Abstract
The adaptations of root growth and rhizosphere processes for soil phosphorus (P) acquisition have been investigated intensively in wheat (Triticum aestivum). However, only a few studies paid attention to these responses to shoot P status. This study aimed at investigating the responses of root morphology and P-mobilizing exudation to increasing shoot P concentration. A broad range of wheat shoot P concentrations (1.0–7.1 mg per g dry weight) was set up with 11 rates of P supply: 0–1200 mg P per kg soil. Root morphology and exudation parameters were measured after 37 days of plant growth. Shoot dry biomass reached a maximum when shoot P concentration was 4.63 mg per g dry weight. The maximum shoot P concentration for total root length, specific root length and the proportion of fine root (diameter ≤ 0.2 mm) length to total root length was 3 mg per g dry weight. Rhizosphere acidification was positively correlated with shoot P concentration when this was <5 mg per g dry weight. Shoot P concentration did not change acid phosphatase activity in the rhizosphere. Citrate concentration in the rhizosphere was suppressed by increasing shoot P concentration. In contrast, malate concentration in the rhizosphere showed a positive correlation with shoot P concentration. In conclusion, wheat root morphological and P-mobilizing exudation traits showed different behaviours with increasing P deficiency stress. Maintaining root biomass and length is the major strategy rather than root exudation for wheat to cope with extreme P deficiency.
Keywords: Rhizosphere, root, shoot phosphorus concentration, wheat
Introduction
Phosphorus (P) is one of the essential elements for plant growth and it is involved in many critical biochemical processes such as photosynthesis and respiration (Raghothama 1999). Soil P deficiency is a major constraint to crop yield in many parts of the world (Vance et al. 2003). In China, soil P in over 50 % of arable land is less than the agronomic optimum (Li et al. 2015b). Since phosphate rock may be exhausted in the next 50–100 years, P is a disappearing nutrient (Cordell et al. 2009; Gilbert 2009; Fixen and Johnston 2012; Johnston et al. 2014). Due to high P sorption of most soils, <20 % of applied fertilizer P may be uptaken by crops during the first growing season (Jones 1998; Zhang et al. 2008). Sorbed P in soil as legacy P has accumulated on many arable lands (Li et al. 2015b; Rowe et al. 2016). There is an urgent need to improve the biological potential of plants to efficiently utilize soil P.
Since phosphate is highly immobile in soil, diffusion is the main way that phosphate anions can reach the root surface (Lambers et al. 2008). Although the diffusion coefficient of phosphate in the soil is much less than that of other nutrients, the diffusion can be increased by increasing the phosphate concentration in the soil solution (Lambers et al. 2006). Moreover, dense root branching shortens diffusion distance of phosphate to the root surface and increases root interception (Lambers et al. 2006; Postma et al. 2014). In order to cope with P limitation, plants have evolved many morphological and physiological adaptations to enhance the roots’ P-uptake surface or mobilize unavailable soil P (Raghothama 1999; Vance et al. 2003).
Phosphorus-deficient plants often have a relatively greater root biomass and a larger root/shoot ratio than P-sufficient ones (Brouwer 1983; Lambers et al. 2006). The inhibition of primary root growth and proliferation of lateral root formation are enhanced by P deficiency, resulting in a shallow root system and increased total root length (Li et al. 2009; Péret et al. 2011; Niu et al. 2012). These traits help roots to exploit top soil efficiently where P is often rich (Niu et al. 2012). Plants tend to allocate more carbohydrates to roots which modified root/shoot ratio (Hermans et al. 2006). Wheat (Triticum aestivum) produces more fine roots in low P soil compared with high P soil (Yuan et al. 2016). It allows wheat to form a larger root surface area utilizing less carbon. Compared with maize (Zea mays), wheat has a similar level of response of root morphology to P deficiency in calcareous soil but a lower level of response in acid soil (Lyu et al. 2016).
Plants modify rhizosphere properties to increase soil P concentration in soil solution through root exudation including H+/OH−, carboxylates, phosphatase enzymes in P-limiting conditions (Hinsinger et al. 2003; Vance et al. 2003; Lambers et al. 2006). Rhizosphere acidification of P-deficient plants is well documented in many previous studies (Dinkelaker et al. 1989; Li et al. 2015a). The strong rhizosphere acidification of faba bean (Vicia faba) not only improves its own P uptake but also benefits P uptake of neighbouring maize plants in calcareous soil (Li et al. 2007). In contrast, faba bean has a greater OH− release in acid soil (Li et al. 2015a). Carboxylates (e.g. citrate, malate) play an important role in mobilization of soil P (Lambers et al. 2008). Carboxylates exudation of roots is greatly induced by P deficiency (Hoffland et al. 1989a; Keerthisinghe et al. 1998). Acid phosphatases efficiently hydrolyse organic P compounds in soils (Tarafdar et al. 2001). The activity of these enzymes is much greater in rhizosphere of P-deficient plants compared with that in bulk soil (Gilbert et al. 1999; Wasaki et al. 2003).
Lyu et al. (2016) summarized root responses of major crops to P deficiency and grouped them into two categories: root morphology-based and physiology-based. Cereals often have a stronger root morphological responses than legumes to P deficiency, but legumes prefer to modify the root physiological process to mobilize soil P. This difference leads to complementary effects on P acquisition in intercropping systems (Li et al. 2018). Many factors can modify root responses to P deficiency, such as shoot P status and soil types (AđAlsteinsson et al. 1994; Shane et al. 2003; Erel et al. 2017). Shoot P status is the dominant factor to regulate these morphological and physiological adaptations to P deficiency (AđAlsteinsson et al. 1994; Shane et al. 2003; Li et al. 2008b). Shoot P concentration of wheat exerts a great role in regulating P influx rate and proportion of P transport to the shoot (AđAlsteinsson et al. 1994). Shoot P status regulates cluster-root growth and citrate exudation in white lupin (Lupinus albus) (Li et al. 2008b). However, only a few studies paid attention to trajectories of these responses with various shoot P status (Shane et al. 2003; Li et al. 2008b).
Wheat is one of the major food crops in the world, and consumes much more P fertilizer than rice and maize every year (Yuan et al. 2016). Therefore, improving P use efficiency of wheat is important for saving rock P reserves and reducing P loss to the environment. In this study, a broad range of shoot P status of wheat was created by applying different rates of P fertilizers. The objective was to investigate the responses of root morphology and P-mobilizing exudations in wheat to various shoot P status.
Methods
Experiment set-up
An experiment was conducted in a greenhouse with natural light at 28/16 °C (day/night, average temperature) and 45–55 % relative air humidity from September to October 2014. A calcareous silt loam soil was collected from Shangzhuang, Beijing, China (40°05′40″N, 116°12′32″E). The soil was air-dried and sieved to pass 2-mm screen, then mixed thoroughly. Soil properties were as follows: 8.40 (pH, soil:water ratio, 1:2.5), 11.5 g kg−1 (organic C); 0.51 g kg−1 (total N), 8.5 mg kg−1 (Nmin, NO3− and NH4+), 0.69 g kg−1 (total P), 1.68 mg kg−1 (Olsen-P), 14.6 g kg−1 (total K), 82.4 mg kg−1 (NH4Ac exchangeable K). Each pot (volume of 1 L) was filled with 1 kg of air-dried soil. The nutrients were added to the soil as basal fertilizers at the following rates (mg kg−1): 1686.67 (Ca(NO3)2·4H2O), 335.10 (K2SO4), 125.67 (CaCl2), 43.34 (MgSO4·7H2O), 5.80 (EDTA-FeNa), 6.67 (MnSO4·4H2O), 10 (ZnSO4·7H2O), 2.0 (CuSO4·5H2O), 0.67 (H3BO3), 0.26 ((NH4)6Mo7O24·4H2O). Phosphorus was added to soil as KH2PO4 at a series of rates: 0, 2.5, 5, 10, 25, 50, 75, 150, 300, 600 and 1200 mg P per kg soil.
The wheat cultivar Yunmai 42 was selected for this study and the seeds were surface sterilized (30 min in a 10 % (v/v) H2O2 solution). They were germinated on the wet filter paper at 25 °C for 24 h in the dark. Six seeds were sown into each pot with 5 replicates per treatment. After 1 week, the seedlings were thinned to 3 plants per pot. During the whole experiment period, soil moisture in pots was kept at ~70 % field capacity by weighting.
Sample collection and analysis
Plants were harvested after 37 days of growth in jointing stage, when visual growth differences among the P rate treatments were obvious [seeSupporting Information—Fig. S1]. The wheat shoots were cut at soil surface. Roots adhered with soil were immerged into 0.2 mM CaCl2 solution as a trap solution, and they were shaken gently to collect the suspension solution of rhizosphere soil, which can be used to determine the pH, carboxylates and acid phosphatase in rhizosphere (Shen et al. 2003; Wang et al. 2007). During sampling, damage of fine roots and root hair should be avoided as much as possible. After sampling the rhizosphere exudation, all visible roots in each pot were then carefully picked out and stored in an ice cube box before they were transferred to the laboratory. In the laboratory, root samples were carefully cleaned using tap water and stored in a refrigerator before measurement of root morphological parameters. The bulk soil was also sampled. After air-drying, soil samples were ground to pass through a 2-mm sieve for analysis of bulk soil pH.
For carboxylates exudations determination, a 10-mL subsample of the rhizosphere extract was kept in a vial with addition of microbial inhibitor Micropur (Sicheres Trinkwasser, Rastatt, Germany) at 0.01 g L−1 and also three drops of concentrated phosphoric acid at −20 °C for high-performance liquid chromatography (HPLC) analysis. Carboxylates concentration in rhizosphere was measured using a reversed-phase HPLC (Shen et al. 2003). The chromatographic separation was carried out on a 250 × 4.6 mm reversed-phase column (Alltima C18, 5 µm; Alltech Associates, Inc., Deerfield, IL, USA). The mobile phase was 25 mmol L−1 KH2PO4 (pH 2.25) with a flow rate of 1 mL min−1 at 31 °C, and detection of the carboxylates was performed at 214 nm. For rhizosphere pH determination, the pH of the trap solution was measured, and adjusted to soil:water ratio of 1:2.5 based on the amount of rhizosphere soil (Li et al. 2010b). Acid phosphatase activity was determined using the spectrophotometric method based on the measuring of p-nitrophenol (PNP) absorbance at 405 nm (Alvey et al. 2001). The pH of bulk soil was measured after extraction in deionized water for 1 min at a soil:water ratio of 1:2.5.
Cleaned root samples were dispersed in water in a transparent array (30 × 20 × 3 cm) and scanned at a resolution of 400 dpi (Epson Expression 1600, Seiko Epson, Nagano, Japan). The root traits were determined by analysis of images using WinRHIZO software (Regent Instrument, Quebec, Canada). The shoots and roots were washed with the deionized water and then oven-dried at 70 °C for 3 days. After being weighed, plant materials were ground to powder for nutrient analysis. To determine the plant total P concentration, dried samples were milled and subsequently digested with concentrated H2SO4 and H2O2 using the molybdate-blue colorimetric method (Murphy and Riley 1962). Total P was measured with the vanado-molybdate method (Westerman 1990) from the P concentration in the digest.
Data analysis
Analysis of variance (ANOVA) was conducted using the one-way ANOVA model in the SAS statistical software (SAS 8.1, USA). Significant differences among means were determined by LSD at the P ≤ 0.05 probability level. Relationships between root/shoot ratio, shoot P concentration, rhizosphere physiological traits and root morphological traits were plotted using the SigmaPlot statistical software (SigmaPlot 10.0, USA).
The linear-plateau model was used for analysis of the relationship between shoot dry biomass, root dry biomass, root/shoot ratio, total root length and shoot P concentration. The unimodal model was established for analysis of relationship between specific root length, proportion of wheat root length in different diameter classes, rhizosphere change value and shoot P concentration. When it reached the maximum then the data witnessed a sharp decrease trend, so we chose the unimodal model. Linear model was used for analysis of the relationship between citrate, malate and shoot P concentration when there is no critical point.
Results
Plant growth and P uptake as affected by P rates
Shoot biomass increased significantly when the P addition rate was >10 mg kg−1, and eventually reached 1.68 g dry weight per pot at a P addition rate of 1200 mg kg−1, which was 5.72 times higher than that in the treatment without P addition (Table 1). Shoot P concentration also increased from 1.0 to 7.1 mg P per g dry weight with increasing P addition rates. The total root length and specific root length increased with increasing P supply when P addition rate was <25 mg kg−1. The specific root length declined slightly when P addition rates increased from 75 to 1200 mg kg−1. Roots were divided into three categories based on root diameter including fine roots (diameter < 0.2 mm), medium-sized roots (diameter between 0.2 and 0.4 mm) and thick roots (diameter > 0.4 mm). The proportion of fine roots length (diameter < 0.2 mm) to total root length reached a maximum (84.6 %) at P addition rate of 50 mg kg−1. Both lower and higher P addition rates reduced the proportion of fine root length to total root length (PFR), which was the lowest (69.5 %) in the treatment without P addition. The proportion of medium-sized root length to total root length (PMR), ranged from 13.7 to 24.3 %, was the highest in the treatment without P addition. The proportion of thick root to total root length (PTR) was <10 % in all the treatments.
Table 1.
Biomass, P concentration, total and specific root length and proportion of root length with different diameters to total root length of wheat grown with different P supplies. Each value is the mean (±SE) of five replicates. Different letters in a given column denote significant differences among P rates (P ≤ 0.05).
| P rate (mg kg−1) | Shoot biomass (g per pot) | Root biomass (g per pot) | Shoot P concentration (mg g−1) | Total root length (m per pot) | Specific root length (m g−1) | Proportion of root length in different diameters to total root length (%) | ||
|---|---|---|---|---|---|---|---|---|
| <0.2 mm | 0.2–0.4 mm | >0.4 mm | ||||||
| 0 | 0.25 ± 0.01f | 0.15 ± 0.00f | 0.98 ± 0.03h | 26.3 ± 1.2d | 173 ± 6f | 69.5 ± 0.7g | 24.3 ± 0.6a | 6.2 ± 0.3bc |
| 2.5 | 0.30 ± 0.01f | 0.18 ± 0.01ef | 0.97 ± 0.03h | 32.2 ± 1.5dc | 180 ± 3f | 70.9 ± 1.0gf | 23.6 ± 0.8ab | 5.5 ± 0.2cd |
| 5 | 0.28 ± 0.02f | 0.18 ± 0.01ef | 1.09 ± 0.06h | 37.5 ± 2.7dc | 210 ± 8e | 73.8 ± 0.9efg | 21.3 ± 0.6bc | 4.9 ± 0.4de |
| 10 | 0.32 ± 0.02f | 0.19 ± 0.01e | 1.23 ± 0.03h | 43.3 ± 4.3c | 224 ± 9de | 76.4 ± 1.3cd | 19.4 ± 1.1cd | 4.2 ± 0.3efg |
| 25 | 0.68 ± 0.04e | 0.26 ± 0.02d | 2.35 ± 0.06g | 78.4 ± 6.1ab | 295 ± 8a | 81.3 ± 1.1b | 15.3 ± 1.1efg | 3.4 ± 0.2g |
| 50 | 1.06 ± 0.05d | 0.28 ± 0.01d | 3.18 ± 0.05f | 80.6 ± 8.0ab | 289 ± 18ab | 84.6 ± 0.9a | 11.9 ± 0.6h | 3.5 ± 0.3fg |
| 75 | 1.25 ± 0.03c | 0.32 ± 0.01ab | 3.68 ± 0.09e | 84.0 ± 4.7a | 265 ± 15abc | 81.8 ± 0.8ab | 13.7 ± 0.8gh | 4.5 ± 0.2def |
| 150 | 1.49 ± 0.03b | 0.29 ± 0.01bcd | 4.71 ± 0.13d | 75.7 ± 5.2ab | 261 ± 8bc | 78.9 ± 1.6bc | 15.0 ± 1.1fg | 6.1 ± 0.5c |
| 300 | 1.44 ± 0.05b | 0.28 ± 0.02cd | 5.08 ± 0.19c | 69.6 ± 7.0b | 244 ± 10cd | 76.1 ± 1.1cd | 16.6 ± 0.7ef | 7.3 ± 0.4ab |
| 600 | 1.45 ± 0.05b | 0.31 ± 0.01abc | 5.83 ± 0.15b | 79.3 ± 2.4ab | 252 ± 8cd | 75.1 ± 0.9de | 17.5 ± 0.7de | 7.4 ± 0.2a |
| 1200 | 1.68 ± 0.12a | 0.34 ± 0.01a | 7.12 ± 0.05a | 82.9 ± 5.3ab | 245 ± 15cd | 72.5 ± 1.8efg | 19.4 ± 1.1cd | 8.2 ± 0.8a |
Relationship between plant growth and shoot P concentration
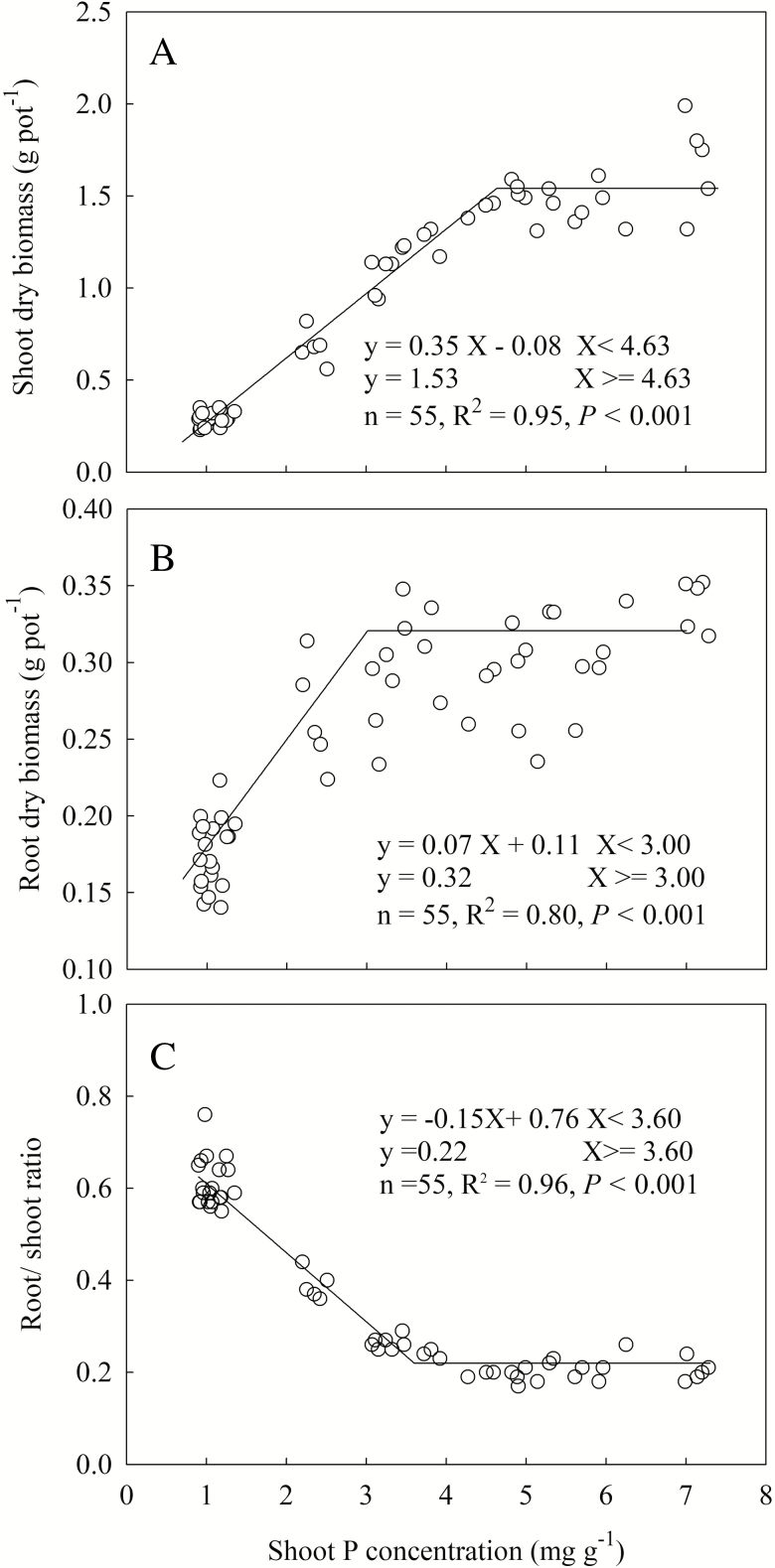
The relationship between shoot biomass and shoot P concentration was fitted well by a linear-plateau model (R2 = 0.96, P < 0.001) (Fig. 1A). Shoot biomass reached a plateau when shoot P concentration was 4.63 mg P per g and above, below which shoot biomass continuously decreased with decreasing shoot P concentration. A linear-plateau model also fitted the relationship between root biomass and shoot P concentration (R2 = 0.80, P < 0.001) (Fig. 1B). With root biomass increasing with shoot P concentration, and when shoot P concentration surpassed 3.00 mg P per g, root growth levelled off, which was earlier than that of shoot biomass. The root/shoot ratio declined substantially with increasing shoot P concentration, reaching a plateau at 3.60 mg P per g (Fig. 1C).
Figure 1.
Shoot biomass (A), root biomass (B) and root/shoot ratio (C) in response to shoot P concentration. Data point represents individual replicate.
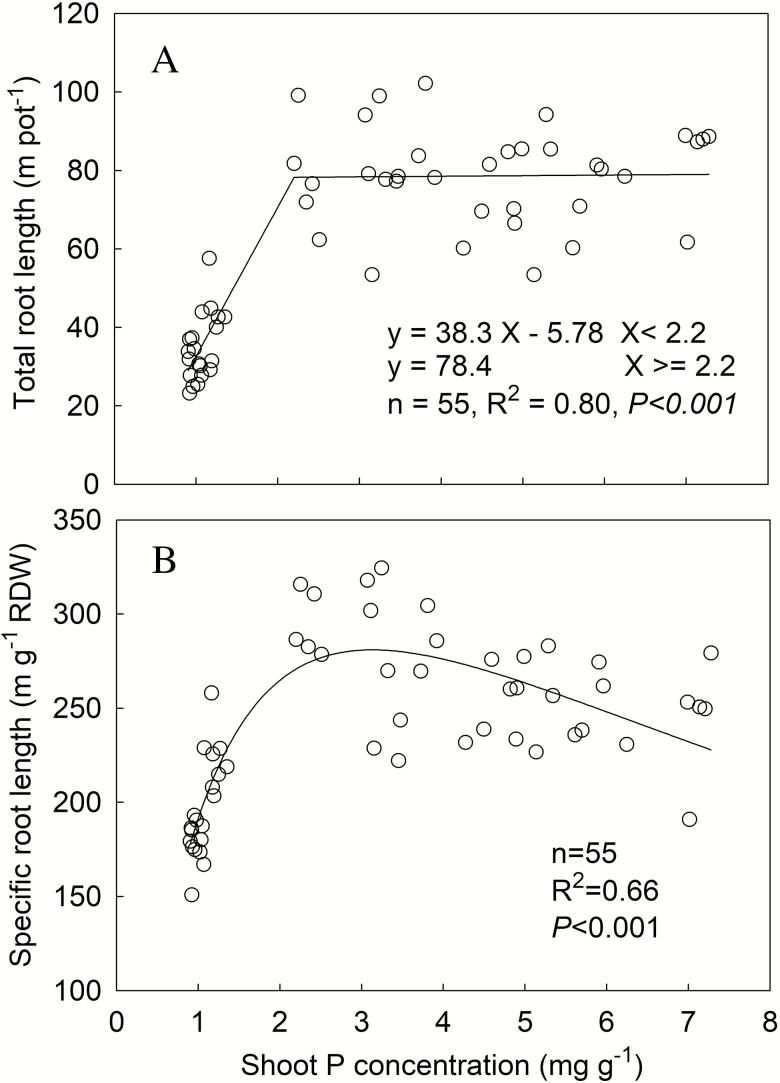
The relationship between total root length and shoot P concentration was also fitted well by a linear-plateau model (R2 = 0.80, P < 0.001) (Fig. 2A). The total root length was positively correlated with shoot P concentration when it was <2.2 mg P per g, above which total root length did not significantly change and was maintained at 78.9 m per pot with increasing shoot P concentration. The change of specific root length with increasing shoot P concentration showed a unimodal pattern (R2 = 0.66, P < 0.001). The specific root length increased from 177 to a peak of 281 m per g root dry weight at 3 mg g−1 for shoot P concentration and then continuously decreased to 228 m per g root dry weight (Fig. 2B).
Figure 2.
Total root length (A) and specific root length (B) in response to shoot P concentration. Data point represents individual replicate. RDW, root dry weight.
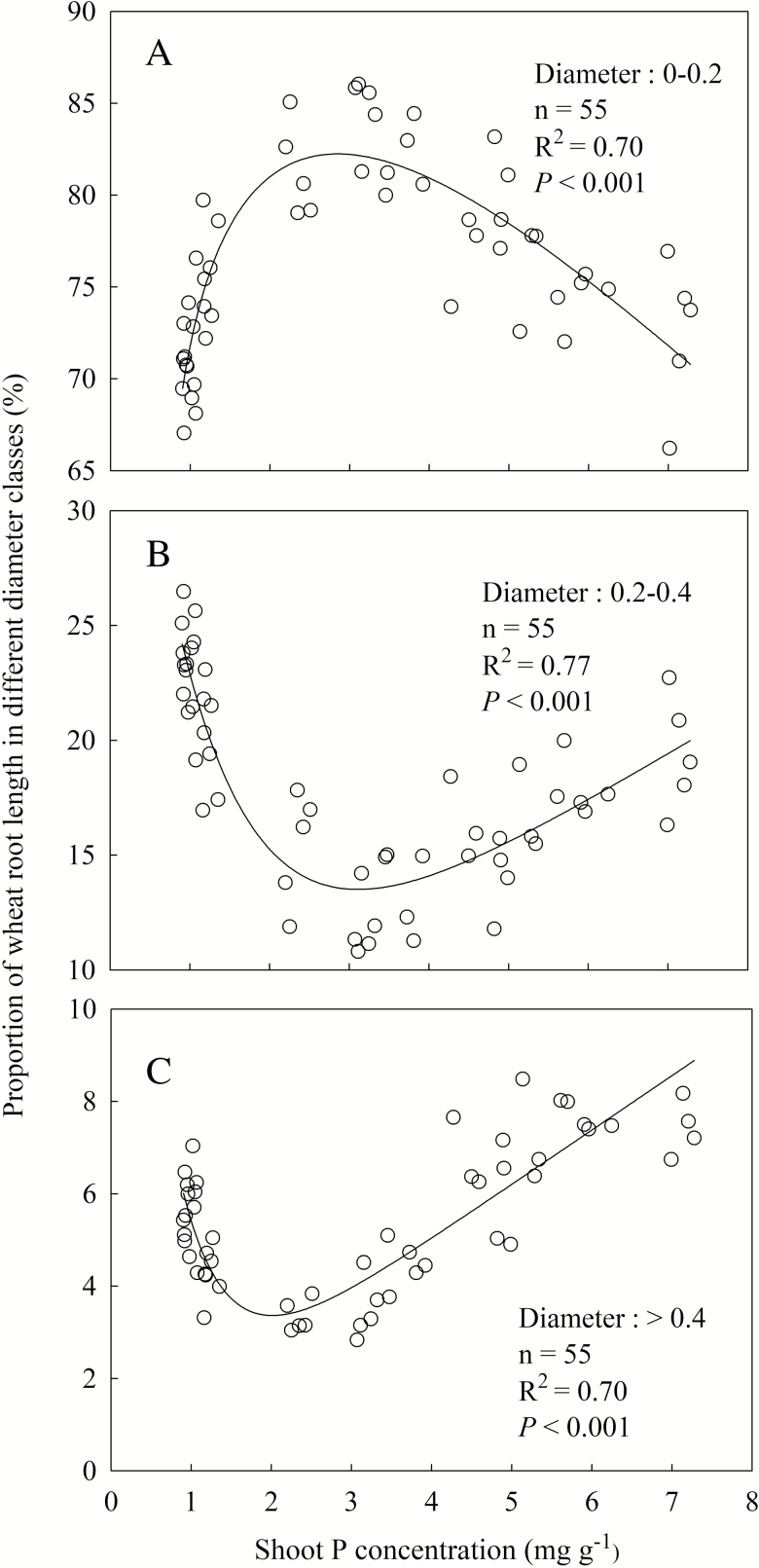
The PFR showed a unimodal pattern with increasing shoot P concentration (R2 = 0.70, P < 0.001), which was similar to that of specific root length (Fig. 3A). The maximum shoot P concentration was 3 mg g−1, below which PFR increased with decreasing shoot P concentration, and above which, PFR decreased. The PMR and PTR showed an opposite pattern to PFR with increasing shoot P concentration (Fig. 3B and C). The PMR and PTR reached a minimum when shoot P concentration was 3 and 2 mg g−1, respectively, above which the proportions continuously increased with increasing shoot P concentration.
Figure 3.
The proportion of root length with different diameter classes to total root length in response to increasing shoot P concentration. (A) Fine root in root diameter class: 0–0.2 mm; (B) medium-sized root in root diameter class: 0.2–0.4 mm; (C) thick root in root diameter class: >0.4 mm. Data point represents individual replicate.
Relationship between rhizosphere processes and P addition rates
The bulk soil pH at harvest was ~8.2 in the treatments ranging from P0 to P600, except the treatment with 1200 mg kg−1 showing a slight reduction by 0.28 unit (Table 2). The rhizosphere pH was not significantly different from the bulk soil pH when the amount of P addition was below 10 mg kg−1. A significant acidification of the rhizosphere was observed when P addition rates were >25 mg kg−1. The pH decreased to the minimum level when P addition rate was above 150 mg kg−1. The maximum acid phosphatase activity in the rhizosphere was observed when P addition rate was 25 mg kg−1, which was 234 µg PNP per h per g soil on average. In contrast, the minimum value was 91.5 µg PNP per h per g soil when P addition rate was 75 mg kg−1. The citrate and malate concentration in the rhizosphere ranged from 107 to 244 and 0 to 313 nmol per g soil, respectively. The highest concentration was observed when P addition rate was 1200 mg P per kg soil for malate and was 10 mg P per kg soil for citrate.
Table 2.
Soil pH, acid phosphatase activity, and citrate and malate concentration in the rhizosphere of wheat grown with different P supplies. Each value is the mean (±SE) of five replicates. Different letters in a given column denote significant differences between P rates (P ≤ 0.05).
| P rate (mg P per kg soil) | Rhizosphere soil pH | Bulk soil pH | Rhizosphere soil pH change | Acid phosphatase activity (μg PNP per h per g soil) | Citrate (nmol per g soil) | Malate (nmol per g soil) |
|---|---|---|---|---|---|---|
| 0 | 8.00 ± 0.05a | 8.19 ± 0.01ab | 0.18 ± 0.05d | 117 ± 3cd | 237 ± 29bcd | 0 ± 0d |
| 2.5 | 8.02 ± 0.02a | 8.20 ± 0.00a | 0.18 ± 0.02d | 162 ± 18bc | 244 ± 15abc | 0 ± 0d |
| 5 | 7.97 ± 0..05a | 8.19 ± 0.01ab | 0.22 ± 0.05d | 203 ± 31ab | 260 ± 34ab | 44 ± 44d |
| 10 | 7.91 ± 0.04a | 8.22 ± 0.01a | 0.31 ± 0.05d | 175 ± 18abc | 295 ± 29a | 31 ± 31d |
| 25 | 7.62 ± 0.05b | 8.20 ± 0.01a | 0.59 ± 0.06c | 234 ± 31a | 194 ± 16cde | 143 ± 43c |
| 50 | 7.56 ± 0.04b | 8.19 ± 0.01ab | 0.63 ± 0.04c | 190 ± 51ab | 195 ± 22cde | 185 ± 16bc |
| 75 | 7.42 ± 0.04c | 8.22 ± 0.01a | 0.80 ± 0.05b | 92 ± 6d | 167 ± 16ef | 192 ± 19bc |
| 150 | 7.36 ± 0.03cd | 8.20 ± 0.00a | 0.84 ± 0.03ab | 122 ± 16cd | 168 ± 25def | 259 ± 40ab |
| 300 | 7.33 ± 0.06cd | 8.15 ± 0.02b | 0.82 ± 0.06ab | 153 ± 13bcd | 138 ± 17ef | 214 ± 23bc |
| 600 | 7.27 ± 0.05d | 8.21 ± 0.04a | 0.95 ± 0.06a | 146 ± 16bcd | 121 ± 15f | 261 ± 39ab |
| 1200 | 7.28 ± 0.03d | 7.91 ± 0.04c | 0.63 ± 0.02c | 166 ± 15abc | 107 ± 16f | 313 ± 28a |
Relationship between rhizosphere processes and shoot P concentration
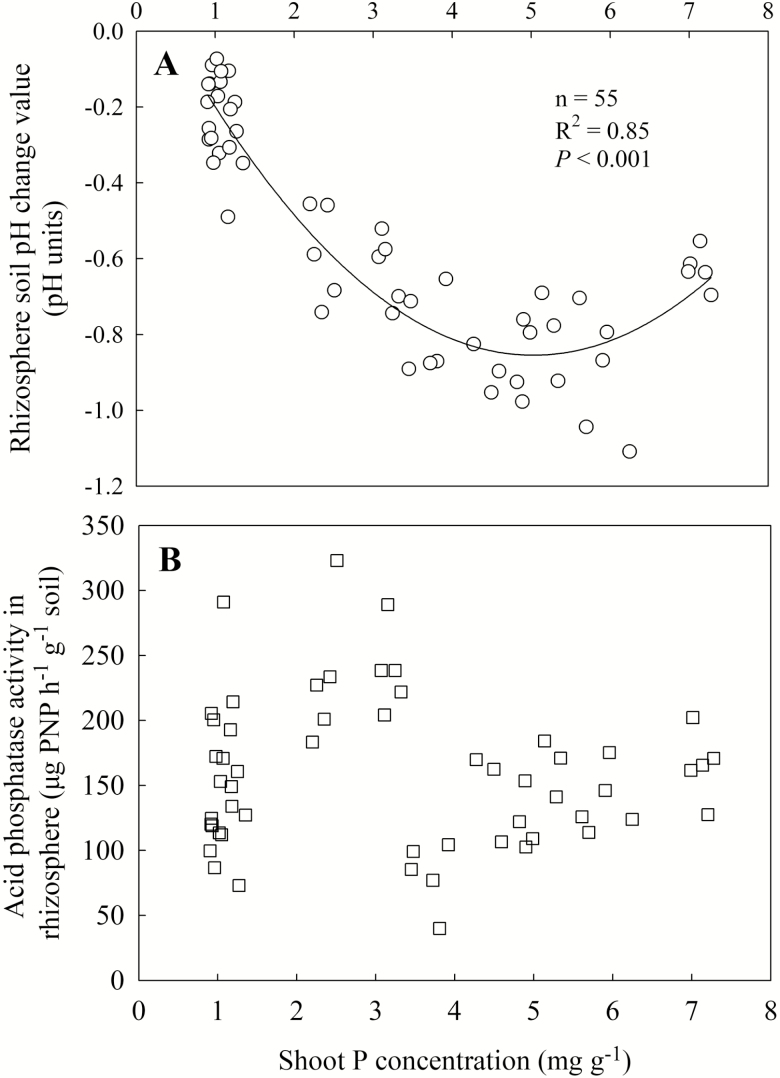
There was a significant acidification in the rhizosphere of wheat at a high shoot P concentration till 5.0 mg g−1, at which rhizosphere pH decreased by 0.8 unit (Fig. 4A). With shoot P concentration increased to 7.0 mg g−1, rhizosphere acidification was slightly reduced to 0.7 unit.
Figure 4.
Rhizosphere soil pH change (bulk soil pH minus rhizosphere soil pH) (A) and acid phosphatase activity in rhizosphere (B) in response to shoot P concentration. Data point represents individual replicate.
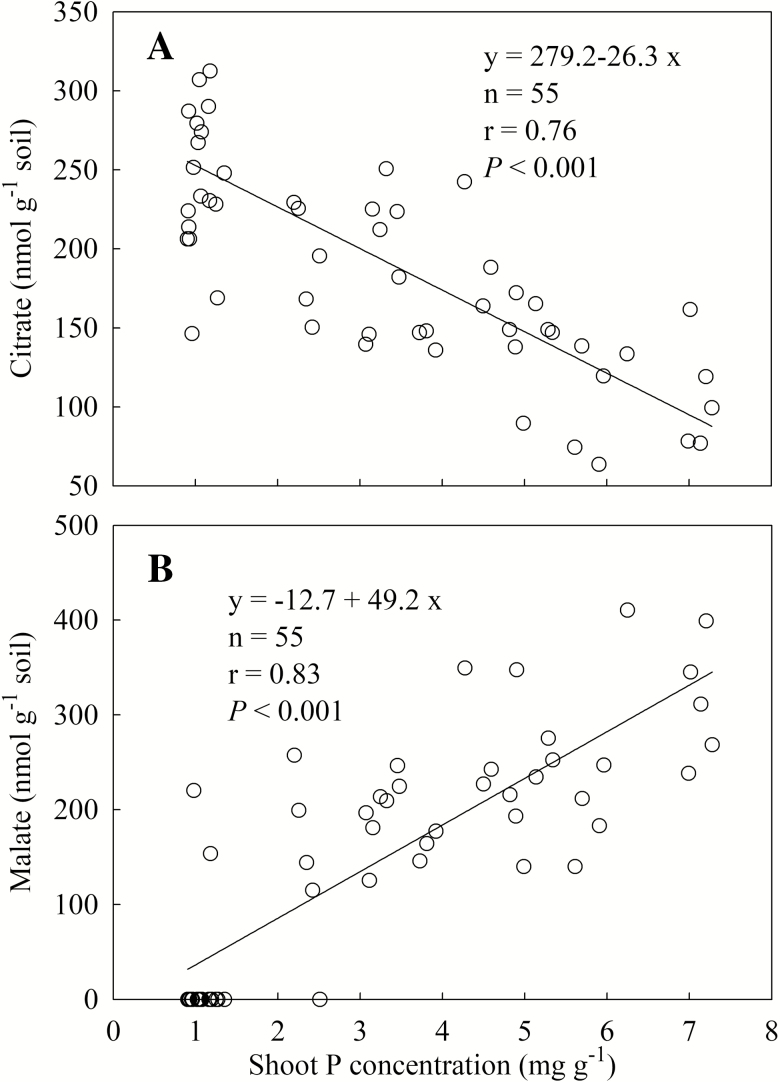
There was a large variation in acid phosphatase activity in the rhizosphere (from 49 to 310 µg PNP per h per g soil), especially, when shoot P concentration was around 1.0 mg g−1 (Fig. 4B). No correlation was observed between acid phosphatase activity and shoot P concentration. There was a negative correlation between citrate concentration in the rhizosphere and shoot P concentration (R2 = 0.58, P < 0.001) (Fig. 5A). In contrast, the malate concentration in the rhizosphere showed a positive correlation with shoot P concentration (Fig. 5B).
Figure 5.
Concentration of citrate (A) and malate (B) in the rhizosphere in response to shoot P concentration. Data point represents individual replicate.
Discussion
Plant growth and P uptake
There is a critical shoot P concentration for plant growth, and it depends on growth stages (Jones 1983; Sandaña and Pinochet 2014; Bélanger et al. 2015). The range of critical shoot P concentration for wheat growth is quite wide, being 1.3–2.8 mg g−1 for young whole shoots and 2.7–3.6 mg g−1 for recently matured leaves (Rashid et al. 2005). A high critical shoot P concentration is observed by Reuter et al. (1997) that is 3.8–4.5 mg g−1 for young whole shoots and 4.4–4.7 % for recently matured leaf blades. Differences of genotypes and achieved yields are presumed to contribute to this discrepancy. In this study, a broad range of shoot P concentration was created by adding a series of P amounts into soil, and the critical shoot P concentration was 4.63 mg g−1 for shoot growth and was only 3.00 mg g−1 for root growth. This indicated that shoot growth suffered P deficiency stress earlier than root at early growth stage. Maintaining root growth facilitates P-deficient plants to absorb soil P, which is an effective adaptation strategy to copy with P deficiency stress. This is in contrast to the root response of white lupin (Li et al. 2010a), which prefers to enhance exudation of carboxylates and acid phosphatases to mobilize soil P than produce more roots to increase soil P adsorption surface under P-deficient conditions (Lyu et al. 2016). Moreover, the cluster-root induced by P deficiency concentrates P-solubilizing compounds released by rootlets in a limited area, and then magnifies P-mobilizing capacity of roots (Hocking et al. 1998; Watt and Evans 1999).
Root morphological traits
In order to enhance P acquisition under limited P supply situations, plant often modifies root morphological traits to increase the ability of root to absorb P from soil (Schjørring and Nielsen 1987). Larger root system provides greater adsorption surface for soil nutrients, which is particularly important for soil P as a less mobile ion (Singh Gahoonia and Nielsen 2004). It was confirmed by Brück et al. (1992) when comparing plant P uptake between ‘rootless’ maize mutant and wild type. More biomass is allocated to roots when plant is suffering P deficiency (Lambers et al. 2006). The P-deficient wheat had a larger root/shoot ratio than P-sufficient plants with >50 mg P per kg addition in this study (Table 1). Root/shoot ratio was enhanced by increasing P stress, indicating that biomass allocation between shoots and roots is systemically regulated by shoot P status. Reduction of root biomass caused by P deficiency (shoot P concentration is below 3.00 mg g−1) did not result in reduction of total root length until shoot P concentration decreased to 2.2 mg g−1. Wheat had the highest specific root length within this range of shoot P concentration because of fine roots proliferation, indicating that wheat produces more root length with less root biomass. It is consistent with the results of Yuan et al. (2016) who found that P deficiency enhanced root length density and reduced root biomass at the same time, and intensity of these responses was dependent on soil types (Yuan et al. 2016). With increasing P deficiency (<2.2 mg g−1), root length was finally reduced. This is opposite to the previous research that sugar beet has a greater root length in low-P plots than high-P plots (Steingrobe 2001). In contrast, Ma and Rengel (2008) observed a similar pattern as this study. This response should be P deficiency intensity dependent: light P deficiency only reduces root biomass but not root length and extreme P deficiency reduces both. A smaller root diameter results in larger root adsorption surface per unit biomass (Atkinson 1990). The P deficiency enhances specific root length and fine root production in some cases (Christie and Moorby 1975; Schroeder and Janos 2005). However, this response was observed only when shoot P concentration was >3 mg g−1 in this study, below which the fine root production was suppressed by P deficiency. A root proliferation was induced by P deficiency at early stage, and finally disappeared with increasing stress intensity caused by plant growth (Marschner 2011). We possibly missed early response of wheat after a long growth period (37 days after planting) in low P soil. Decrease of fine root growth was possibly due to reduction of the numbers of lateral roots and lateral root primordia caused by extreme P deficiency (Li et al. 2012). This response is co-regulated by DNA replication, transcription, protein synthesis and degradation and cell growth. Although fine roots are more efficient than thick ones, carbon cost of fine roots may be much greater because of more frequent turnover (Persson 1983). Hence, it is speculated that the fine roots of wheat were more inhibited at extremely low P condition compared with thick roots (diameter > 0.2 mm).
The release of root exudates
To increase P mobilization, root morphological changes always follow with physiological changes, such as root exudation (Vance et al. 2003; Lambers et al. 2006). Soil pH plays a prominent role in chemical equilibrium of soil P that determines soil P bioavailability (Hinsinger 2001; Hinsinger et al. 2003). Soil acidification enhances dissolution of Ca phosphates to increase soil P bioavailability in calcareous soil (Hinsinger 2001; Li et al. 2015a). Many plant species reduce medium pH in low P condition, such as white lupin, tomato (Lycopersicon esculentum L.) and chickpea (Cicer arietinum L.) (Neumann and Römheld 1999). However, rhizosphere acidification of P-deficient wheat was weak in the previous studies (Neumann and Römheld 1999; Li et al. 2008a). No significant acidification was observed for extreme P-deficient wheat as well in this study. On the contrary, rhizosphere acidification strengthened with the increasing shoot P concentration till 5.0 mg g−1 (Fig. 4A), which was possibly due to excessive uptake of cations by root than anions (Hinsinger et al. 2003). In this study, P was added into soil as KH2PO4; thus, K as an accompany ion was inevitably added. Wheat absorbed much more K per unit root length with high P additions than low P additions [seeSupporting Information—Fig. S2], which led to rhizosphere acidification of wheat with sufficient P supply, as shown in the previous study (Wen et al. 2017). The P-deficient wheat did not depend on rhizosphere acidification to mobilize soil P, which was consistent with the previous study (Li et al. 2008a). Phosphatase activity in the rhizosphere has a tight positive correlation with the depletion of soil organic P (Tarafdar and Jungk 1987). The activity of acid phosphatase is often high in the rhizosphere of P-deficient plants (Gilbert et al. 1999; Wasaki et al. 2003; Ciereszko et al. 2011), which was up to eight times greater than bulk soil (Tarafdar and Jungk 1987). The relative expression levels of purple acid phosphatase genes (PAP15 and PAP16) of wheat were down-regulated with increasing soil P supply at flowering stage (Teng et al. 2013). However, phosphatase activity in the rhizosphere of maize had a positive linear correlation with shoot P concentration (Wen et al. 2017). This response may facilitate recapture of some organic P lost from roots into the rhizosphere. In this study, we failed to find correlation between acid phosphatase activity and P addition rates or shoot P concentration. In unsterilized condition, acid phosphatase in the rhizosphere is partly from soil microorganisms (Tarafdar et al. 1992). The change of acid phosphatase secretion of roots induced by variable shoot P concentration may be covered by secretion of soil microorganisms in this study.
Phosphorus-deficient plants exude carboxylates into rhizosphere to mobilize soil P through complexing metal cations-bound phosphate and displacing phosphate from soil mineral surface by ligand exchange (Hoffland et al. 1989b; Gerke et al. 2000; Jones et al. 2003). Predominant carboxylate composition is malate and citrate in the rhizosphere of wheat, and malate comprised over 85 % of total carboxylate (Pearse et al. 2006). However, carboxylate is not observed in the rhizosphere of wheat in other studies (Li et al. 2008a; Rose et al. 2010). We found malate and citrate in the rhizosphere of wheat, which is consistent with the results of Pearse et al. (2006). The carboxylate exudation of wheat is shoot P status-dependent. Increasing shoot P concentration diminished citrate accumulation, but induced malate accumulation synchronously. It is opposite to the results of Pearse et al. (2006) that wheat produced similar amounts of carboxylates between P-deficient and -sufficient plants (Pearse et al. 2006). Maize enhances carboxylates exudation with increasing shoot P concentration in some studies (Liu et al. 2004; Li et al. 2010b, Wen et al. 2017). This response may not be an adaptation to P deficiency but a by-product of high root activity of plants. Thus, only citrate exudation of wheat was regarded as an adaptive response to P deficiency in this study.
It should be noted that damage of root and root hair during the sampling could change root exudation, and then change carboxylate concentration in rhizosphere no matter how root sampling is carefully processed. This may cause overestimation of carboxylate concentration in rhizosphere in this study.
In summary, plant growth was suppressed by P deficiency in the following order: shoot biomass > root/shoot ratio > root biomass > total root length. It indicated that shoot growth was more sensitive to shoot P status than roots. As a root morphology-based crop, wheat did not have consistent root physiological traits to cope with P deficiency. Shoot P concentration as a dominant factor regulated these adaptations of soil P acquisition for wheat.
Conclusions
Root morphological and physiological traits of wheat showed different behaviours with increasing P deficiency. Phosphorus-deficient wheat preferred to maintain root growth than shoot by allocating more carbon to root, and root length by producing more fine roots even root biomass decreased. Extreme P deficiency (shoot P concentration < 3 mg g−1) reduced more fine roots production than thick roots. Wheat did not increase rhizosphere acidification and acid phosphatase secretion in low P soil. Citrate not malate exudation of roots as an adaptive response was enhanced by P deficiency. It is concluded that maintaining root biomass and length is the major strategy for wheat to cope with extreme P deficiency rather than root exudation.
Contributions by the Authors
Q.S., H.L. and J.S. designed the experiments; Q.S. and Z.W. performed the experiment and analysed the data; the authors interpreted the data together; Q.S. wrote the first draft of the manuscript; H.L. coordinated the research and supported all the technical support. All authors participated in writing the manuscript.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgements
We thank the Associate Editor and two anonymous reviewers for their valuable comments. We also thank Prof. Hans Lambers from University of Western Australia for helpful comments on an earlier draft of this manuscript.
Sources of Funding
This study was financially supported by National Key R&D Program of China (2017YFD0200200 and 2017YFD0200202), the National Natural Science Foundation of China (NSFC) (31330070 and 31210103906), the Innovative Group Grant of the National Natural Science Foundation of China (31421092), the National Basic Research Program (973-2015CB150405).
Supporting Information
The following additional information is available in the online version of this article—
Figure S1. The response of wheat shoots to different P fertilization rates (in mg P per kg soil) at the harvest time (37 days after planting).
Figure S2. The rhizosphere soil pH change (bulk soil pH minus rhizosphere soil pH) with K acquisition efficiency (total K uptake divide total root length). Each symbol represents the mean of five replicates (±SD). The numbers above each symbol indicate P added rates (mg kg−1).
Literature Cited
- AđAlsteinsson S, Schjørring JK, Jensen P. 1994. Regulation of phosphate influx in winter wheat: root-shoot phosphorus interactions. Journal of Plant Physiology 143:681–686. [Google Scholar]
- Alvey S, Bagayoko M, Neumann G, Buerkert A. 2001. Cereal/legume rotations affect chemical properties and biological activities in two west African soils. Plant and Soil 231:45–54. [Google Scholar]
- Atkinson D. 1990. Influence of root system morphology and development on the need for fertilizers and the efficiency of use. In: Baligar VC and Duncan RR, eds. Crops as enhancers of nutrient use. San Diego, CA: Academic Press, 411–451. [Google Scholar]
- Bélanger G, Ziadi N, Pageau D, Grant C, Högnäsbacka M, Virkajärvi P, Hu Z, Lu J, Lafond J, Nyiraneza J. 2015. A model of critical phosphorus concentration in the shoot biomass of wheat. Agronomy Journal 107:963–970. [Google Scholar]
- Brouwer R. 1983. Functional equilibrium: sense or nonsense?Netherlands Journal of Agricultural Science 31:335–348. [Google Scholar]
- Brück H, Becker H, Sattelmacher B. 1992. Phosphate efficiencies of two maize inbred lines. In: Root ecology and its practical applications. 3rd ISRR Symposium, Vienna, Austria, 193–196. [Google Scholar]
- Christie E, Moorby J. 1975. Physiological responses of semiarid grasses. I. The influence of phosphorus supply on growth and phosphorus absorption. Crop and Pasture Science 26:423–436. [Google Scholar]
- Ciereszko I, Szczygła A, Żebrowska E. 2011. Phosphate deficiency affects acid phosphatase activity and growth of two wheat varieties. Journal of Plant Nutrition 34:815–829. [Google Scholar]
- Cordell D, Drangert J-O, White S. 2009. The story of phosphorus: global food security and food for thought. Global Environmental Change 19:292–305. [Google Scholar]
- Dinkelaker B, Römheld V, Marschner H. 1989. Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant, Cell & Environment 12:285–292. [Google Scholar]
- Erel R, Bérard A, Capowiez L, Doussan C, Arnal D, Souche G, Gavaland A, Fritz C, Visser EJW, Salvi S, Le Marié C, Hund A, Hinsinger P. 2017. Soil type determines how root and rhizosphere traits relate to phosphorus acquisition in field-grown maize genotypes. Plant and Soil 412:115–132. [Google Scholar]
- Fixen PE, Johnston AM. 2012. World fertilizer nutrient reserves: a view to the future. Journal of the Science of Food and Agriculture 92:1001–1005. [DOI] [PubMed] [Google Scholar]
- Gerke J, Beißner L, Römer W. 2000. The quantitative effect of chemical phosphate mobilization by carboxylate anions on P uptake by a single root. I. The basic concept and determination of soil parameters. Journal of Plant Nutrition and Soil Science 163:207–212. [Google Scholar]
- Gilbert N. 2009. Environment: the disappearing nutrient. Nature 461:716–718. [DOI] [PubMed] [Google Scholar]
- Gilbert G, Knight J, Vance C, Allan D. 1999. Acid phosphatase activity in phosphorus-deficient white lupin roots. Plant, Cell & Environment 22:801–810. [Google Scholar]
- Hermans C, Hammond JP, White PJ, Verbruggen N. 2006. How do plants respond to nutrient shortage by biomass allocation?Trends in Plant Science 11:610–617. [DOI] [PubMed] [Google Scholar]
- Hinsinger P. 2001. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant and Soil 237:173–195. [Google Scholar]
- Hinsinger P, Plassard C, Tang C, Jaillard B. 2003. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant and Soil 248:43–59. [Google Scholar]
- Hocking PJ, Keerthisinghe G, Smith FW, Randall PJ. 1998. A comparison of the ability of different crop species to access poorly-available soil phosphorus. In: Ando P, Fujita K, Mae T, Matsumoto H, Mori S, Sekiya J, eds. Plant nutrition for sustainable food production & environment. Dordrecht, The Netherlands: Kluwer Academic Publishers, 305–308. [Google Scholar]
- Hoffland E, Findenegg GR, Nelemans JA. 1989a. Solubilization of rock phosphate by rape: II. Local root exudation of organic acids as a response to P-starvation. Plant and Soil 113:161–165. [Google Scholar]
- Hoffland E, Findenegg GR, Nelemans JA. 1989b. Solubilization of rock phosphate by rape. Plant and Soil 113:155–160. [Google Scholar]
- Johnston AE, Poulton PR, Fixen PE, Curtin D. 2014. Phosphorus: its efficient use in agriculture. Advances in Agronomy 123:177–228. [Google Scholar]
- Jones CA. 1983. A survey of the variability in tissue nitrogen and phosphorus concentrations in maize and grain sorghum. Field Crops Research 6:133–147. [Google Scholar]
- Jones DL. 1998. Organic acids in the rhizosphere–a critical review. Plant and Soil 205:25–44. [Google Scholar]
- Jones D, Dennis P, Owen A, Van Hees P. 2003. Organic acid behavior in soils–misconceptions and knowledge gaps. Plant and Soil 248:31–41. [Google Scholar]
- Keerthisinghe G, Hocking P, Ryan P, Delhaize E. 1998. Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.). Plant, Cell & Environment 21:467–478. [Google Scholar]
- Lambers H, Chapin FS, Pons TL. 2008. Plant physiological ecology. New York: Springer. [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. 2006. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany 98:693–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Kuper TW, van der Werf W, Zhang J, Li H, Zhang F, Hollland E. 2018. Testing for complementarity in phosphorus resource use by mixtures of crop species. Plant and Soil. doi: 10.1007/s11104-018-3732-4. [DOI] [Google Scholar]
- Li G, Li H, Leffelaar PA, Shen J, Zhang F. 2015a. Dynamics of phosphorus fractions in the rhizosphere of fababean (Vicia faba L.) and maize (Zea mays L.) grown in calcareous and acid soils. Crop and Pasture Science 66:1151–1160. [Google Scholar]
- Li L, Li S, Sun J, Zhou L, Bao X, Zhang H, Zhang F. 2007. Diversity enhances agricultural productivity via rhizosphere phosphorus facilitation on phosphorus-deficient soils. Proceedings of the National Academy of Sciences USA 104:11192–11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Liu J, Li G, Shen J, Bergström L, Zhang F. 2015b. Past, present, and future use of phosphorus in Chinese agriculture and its influence on phosphorus losses. Ambio 44:274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shen J, Zhang F, Clairotte M, Drevon J, Le Cadre E, Hinsinger P. 2008a. Dynamics of phosphorus fractions in the rhizosphere of common bean (Phaseolus vulgaris L.) and durum wheat (Triticum turgidum durum L.) grown in monocropping and intercropping systems. Plant and Soil 312:139–150. [Google Scholar]
- Li H, Shen J, Zhang F, Lambers H. 2010a. Localized application of soil organic matter shifts distribution of cluster roots of white lupin in the soil profile due to localized release of phosphorus. Annals of Botany 105:585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shen J, Zhang F, Marschner P, Cawthray G, Rengel Z. 2010b. Phosphorus uptake and rhizosphere properties of intercropped and monocropped maize, faba bean, and white lupin in acidic soil. Biology and Fertility of Soils 46:79–91. [Google Scholar]
- Li H, Shen J, Zhang F, Tang C, Lambers H. 2008b. Is there a critical level of shoot phosphorus concentration for cluster-root formation in Lupinus albus?Functional Plant Biology 35:328–336. [DOI] [PubMed] [Google Scholar]
- Li J, Xie Y, Dai A, Liu L, Li Z. 2009. Root and shoot traits responses to phosphorus deficiency and QTL analysis at seedling stage using introgression lines of rice. Journal of Genetics and Genomics 36:173–183. [DOI] [PubMed] [Google Scholar]
- Li Z, Xu C, Li K, Yan S, Qu X, Zhang J. 2012. Phosphate starvation of maize inhibits lateral root formation and alters gene expression in the lateral root primordium zone. BMC Plant Biology 12:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Mi G, Chen F, Zhang J, Zhang F. 2004. Rhizosphere effect and root growth of two maize (Zea mays L.) genotypes with contrasting P efficiency at low P availability. Plant Science 167:217–223. [Google Scholar]
- Lyu Y, Tang H, Li H, Zhang F, Rengel Z, Whalley WR. 2016. Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Frontier in Plant Science 7:1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Rengel Z. 2008. Phosphorus acquisition and wheat growth are influenced by shoot phosphorus status and soil phosphorus distribution in a split-root system. Journal of Plant Nutrition and Soil Science 171:266–271. [Google Scholar]
- Marschner H. 2011. Marschner’s mineral nutrition of higher plants. New York: Academic Press. [Google Scholar]
- Murphy J, Riley JP. 1962. A modified single solution method for the determination of phosphate in natural waters. Analytica Chimica Acta 27:31–36. [Google Scholar]
- Neumann G, Römheld V. 1999. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant and Soil 291:121–130. [Google Scholar]
- Niu Y, Chai R, Jin G, Wang H, Tang C, Zhang Y. 2012. Responses of root architecture development to low phosphorus availability: a review. Annals of Botany 112:391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Clément M, Nussaume L, Desnos T. 2011. Root developmental adaptation to phosphate starvation: better safe than sorry. Trends in Plant Science 16:442–450. [DOI] [PubMed] [Google Scholar]
- Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MD, Lambers H. 2006. Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant and Soil 288:127–139. [Google Scholar]
- Persson HÅ. 1983. The distribution and productivity of fine roots in boreal forests. Plant and Soil 71:87–101. [Google Scholar]
- Postma JA, Dathe A, Lynch JP. 2014. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiology 166:590–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghothama KG. 1999. Phosphate acquisition. Annual Review of Plant Physiology and Plant Molecular Biology 50:665–693. [DOI] [PubMed] [Google Scholar]
- Rashid A, Awan Z, Ryan J. 2005. Diagnosing phosphorus deficiency in spring wheat by plant analysis: proposed critical concentration ranges. Communications in Soil Science and Plant Analysis 36:609–622. [Google Scholar]
- Reuter DJ, Elliott DE, Reddy GD, Abbott RJ. 1997. Phosphorus nutrition of spring wheat (Triticum aestivum L.). 4. Calibration of plant phosphorus test criteria from rain-fed field experiments. Australian Journal of Agricultural Research 48:899–912. [Google Scholar]
- Rose TJ, Hardiputra B, Rengel Z. 2010. Wheat, canola and grain legume access to soil phosphorus fractions differs in soils with contrasting phosphorus dynamics. Plant and Soil 326:159–170. [Google Scholar]
- Rowe H, Withers PJ, Baas P, Chan NI, Doody D, Holiman J, Jacobs B, Li H, MacDonald GK, McDowell R. 2016. Integrating legacy soil phosphorus into sustainable nutrient management strategies for future food, bioenergy and water security. Nutrient Cycling in Agroecosystems 104:393–412. [Google Scholar]
- Sandaña P, Pinochet D. 2014. Grain yield and phosphorus use efficiency of wheat and pea in a high yielding environment. Journal of Soil Science and Plant Nutrition 14:973–986. [Google Scholar]
- Schjørring JK, Nielsen NE. 1987. Root length and phosphorus uptake by four barley cultivars grown under moderate deficiency of phosphorus in field experiments. Journal of Plant Nutrition 10:1289–1295. [Google Scholar]
- Schroeder MS, Janos DP. 2005. Plant growth, phosphorus nutrition, and root morphological responses to arbuscular mycorrhizas, phosphorus fertilization, and intraspecific density. Mycorrhiza 15:203–216. [DOI] [PubMed] [Google Scholar]
- Shane M, De Vos M, De Roock S, Lambers H. 2003. Shoot P status regulates cluster-root growth and citrate exudation in Lupinus albus grown with a divided root system. Plant, Cell & Environment 26:265–273. [Google Scholar]
- Shen J, Rengel Z, Tang C, Zhang F. 2003. Role of phosphorus nutrition in development of cluster roots and release of carboxylates in soil-grown Lupinus albus. Plant and Soil 248:199–206. [Google Scholar]
- Singh Gahoonia T, Nielsen NE. 2004. Root traits as tools for creating phosphorus efficient crop varieties. Plant and Soil 260:47–57. [Google Scholar]
- Steingrobe B. 2001. Root renewal of sugar beet as a mechanism of P uptake efficiency. Journal of Plant Nutrition and Soil Science 164:533–539. [Google Scholar]
- Tarafdar J, Jungk A. 1987. Phosphatase activity in the rhizosphere and its relation to the depletion of soil organic phosphorus. Biology and Fertility of Soils 3:199–204. [Google Scholar]
- Tarafadar JC, Rao AV, Praveen-Kumar. 1992. Effects of different phosphatase-producing fungi on growth and nutrition of mung beans [Vigna radiata (L.) Wilczek] in an arid soil. Biology and Fertility of Soils 13:35–38. [Google Scholar]
- Tarafdar JC, Yadav RS, Meena SC. 2001. Comparative efficiency of acid phosphatase originated from plant and fungal sources. Journal of Plant Nutrition and Soil Science 164:279–282. [Google Scholar]
- Teng W, Deng Y, Chen XP, Xu XF, Chen RY, Lv Y, Zhao YY, Zhao XQ, He X, Li B, Tong YP, Zhang FS, Li ZS. 2013. Characterization of root response to phosphorus supply from morphology to gene analysis in field-grown wheat. Journal of Experimental Botany 64:1403–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. 2003. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. The New Phytologist 157:423–447. [DOI] [PubMed] [Google Scholar]
- Wang BL, Shen JB, Zhang WH, Zhang FS, Neumann G. 2007. Citrate exudation from white lupin induced by phosphorus deficiency differs from that induced by aluminum. The New Phytologist 176:581–589. [DOI] [PubMed] [Google Scholar]
- Wasaki J, Yamamura T, Shinano T, Osaki M. 2003. Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency. Plant and Soil 248:129–136. [Google Scholar]
- Watt M, Evans JR. 1999. Linking development and determinacy with organic acid efflux from proteoid roots of white lupin grown with low phosphorus and ambient or elevated atmospheric CO2 concentration. Plant Physiology 120:705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Li H, Shen J, Rengel Z. 2017. Maize responds to low shoot P concentration by altering root morphology rather than increasing root exudation. Plant and Soil 416:377–389. [Google Scholar]
- Westerman RL. 1990. Soil testing and plant analysis, 3rd edn Madison, WI: Soil Science Society of America. [Google Scholar]
- Yuan H, Blackwell M, Mcgrath S, George T, Granger S, Hawkins J, Dunham S, Shen J. 2016. Morphological responses of wheat (Triticum aestivum L.) roots to phosphorus supply in two contrasting soils. The Journal of Agricultural Science 154:98–108. [Google Scholar]
- Zhang W, Ma W, Ji Y, Fan M, Oenema O, Zhang F. 2008. Efficiency, economics, and environmental implications of phosphorus resource use and the fertilizer industry in China. Nutrient Cycling in Agroecosystems 80:131–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.