, and C. difficile colitis and clostridial infection in the other). Opportunistic infections resulted in vedolizumab discontinuation in only 7.8% of patients (4 of 51).
Post-marketing data
The number of PY of exposure to vedolizumab in the post-marketing setting is an approximation based on shipping data; it is assumed that each patient received vedolizumab 300 mg (1 vial) every 8 weeks. In the context of approximately 114,071 PY of vedolizumab therapy in the post-marketing setting, opportunistic infections were reported in 210 patients (Table 2). Of 217 total events reported in these patients, the most frequent were C. difficile infection (83 nonserious, 44 serious events) and CMV infection (6 nonserious, 14 serious events).
TABLE 2:
Opportunistic Infections in Patients Receiving Vedolizumab in the Post-Marketing Setting
| MedDRA Preferred Term | Number of Nonserious Events | Number of Serious Events | Total |
|---|---|---|---|
| Total | 113 | 104 | 217* |
| Bronchopulmonary aspergillosis | 0 | 1 | 1 |
| C. difficile colitis | 10 | 9 | 19 |
| C. difficile infection | 83 | 44 | 127 |
| Cytomegalovirus colitis | 6 | 9 | 15 |
| Cytomegalovirus infection | 6 | 14 | 20 |
| Cytomegalovirus viremia | 2 | 0 | 2 |
| Cytomegalovirus mononucleosis | 0 | 1 | 1 |
| Disseminated tuberculosis | 0 | 1 | 1 |
| Epstein–Barr virus-associated lymphoma | 0 | 1 | 1 |
| Esophageal candidiasis | 1 | 1 | 2 |
| Fungal esophagitis | 1 | 0 | 1 |
| Herpes esophagitis | 1 | 0 | 1 |
| Herpes zoster infection neurological | 0 | 1 | 1 |
| Herpes zoster disseminated | 0 | 1 | 1 |
| Histoplasmosis disseminated | 0 | 1 | 1 |
| JC virus test positive† | 1 | 8 | 9 |
| Mycobacterium avium complex infection | 1 | 2 | 3 |
| Nocardiosis | 1 | 3 | 4 |
| Opportunistic infection | 0 | 2 | 2 |
| Pneumocystis jirovecii pneumonia | 0 | 3 | 3 |
| Posttransplant lymphoproliferative disorder | 0 | 1 | 1 |
| Presumed ocular histoplasmosis syndrome | 0 | 1 | 1 |
*217 events were reported in 210 patients.
†The background prevalence of anti-JCV antibody seropositivity is approximately 33–91%, based on published rates in multiple patient populations and healthy individuals.44 JCV infection can lead to the development of progressive multifocal leukoencephalopathy.
JC indicates John Cunningham; MedDRA, Medical Dictionary for Regulatory Activities.
The median age of patients who had an opportunistic infection was 39 years (range 9–85 years; where age was reported) and 99 of 183 patients (for whom sex was reported) were male. Of the 210 patients, 99 had a confirmed medication history of prior/concomitant exposure to TNF antagonists, 104 had confirmed prior/concomitant exposure to steroids, and 76 had confirmed prior/concomitant exposure to other immunosuppressive therapies. In most cases, the duration between the first or final vedolizumab dose and the adverse event was not reported. In those reports that contained this information, most opportunistic infections occurred at least 1 month after the first vedolizumab infusion (62 of 79 reports) and within 0 to 13 days of the most recent infusion (15 of 20 reports).
Among the opportunistic infections reported, 104 were considered serious, including 2 events that resulted in death: one patient developed Pneumocystis jirovecii pneumonia due to long-term, high-dose steroid use, and a second patient with steroid-refractory graft-versus-host disease who was using vedolizumab off-label developed an unspecified opportunistic infection in addition to an existing CMV infection. Vedolizumab therapy was continued after the opportunistic infection in 101 of 158 patients (no available data on treatment continuation in 52 patients). There have been no cases of progressive multifocal leukoencephalopathy reported with vedolizumab treatment to date.
Most of the reports were from Canada (85 patients; approximately 5882 PY of vedolizumab exposure) and the United States (78 patients; approximately 61,488 PY of vedolizumab exposure).
Tuberculosis
Vedolizumab clinical trials
Tuberculosis was reported in 1 patient during GEMINI 2 and 4 patients in the LTS study (2 with latent tuberculosis and 3 with pulmonary tuberculosis; incidence rate across all clinical trials: 0.1 per 100 PY; Table 3), all of whom had received vedolizumab.
Table 3:
Tuberculosis in Vedolizumab Clinical Trials
| Study in Which Event Reported (Days From First VDZ Dose to TB) | Sex, Age, Indication | Events | Serious? | Intensity | Other Medical History | Induction treatment in GEMINI 1/2/3 (first 6 wk) | Maintenance Treatment in GEMINI 1/2/3 (≤52 wk) | Relationship to VDZ (Investigator’s Assessment) | Concomitant Medication | Prior Medication | Was VDZ discontinued? | Country (total VDZ exposure in country) | Incidence of TB in country of origin (per 100,000 people) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GEMINI LTS (938) | Male, 19 y, Crohn’s disease | Latent TB*† Pharyngitis | No‡ | Moderate | Tonsillectomy | Placebo (GEMINI 3) | NA§ | No | Azathioprine, loperamide, mesalazine, omeprazole | Azithromycin | Yes | Slovakia (56.2 PY) | 6.5 |
| GEMINI 2 (46) | Male, 38 y, Crohn’s disease | Latent TB* | Yes | Mild | NR | Vedolizumab | Vedolizumab Q4W | Yes | Mesalazine | Azathioprine, mesalazine, prednisone | Yes | Czech Republic (314.9 PY) | 5.2 |
| GEMINI LTS (105) | Female, 30 y, ulcerative colitis | Pulmonary TB | Yes | Moderate | NR | Vedolizumab (GEMINI 1) | Placebo (GEMINI 1) | No | Not reported | Azathioprine, Bacillus subtilis/ Streptococcus faecalis (Medilac-S), infliximab, mesalazine, prednisone | Yes, but prior to onset of TB because of lack of efficacy | Republic of Korea (110.4 PY) | 80 |
| GEMINI LTS (72 and 82) | Male, 31 y, Crohn’s disease | Pulmonary TB (2 events) | Event 1: Yes Event 2: No‡ |
Event 1: Moderate Event 2: Mild |
Gastrointestinal TB Anemia Peritonitis |
Placebo (GEMINI 2) | Placebo (GEMINI 2) | Yes | Budesonide | Unspecified anti-TB drugs, mesalazine, methylprednisolone | Yes | India (145.0 PY) | 217 |
| GEMINI LTS (141) | Female, 23 y, Crohn’s disease | Pulmonary TB | Yes | Moderate | Hiatal hernia Craniocerebral injury Craniotomy Anemia Thrombocytosis Hypoalbuminemia Arthritis Chronic gastritis Recurrent peptic ulcer Gastroesophageal reflux disease Rhinopharyngitis |
Vedolizumab (GEMINI 2) | Vedolizumab Q8W (GEMINI 2) | Yes | Azathioprine, desogestrel-ethinyl estradiol, mesalazine, prednisone | Ciprofloxacin, ferrous sulfate, folic acid, infliximab, iron preparations, mesalazine, omeprazole, proton pump inhibitors | Yes | Russian Federation (170.1 PY) | 80 |
*Not reported as part of the patient’s concomitant or previous medical history (diagnosed after the start of treatment with VDZ).
†On the date of event onset, the patient had a non-productive cough and physical examination results were normal. The diagnosis of latent TB infection was confirmed by Mycobacterium tuberculosis complex tests.
‡Two events of TB were assessed by the investigator as nonserious.
§GEMINI 3 was only 10 weeks in duration (no maintenance phase).
LTS indicates long-term safety; NA, not applicable; NR, not reported; PY, patient-years; Q4W, once every 4 weeks; Q8W, once every 8 weeks; TB, tuberculosis; VDZ, vedolizumab.
The median age of patients with tuberculosis was 30 years (range 19–38 years) and of the 5 patients, 2 were female. The median time from the first infusion to the occurrence of tuberculosis was 105 days (range 46–938 days). Two of the 5 patients had prior exposure to TNF antagonists (a 30-year old who had received infliximab and a 23-year old who had received infliximab [the dates of TNF antagonist exposure were not reported]).
All adverse events were reported as mild or moderate in intensity, none were fatal, and none of the patients had extra-pulmonary tuberculosis (Table 3). Tuberculosis resulted in discontinuation of vedolizumab treatment in 4 of the 5 patients (vedolizumab discontinuation under these circumstances was mandated by the clinical trial protocols). The fifth patient had discontinued vedolizumab before the onset of tuberculosis because of a lack of treatment efficacy. All 5 adverse events of tuberculosis were reported from countries known to be moderately or highly endemic for tuberculosis, including Czech Republic, India, Republic of Korea, Russian Federation, and Slovakia10 (1 patient in each country; the total vedolizumab exposure by country was 314.9 PY, 145.0 PY, 110.4 PY, 170.1 PY, and 56.2 PY, respectively).
Post-marketing data
In the context of approximately 114,071 PY of vedolizumab therapy, 7 adverse events of tuberculosis were reported in 7 patients (Table 4).
TABLE 4:
Tuberculosis Patients Receiving Vedolizumab in the Post-Marketing Setting
| Sex, Age, Indication | Events | Days From First VDZ Dose to TB | Serious? | Other Medical History | Concomitant Medication | Prior Medication | Was VDZ Discontinued? | Country (Total VDZ Exposure in Country) | Incidence of TB in Country of Origin (per 100,000 People) |
|---|---|---|---|---|---|---|---|---|---|
| Male, NR, ulcerative colitis | Latent TB,* Rash |
Not specified | No† |
Mycobacterium tuberculosis complex test (result NR) Rash with unspecified biologic |
NR | Adalimumab | Yes | US (~61,488 PY) | 3.2 |
| Female, 33, hemorrhagic rectocolitis‡ | Disseminated TB, Dyspnea | 48 | Yes | TB vaccine TB |
NR | Prednisolone (Solupred), infliximab (Remicade), interferon | Yes, but restarted after the event | France (~5524 PY) | 8.2 |
| Male, 20, Crohn’s disease | Cutaneous TB | 97 | Yes | Cutaneous TB (healed in the past) | NR | NR | Yes, but restarted after the event | Germany (~14,222 PY) | 8.1 |
| Female, NR, NR | TB,§ C. difficile infection | NR | Yes | NR | NR | NR | NR | US (~61,488 PY) | 3.2 |
| Male, 46, ulcerative colitis | TB§ | 196 | Yes | None║ | NR | NR | Yes | US (~61,488 PY) | 3.2 |
| NR, NR, IBD (unspecified) | Pulmonary TB | NR | Yes | Failed TNF antagonist therapy (unspecified) | NR | TNF antagonist therapy (unspecified) | Yes | US (~61,488 PY) | 3.2 |
| Female, NR, NR | Latent TB* | 154 | No† | NR | Augmentin | Adalimumab, infliximab | No | US (~61,488 PY) | 3.2 |
*Not reported as part of the patient’s concomitant or previous medical history (diagnosed after the start of treatment with VDZ).
†Reported as not serious despite TB being a significant event.
‡VDZ is not approved for use in hemorrhagic rectocolitis.
§Not reported whether patient had symptomatic or active TB.
║No other medical history reported.
IBD indicates inflammatory bowel disease; NR, not reported; PY, patient-years; TB, tuberculosis; TNF, tumor necrosis factor; VDZ, vedolizumab.
Age was reported for 3 patients (20, 33, and 46 years). Three patients were female and 3 were male (sex not reported for 1 patient). Four patients with tuberculosis had received TNF antagonists prior to treatment with vedolizumab (1 patient last received treatment with infliximab 98 days before being hospitalized with suspicion of tuberculosis; 1 discontinued treatment with infliximab 175 days before the onset of tuberculosis and adalimumab approximately 14 months before the onset of the event; 1 received adalimumab [treatment dates not reported]; and 1 received an unspecified TNF antagonist [treatment dates not reported]). Where reported, the time range from the first vedolizumab infusion to the occurrence of tuberculosis was 48–196 days.
Although tuberculosis is a significant medical event, 2 adverse events were assessed by the reporter as nonserious. Three patients discontinued treatment with vedolizumab after the diagnosis of tuberculosis, 1 continued treatment after diagnosis, 2 discontinued treatment and later restarted (the product label recommends discontinuation until tuberculosis is resolved), and in 1 of the patients the outcome was not reported.
The adverse events of tuberculosis occurred in the United States (5 patients), France (1 patient), and Germany (1 patient); the vedolizumab exposure by country was approximately 61,488 PY, 5524 PY, and 14,222 PY, respectively. Further details of the tuberculosis case reports received are described in the supplementary material (see case narratives, Supplemental Digital Content 2 and 3).
Safety Profile in Patients With History of or Concurrent HBV or HCV Infection
In the context of approximately 114,071 PY of vedolizumab post-marketing exposure, 29 patients with a history of or concurrent HBV or HCV infection were identified in the Global Safety Database. Fourteen patients had a medical history of hepatitis B (of these, 3 were confirmed as having chronic HBV infection; the infection status of the other 11 patients was unknown), and 15 patients had a medical history of hepatitis C. Of the 29 patients, 10 patients were diagnosed with ulcerative colitis, 17 patients with Crohn’s disease, and the indication was not specified for 2 patients. Patient age ranged from 24 to 75 years, and 18 patients were male.
Most patients (16 of 29; 55.2%) had prior or concomitant exposure to TNF antagonists. Patients were from Canada (18 patients), the United States (6 patients), Israel (2 patients), and Spain, Sweden, and Switzerland (1 patient each). Total vedolizumab exposure by country was approximately 5882 PY, 61,488 PY, 1510 PY, 2135 PY, 1852 PY, and 1538 PY, respectively.
A total of 109 adverse events (Table, Supplemental Digital Content 4) were reported in these 29 patients, most of which were nonserious (25 serious nonfatal events and 84 nonserious events). The most frequently reported event was increased blood pressure (7 patients with transient increased blood pressure associated with their infusion visit), followed by nausea (4 patients). Vedolizumab treatment was continued in 22 of 27 patients with available data (treatment continuation/discontinuation not reported in 2 patients). No trends were observed in the reported adverse events, which reflected the general vedolizumab safety profile in patients without viral hepatitis.16
Liver-related events were reported in 2 patients. A hepatic neoplasm (nodule in liver found on magnetic resonance imaging) was reported in a 61-year-old patient with Crohn’s disease and a history of hepatitis C, blood transfusion, and 4 bowel resections; this event was assessed by the physician as serious (outcome not reported). A hepatic mass (hypodense lesion in the right hepatic lobe) was reported in a 70-year-old patient with Crohn’s disease and a history of hepatitis C, skin cancer, cholecystectomy, removal of bladder tumor, and right radical orchiectomy. This event was assessed by the physician as not serious (outcome also not reported).
DISCUSSION
In pooled data from phase 3 clinical trials of vedolizumab for the treatment of ulcerative colitis and Crohn’s disease, we found that the rate of opportunistic infections was low, with an incidence of 0.7 per 100 PY (Table 1). The rate of opportunistic infections in the LTS study of vedolizumab was also relatively low, with an incidence of 1.0 per 100 PY. These data (based on a data cut of May 2015) extend the findings reported by Colombel et al. (2017), which used an earlier data cut (June 2013); this correlation suggests that the rates were stable over the study period.17 The most commonly reported infections were C. difficile or CMV infections and esophageal candidiasis; however, few of these infections led to the discontinuation of vedolizumab. In the post-marketing setting, 217 opportunistic infections were reported in 210 patients (99 of whom had prior exposure to TNF antagonists), including 2 opportunistic infections that resulted in death. Tuberculosis was reported in 5 (0.1%) patients in clinical trials and 7 patients in the post-marketing setting. We also identified 29 patients in the vedolizumab Global Safety Database with a history of or concurrent HBV/HCV infection; however, none reported viral reactivation after vedolizumab treatment.
Patients with IBD are at an increased risk of developing C. difficile infection; in one study by Nguyen et al. (2008), the prevalence of C. difficile in patients with ulcerative colitis was 37.3/1000, compared with 10.9/1000 in patients with Crohn’s disease and 4.5/1000 in general medical patients.26 The frequency of opportunistic infections such as C. difficile infections in patients with IBD is further increased with the use of immunosuppressive therapies, including TNF antagonists.4, 5 Patients with IBD who develop C. difficile infections have higher colectomy and mortality rates.27, 28 The occurrence and severity of C. difficile infections in patients with IBD may in part relate to host factors such as immune response and changes in the gut microbiota.29, 30 A recent case-control study found that the use of a TNF antagonist (not controlling for concomitant medications that included azathioprine or 6-mercaptopurine) was associated with an increased risk of opportunistic infection (odds ratio 4.4; 95% CI 1.1–17.0; P = 0.03), which may be due to systemic immunosuppression by TNF antagonists.31 In addition, the risk of an opportunistic infection was found to be increased by the use of steroids in combination with other immunosuppressive agents.31
The results of the present analysis demonstrate that the majority of patients treated with vedolizumab in both clinical trial and post-marketing settings did not experience a serious opportunistic infection, and most patients continued vedolizumab treatment. Comparison between vedolizumab treatment and TNF antagonist therapy was not possible using these data, because the studies were not designed for such an analysis. A history of multiple drug therapies in both the clinical trial and post-marketing settings must be considered when interpreting these results. In the current analysis, all post-marketing opportunistic infections reported from Canada originated from a large, ongoing patient support program and were therefore solicited (as opposed to being spontaneous reports).
A previous analysis of safety data from 6 clinical trials, including 2830 patients with IBD and covering 4811 PY of vedolizumab exposure, showed no increased risk of serious infections or opportunistic infections with vedolizumab compared with placebo. Overall, serious clostridial infections, sepsis, and tuberculosis were reported in ≤0.6% of patients (0.3, 0.2, and 0.1 per 100 PY, respectively).17 The current analysis is based on a larger clinical trial data set—including 3677 patients, covering 6488 PY of vedolizumab exposure and approximately 114,071 PY of post-marketing data—and is consistent with the previous safety data analysis.17
Progressive multifocal leukoencephalopathy is a disabling and potentially fatal neurologic syndrome that can occur in the presence of severe immunosuppression.32 This disease is an uncommon adverse effect of treatment with natalizumab, an α4 integrin antagonist with a mechanism of action that decreases immune surveillance within the central nervous system, thereby increasing the risk of developing progressive multifocal leukoencephalopathy.33 Vedolizumab selectively targets the α4β7 integrin expressed on gut-homing lymphocytes, with non-gut-homing T lymphocytes remaining unaffected.15, 34 No cases of progressive multifocal leukoencephalopathy were reported in the GEMINI trials or in any other reports of vedolizumab use to date.
TNF antagonists and recent hospitalization appear to be risk factors for reactivation of latent tuberculosis in patients with IBD.35 A meta-analysis of data from randomized clinical trials found that treatment with TNF antagonists in patients with IBD more than doubled the risk of tuberculosis (relative risk 2.5; 95% CI 0.6–10.2).5 To minimize the risk of reactivation of latent tuberculosis, clinical practice guidelines recommend screening patients for tuberculosis before initiation of TNF antagonist therapy.36–38 However, recent studies have highlighted an increased risk of tuberculosis even among patients with a negative screening test prior to the initiation of TNF antagonists.8, 39, 40 It has been shown that screening for latent tuberculosis while patients are already on immunosuppressive therapies may lead to a lower rate of positivity for the interferon-gamma release assay.41
Tuberculosis was reported in 5 patients participating in vedolizumab clinical trials and 7 patients in the post-marketing setting (Tables 3 and 4). In the clinical trials, 3 of 5 adverse events were reported from countries with a high incidence of tuberculosis in the general population. The incidence of tuberculosis in patients with IBD treated with vedolizumab in clinical trials was similar to incidence rates in their respective countries of origin (Table 3), with over one-half of patients having had prior exposure to TNF antagonists. However, the sample size is too small to draw a firm conclusion, particularly because it is difficult to reliably assess the incidence or risk of uncommon adverse events such as tuberculosis from a relatively small study population or based on voluntary reports. The geographic distribution of tuberculosis reports in the Global Safety Database reflects a greater exposure to vedolizumab in the United States, France, and Germany (ie, the countries where vedolizumab has been available since 2014). This is compared with fewer tuberculosis reports in countries where vedolizumab is not yet approved or has only recently become available but where there is a higher background prevalence of tuberculosis.
Overall, the current analysis of data from clinical trials and post-marketing surveillance did not suggest an increased risk of tuberculosis with vedolizumab use. In the post-marketing setting, patients with tuberculosis were able to restart vedolizumab therapy after treatment of their tuberculosis (described in Supplemental Digital Content 3).
To our knowledge, this is the first study that addresses the risk of reactivation of HBV or HCV infection with vedolizumab therapy. A total of 29 patients receiving vedolizumab were identified in the Global Safety Database as having had a history of or concurrent HBV or HCV infection. More than one-half of all patients reported prior or concomitant therapy with a TNF antagonist. Of a total of 109 adverse events, most (77%) were nonserious. The most frequently reported adverse events included increased blood pressure and nausea, reflecting the general safety profile observed in patients without HBV/HCV receiving vedolizumab.16 There was no evidence of viral hepatitis reactivation in these case reports. One liver-related event (a hepatic neoplasm) was assessed as serious by the investigator. Among patients with available data, 81% continued vedolizumab treatment despite reporting adverse events.
Certain limitations should be considered when interpreting the LTS clinical trial data, such as the lack of corresponding long-term data for patients on placebo or on an active comparator, such as TNF antagonists, which would have allowed a direct comparison. However, the study included a heterogeneous patient population with some patients who received vedolizumab de novo, some who had completed a previous clinical trial of vedolizumab, and others who had experienced no response to vedolizumab or disease worsening in previous trials. Therefore, the population reflects the real-life situation and may be more representative of patients in clinical practice than of those who participated in the randomized, controlled trials.
Limitations of post-marketing data include the voluntary nature of reporting, incomplete details of the event (eg, the temporal relationship between drug administration and event), incomplete comorbidity and comedication data, and an inconsistent level of detail of patients’ past medical histories. Serious events are more likely to be reported than less serious ones.42, 43 A further potential reporting bias that also needs to be considered is that adverse event reports may be more likely to be submitted when solicited through patient support programs. Nonetheless, the data presented here represent the first post-marketing dataset that highlights the long-term safety of vedolizumab in the real-world setting without the constraints and stringent patient selection of clinical trials.
In conclusion, clinical trial and post-marketing data in patients with IBD receiving vedolizumab showed that the rate of serious opportunistic infection was low, and most patients were able to continue vedolizumab treatment. The frequency of tuberculosis was also low, and there was no evidence of viral reactivation in patients with a history of HBV or HCV infection. The LTS study, ongoing observational studies, and real-world data will be important to provide further insights into the frequency of opportunistic infections and tuberculosis in patients treated with vedolizumab. Such data will help inform treatment decisions in clinical practice.
SUPPLEMENTARY DATA
Supplementary data are available at Inflammatory Bowel Diseases online.
ACKNOWLEDGMENTS
Analytical support was provided by Kelly Williams, formerly of Takeda Development Center Americas, USA. The authors would like to thank William Palo, formerly of Takeda Development Center Americas, USA, for providing statistical support, analysis of data, and participating in initial concept/study discussions. We would also like to acknowledge the following employees of Takeda: Javaria Mona Khalid (Takeda International - UK Branch) for her work on the tuberculosis background information, and Baoguo Jiang (Takeda Development Center Americas, Inc.) and Gary Hantsbarger (Takeda Development Center Americas, Inc.) for providing additional statistical support. Medical writing support was provided by Khalid Siddiqui of Chameleon Communications International Ltd, UK (a Healthcare Consultancy Group Company) and was sponsored by Takeda Pharmaceutical Company Ltd.
Supported by Takeda Pharmaceutical Company Ltd. Medical writing support was provided by Khalid Siddiqui of Chameleon Communications International Ltd, UK (a Healthcare Consultancy Group Company) and was sponsored by Takeda Pharmaceutical Company Ltd.
Siew C. Ng has received financial support for research from AbbVie, Ferring, and Janssen; has received lecture fees from AbbVie, Ferring, Janssen, Menarini, and Takeda; and has participated in advisory boards for AbbVie, Ferring, and Takeda. Ida Normiha Hilmi has participated in a Takeda advisory board. Aimee Blake is an employee of Takeda International - UK Branch. Fatima Bhayat is an employee of Takeda Pharmaceuticals International Co. Shashi Adsul is an employee of Takeda Pharmaceuticals International AG, Zurich, Switzerland. Qasim Rana Khan is an employee of Takeda Pharmaceuticals International AG Singapore. Deng-Chyang Wu has participated in a Takeda advisory board and as a speaker at a Takeda symposium.
REFERENCES
- 1. Bernstein C, Eliakim A, Fedail S, et al. World Gastroenterology Organisation Global Guidelines. Inflammatory Bowel Disease. 2015. http://www.worldgastroenterology.org/guidelines/global-guidelines/inflammatory-bowel-disease-ibd/inflammatory-bowel-disease-ibd-english. Accessed February 23, 2017. [DOI] [PubMed] [Google Scholar]
- 2. Gomollón F, Dignass A, Annese V, et al. ; European Crohn’s and Colitis Organisation (ECCO). 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 3. Bonovas S, Fiorino G, Allocca M, et al. Biologic therapies and risk of infection and malignancy in patients with inflammatory bowel disease: a systematic review and network meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1385–97.e10. [DOI] [PubMed] [Google Scholar]
- 4. Ali T, Kaitha S, Mahmood S, et al. Clinical use of anti-TNF therapy and increased risk of infections. Drug Healthc Patient Saf. 2013;5:79–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ford AC, Peyrin-Biroulet L. Opportunistic infections with anti-tumor necrosis factor-α therapy in inflammatory bowel disease: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2013;108:1268–76. [DOI] [PubMed] [Google Scholar]
- 6. Rahier JF, Magro F, Abreu C, et al. ; ECCO Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–68. [DOI] [PubMed] [Google Scholar]
- 7. McAuliffe ME, Lanes S, Leach T, et al. Occurrence of adverse events among patients with inflammatory bowel disease in the HealthCore Integrated Research Database. Curr Med Res Opin. 2015;31:1655–64. [DOI] [PubMed] [Google Scholar]
- 8. Jauregui-Amezaga A, Turon F, Ordás I, et al. Risk of developing tuberculosis under anti-TNF treatment despite latent infection screening. J Crohns Colitis. 2013;7:208–12. [DOI] [PubMed] [Google Scholar]
- 9. Byun JM, Lee CK, Rhee SY, et al. The risk of tuberculosis in Korean patients with inflammatory bowel disease receiving tumor necrosis factor-α blockers. J Korean Med Sci. 2015;30:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization. Global tuberculosis report. 2016. http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf. Accessed September 19, 2017. [Google Scholar]
- 11. Temel T, Cansu DÜ, Korkmaz C, et al. The long-term effects of anti-TNF-α agents on patients with chronic viral hepatitis C and B infections. Int J Rheum Dis. 2015;18:40–5. [DOI] [PubMed] [Google Scholar]
- 12. Morisco F, Castiglione F, Rispo A, et al. Effect of immunosuppressive therapy on patients with inflammatory bowel diseases and hepatitis B or C virus infection. J Viral Hepat. 2013;20:200–8. [DOI] [PubMed] [Google Scholar]
- 13. Loras C, Gisbert JP, Mínguez M, et al. ; REPENTINA study; GETECCU (Grupo Español de Enfermedades de Crohn y Colitis Ulcerosa) Group Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut. 2010;59:1340–6. [DOI] [PubMed] [Google Scholar]
- 14. Soler D, Chapman T, Yang LL, et al. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009;330:864–75. [DOI] [PubMed] [Google Scholar]
- 15. Fedyk ER, Wyant T, Yang LL, et al. Exclusive antagonism of the α4β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm Bowel Dis. 2012;18:2107–19. [DOI] [PubMed] [Google Scholar]
- 16. Takeda Pharma A/S. ENTYVIO® (vedolizumab) summary of product characteristics. 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002782/WC500168528.pdf. Accessed September 20, 2017. [Google Scholar]
- 17. Colombel JF, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 19. Sandborn WJ, Feagan BG, Rutgeerts P, et al. ; GEMINI 2 Study Group Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369:711–21. [DOI] [PubMed] [Google Scholar]
- 20. Loftus EV Jr, Colombel JF, Feagan BG, et al. Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis. 2017;11:400–11. [DOI] [PubMed] [Google Scholar]
- 21. Vermeire S, Loftus EV Jr, Colombel JF, et al. Long-term efficacy of vedolizumab for Crohn’s disease. J Crohns Colitis. 2017;11:412–24. [DOI] [PubMed] [Google Scholar]
- 22. Sands BE, Feagan BG, Rutgeerts P, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014;147:618–27.e3. [DOI] [PubMed] [Google Scholar]
- 23. Parikh A, Fox I, Leach T, et al. Long-term clinical experience with vedolizumab in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1691–9. [DOI] [PubMed] [Google Scholar]
- 24. Mahadevan U, Vermeire S, Lasch K, et al. Vedolizumab exposure in pregnancy: outcomes from clinical studies in inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:941–50. [DOI] [PubMed] [Google Scholar]
- 25. US Food and Drug Administration. Investigational New Drug Application, 21 C.F.R. § 312.32. 2016. http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm. Accessed June 8, 2017. [Google Scholar]
- 26. Nguyen GC, Kaplan GG, Harris ML, et al. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103:1443–50. [DOI] [PubMed] [Google Scholar]
- 27. Goodhand JR, Alazawi W, Rampton DS. Systematic review: Clostridium difficile and inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:428–41. [DOI] [PubMed] [Google Scholar]
- 28. Khanna S, Pardi DS. IBD: poor outcomes after Clostridium difficile infection in IBD. Nat Rev Gastroenterol Hepatol. 2012;9:307–8. [DOI] [PubMed] [Google Scholar]
- 29. Hughes M, Qazi T, Berg A, et al. Host immune response to Clostridium difficile infection in inflammatory bowel disease patients. Inflamm Bowel Dis. 2016;22:853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khanna S, Vazquez-Baeza Y, González A, et al. Changes in microbial ecology after fecal microbiota transplantation for recurrent C. difficile infection affected by underlying inflammatory bowel disease. Microbiome. 2017;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Toruner M, Loftus EV Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–36. [DOI] [PubMed] [Google Scholar]
- 32. Adang L, Berger J. Progressive multifocal leukoencephalopathy. F1000Res. 2015;4:1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Major EO. Progressive multifocal leukoencephalopathy in patients on immunomodulatory therapies. Annu Rev Med. 2010;61:35–47. [DOI] [PubMed] [Google Scholar]
- 34. Milch C, Wyant T, Xu J, et al. Vedolizumab, a monoclonal antibody to the gut homing α4β7 integrin, does not affect cerebrospinal fluid T-lymphocyte immunophenotype. J Neuroimmunol. 2013;264:123–6. [DOI] [PubMed] [Google Scholar]
- 35. Riestra S, de Francisco R, Arias-Guillén M, et al. Risk factors for tuberculosis in inflammatory bowel disease: anti-tumor necrosis factor and hospitalization. Rev Esp Enferm Dig. 2016;108:541–9. [DOI] [PubMed] [Google Scholar]
- 36. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501–23; quiz 524. [DOI] [PubMed] [Google Scholar]
- 37. Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–83; quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 38. Mowat C, Cole A, Windsor A, et al. ; IBD Section of the British Society of Gastroenterology Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. [DOI] [PubMed] [Google Scholar]
- 39. Abitbol Y, Laharie D, Cosnes J, et al. ; GETAID Negative screening does not rule out the risk of tuberculosis in patients with inflammatory bowel disease undergoing anti-TNF treatment: a descriptive study on the GETAID cohort. J Crohns Colitis. 2016;10:1179–85. [DOI] [PubMed] [Google Scholar]
- 40. Carpio D, Jauregui-Amezaga A, de Francisco R, et al. ; GETECCU Tuberculosis in anti-tumour necrosis factor-treated inflammatory bowel disease patients after the implementation of preventive measures: compliance with recommendations and safety of retreatment. J Crohns Colitis. 2016;10:1186–93. [DOI] [PubMed] [Google Scholar]
- 41. Wong SH, Gao Q, Tsoi KK, et al. Effect of immunosuppressive therapy on interferon γ release assay for latent tuberculosis screening in patients with autoimmune diseases: a systematic review and meta-analysis. Thorax. 2016;71:64–72. [DOI] [PubMed] [Google Scholar]
- 42. Sharrar RG, Dieck GS. Monitoring product safety in the postmarketing environment. Ther Adv Drug Saf. 2013;4:211–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Biswas P. Pharmacovigilance in Asia. J Pharmacol Pharmacother. 2013;4:S7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sørensen PS, Bertolotto A, Edan G, et al. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler. 2012;18:143–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.