SUMMARY
Adult stem cells usually reside in specialized niche microenvironments. Accumulating evidence indicates that competitive niche occupancy favors stem cells with oncogenic mutations, also known as tumor-like stem cells. However, the mechanisms that regulate tumor-like stem cell niche occupancy are largely unknown. Here we use Drosophila ovarian germline stem cells as a model, and use bam mutant cells as tumor-like stem cells. Interestingly, we find that autophagy is low in wild type stem cells, but elevated in bam mutant stem cells. Significantly, autophagy is required for niche occupancy by bam mutant stem cells. Although loss of either atg6 or Fip200 alone in stem cells did not impact their competitiveness, loss of these conserved regulators of autophagy decreased bam mutant stem cell niche occupancy. In addition, starvation enhances the competition of bam mutant stem cells for niche occupancy in an autophagy-dependent manner. Of note, loss of autophagy slows the cell cycle of bam mutant stem cells, and does not influence stem cell death. In contrast to canonical epithelial cell competition, loss of regulators of tissue growth, either the insulin receptor or cyclin-dependent kinase 2 function, influences the competition of bam mutant stem cells for niche occupancy. Additionally, autophagy promotes the tumor-like growth of bam mutant ovaries. Autophagy is known to be induced in a wide variety of tumors. Therefore, these results suggest that specifically targeting autophagy in tumor-like stem cells has potential as a therapeutic strategy.
Keywords: autophagy, stem cell, stem cell niche, Drosophila
Graphical Abstract
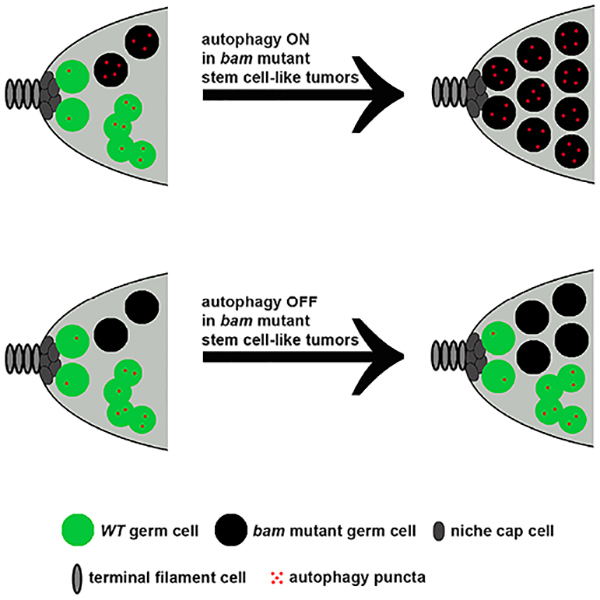
In Brief
Adult stem cells with oncogenic mutations compete for stem cell niche occupancy. Zhao et al. provide evidence indicating that autophagy and regulators of growth promote tumor-like stem cell niche occupancy. Autophagy is required for proper stem cell cycling, and influences ovarian germline tumor-like growth.
INTRODUCTION
Adult stem cells usually exist in specialized microenvironments and dynamically compete with each other to occupy this niche [1]. Departure from the niche is typically associated with the initiation of cell differentiation. If stem cells in the same niche are equivalent, their competition is thought to follow a neutral drift pattern, which means individual stem cells can be stochastically replaced by their neighboring stem cells. This phenomenon has been observed in intestine stem cells [2], cyst stem cells in testes [3], and follicle stem cells in ovaries [4] of Drosophila, as well as epidermal progenitors [5–7], intestine stem cells [8, 9], and male germline stem cells [10] in mice. If a stem cell in the niche possesses a competitive advantage over neighboring stem cells, their competition would follow a biased drift pattern resulting in a clonal expansion of the advantaged stem cell. Importantly, increasing evidence indicates that niche occupancy favors stem cells with oncogenic mutations (hereafter tumor-like stem cells), which is proposed to be important for tumor initiation [3, 11–14]. However, why tumor-like stem cells are more competitive remains largely unknown.
Macroautophagy (hereafter autophagy) is a conserved catabolic process that delivers cargoes, such as damaged organelles and protein aggregates, to lysosomes for recycling [15]. Cytoplasmic cargoes are first delivered to autophagosomes, and the formation of these double membrane vesicles depends on autophagy Atg proteins [16]. Autophagosomes then fuse with lysosomes to form autolysosomes where cargoes are degraded. Growing evidence indicates that autophagy can be stimulated by various metabolic stressors in the tumor microenvironment [17]. Although autophagy suppresses tumorigenesis in some cases, it usually functions to fuel tumor growth [18]. However, the role of autophagy in tumor-like stem cell competition remains unknown.
Drosophila ovarian germline stem cells are an ideal system to study stem cell competition for niche occupancy in vivo. Each fly ovary is composed of 16 to 20 ovarioles that individually produce eggs. The tip structure of each ovariole is called germarium, and two or three germline stem cells reside in the germarium tip that is well defined as a stem cell niche (Figure 1A) [19]. Because each niche only contains either two or three germline stem cells, stem cell competition can be studied at single cell resolution. Additionally, each ovary contains 16 to 20 independent niches enabling robust quantitative analyses. Last but not least, the development of sophisticated genetic approaches has enabled this to become one of the best understood in vivo stem cell systems [20].
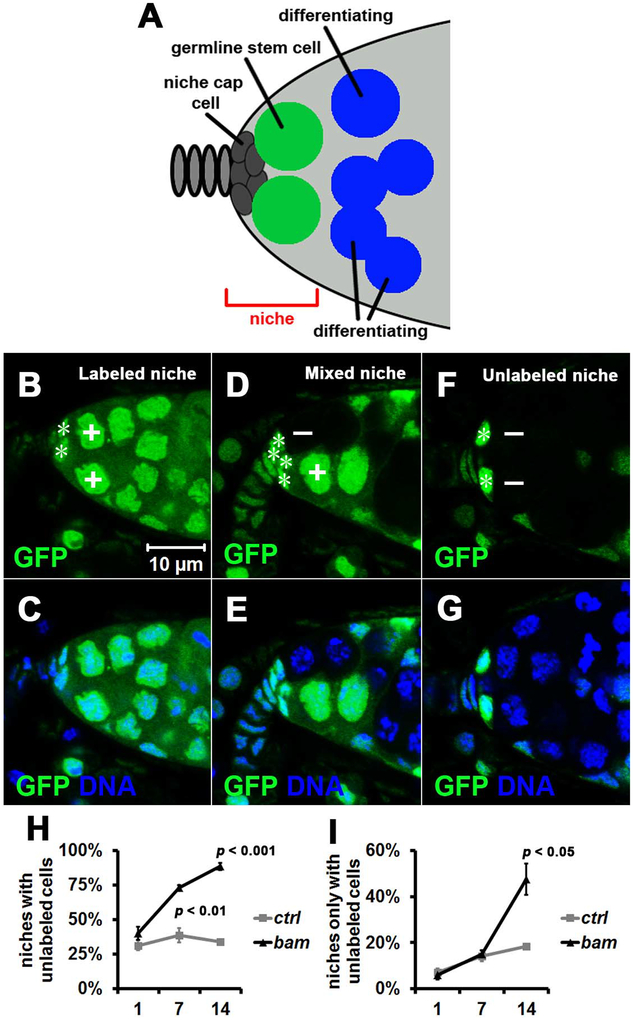
Figure 1. System for Studying the Super-competition of Tumor-like Stem Cells for Niche Occupancy.
(A) Drosophila ovarian stem cell niche cartoon. Niche region is denoted by the red bracket, and it usually contains either two or three germline stem cells (green) that directly contact niche cap cells. Cells (blue) initiate differentiation when exiting the stem cell niche, and are known as cystoblasts and cystocytes.
(B-G) Representative images of stem cell niche types. (B, C) Labeled niche that contains only labeled (GFP+, denoted by +) stem cells. (D, E) Mixed niche that contains both labeled (GFP+) and unlabeled (GFP−, denoted by −) stem cells. (F, G) Unlabeled niche that contains only unlabeled (GFP−) stem cells. Niche cap cells are denoted by the white asterisks. All images are the same magnification.
(H, I) Niche occupancy favors unlabeled bam mutant (bamΔ86, null) stem cells over control (ctrl) FRT82B (chromosome 3R) stem cells. 100 niches were quantified for each replicate, and three independent replicates were performed for each time point. Data represent mean ± standard deviation, and statistical significance was determined by a two-tailed Student’s t test for two samples assuming unequal variances. (H) Percent of niches with unlabeled stem cells (mixed+unlabeled niches, Figure 1D+F) at 1, 7 and 14 days after the last heatshock. (I) Percent of niches only with unlabeled stem cells (unlabeled niches, Figure 1F) at 1, 7 and 14 days after the last heatshock.
See also Figure S1.
Here we use bam mutant germline stem cells as a tumor-like stem cell model for studying stem cell competition for niche occupancy. Interestingly, autophagy is low in wild type stem cells, but elevated in bam mutant stem cells. Importantly, autophagy and regulators of tissue growth promote the competition of bam mutant stem cells for niche occupancy. Autophagy is required for proper cell cycle of bam mutant stem cells, and does not influence stem cell death.
RESULTS
Autophagy Promotes Niche Occupancy by bam Mutant Tumor-like Stem Cells
To compare the competition between genetically different stem cells in the same niche, we used the FLP/FRT system [21] to generate mosaic germline stem cells that are either labeled with green fluorescent protein (GFP) and serve as control stem cells, or gene-specific mutant stem cells that are not labeled with GFP (GFP−). Stem cell niches were divided into three types based on the composition of labeled (GFP+) and unlabeled (GFP−) stem cells: labeled niches that only contain labeled stem cells; mixed niches that contain both labeled and unlabeled stem cells; unlabeled niches that only contain unlabeled stem cells (Figure 1B–G).
Loss of either bag of marbles (bam), benign gonial cell neoplasm (bgcn), brain tumor (brat), star (s), or stem cell tumor (stet) leads to an increase of germline stem cell-like cells in the Drosophila ovary [22–26]. As previously reported, niche occupancy favors germline stem cells with bam mutations (Figure 1H and I) [11], while it did not favor either brat, s, or stet mutant germ cells (Figure S1A–D). Significantly, the fractions of niches that contain bam mutant stem cells increased from 40% to 73% at 7 days, and to 89% at 14 days (Figure 1H). In addition, the fraction of niches with only bam mutant stem cells was significantly increased at 14 days compared with unlabeled control stem cells (Figure 1I). An obvious difference between bam and either brat, s, or stet mutant cells is that bam mutant cells are homogeneous stem cell-like cells while either brat, s, or stet mutant cells contain both stem cell-like and differentiating germ cells (Figure S1E–H) [22, 26]. These results indicate that niche occupancy favors homogeneous tumor-like stem cells.
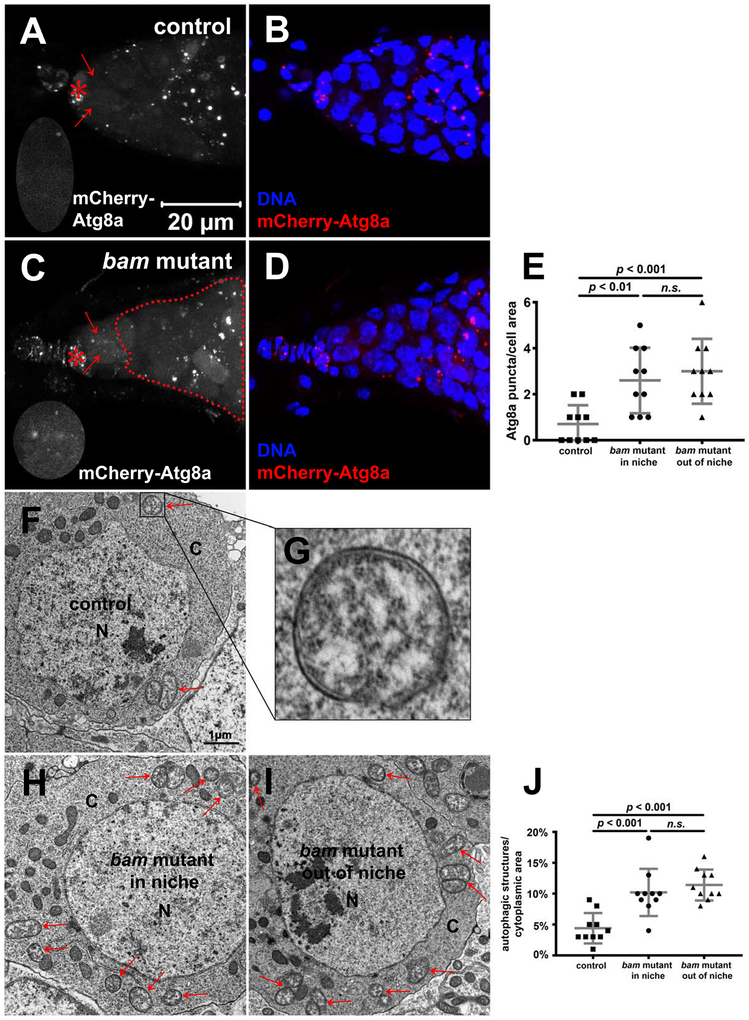
Autophagy can be stimulated by metabolic stress in the tumor microenvironment [17]. Therefore, we asked whether autophagy is altered in bam mutant cells using the mCherry-Atg8a reporter [27]. Control stem cells contained very few mCherry-Atg8a puncta (Figure 2A, B and E) indicating that autophagy is low. However, mCherry-Atg8a puncta were increased in bam mutant stem cells (Figure 2C, D and E). Consistent with these observations, transmission electron microscopy (TEM) analyses revealed that bam mutant stem cells contain significantly more autophagic structures than control stem cells (Figure 2F–J).
Figure 2. Autophagy Is Elevated in bam Mutant Tumor-like Stem Cells.
(A-E) bam mutant tumor-like stem cells contain more mCherry-Atg8a puncta than control stem cells. (A-D) 7-day old bamBG/+ flies were used as the control, and 7-day old bamBG/BG flies were used as bam mutant. bamBG is a null allele. All images are z-stacks that include 7 slices at 1-μm intervals, and the same magnification. Niche cap cells, stem cells, and bam mutant cells out of niche are denoted by red asterisks, red arrows, and the red dotted line, respectively. Niche regions enlarged two times are shown in the lower left corners in A, C. (E) Quantification of the mCherry-Atg8a puncta number in control stem cells, bam mutant stem cells within niche, and bam mutant stem cells out of niche shown in A-D. 10 cells were quantified for each genotype, and statistical significance was determined by a two-tailed Student’s t test for two samples assuming unequal variances.
(F-J) bam mutant tumor-like stem cells contain more autophagic structures than control stem cells. 7-day old bamBG/+ flies were used as the control, and 7-day old bamBG/BG flies were used as bam mutant. (F-I) Autophagic structures are denoted by red arrows. N: nucleus; C: cytoplasm. An autophagosome in F is enlarged in G. F, H, I images are the same magnification. (J) Quantification data in F, H, I. The percent of cytoplasmic area that was occupied by autophagic structures was quantified, and 10 cells were quantified for each genotype. Statistical significance was determined by a two-tailed Student’s t test for two samples assuming unequal variances.
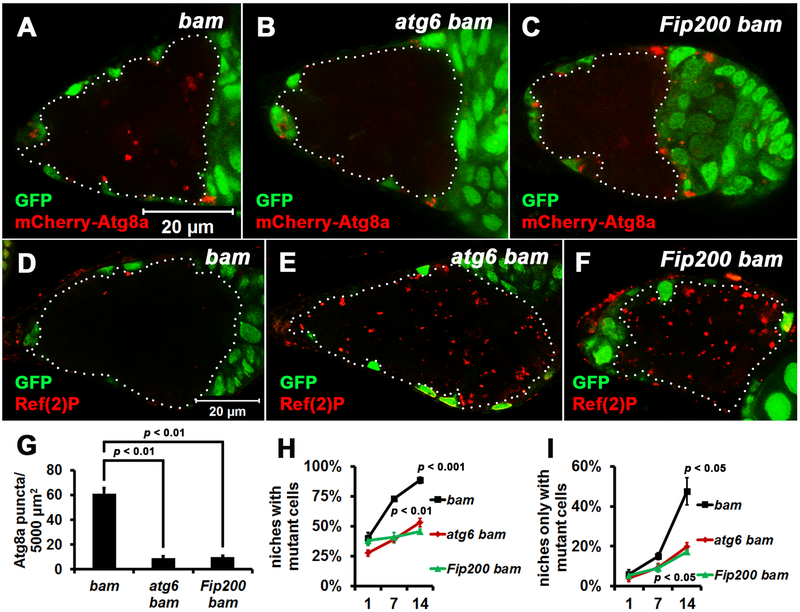
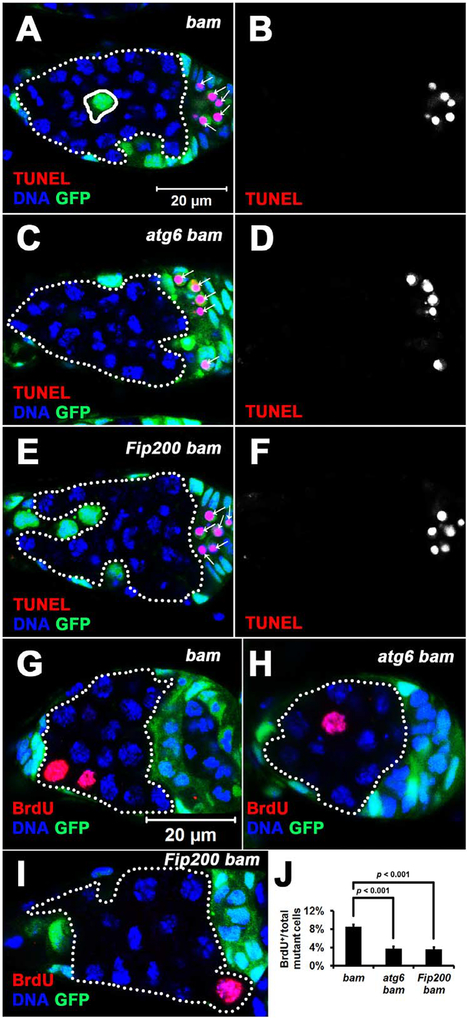
We next sought to address whether autophagy influences niche occupancy by bam mutant stem cells. To block autophagy in bam mutant cells, we generated double mutants of bam with either atg6 or Fip200, both of which are conserved autophagy genes that are required for autophagosome formation [28, 29]. Loss of either atg6 or Fip200 alone in stem cells did not impact their competitiveness (Figure S2A and B), consistent with our data indicating that autophagy is low in normal control stem cells (Figure 2A, E, F, and J). Compared with control bam single mutant cells, either atg6 bam or Fip200 bam double mutant cells contained fewer mCherry-Atg8a autophagy reporter puncta (Figure 3A–C and G). In addition, either atg6 bam or Fip200 bam double mutant cells contained more Ref(2)P protein puncta than control bam single mutant cells (Figure 3D–F). Ref(2)P, Drosophila p62, aids in recruiting ubiquitinated substrates into autophagosomes for degradation, and its accumulation can reflect a decrease in autophagy [30]. Importantly, compared with control bam single mutant cells, either atg6 bam or Fip200 bam double mutant cells had significantly attenuated niche occupancy (Figure 3H and I). To exclude the possible effect of GFP expression on niche occupancy, we performed similar experiments using RFP-labeled cells and obtained similar results (Figure S2C–J). These data indicate that autophagy is required for niche occupancy by bam mutant stem cells.
Figure 3. Autophagy Is Required for Niche Occupancy by bam Mutant Cells.
(A-C) atg bam double mutant cells contain less mCherry-Atg8a puncta than bam single mutant cells. Homozygous bamΔ86, atg6Δ1 bamΔ86, and Fip2003F5 bamΔ86 mutant cells (GFP− cells outlined by white dotted lines) were analyzed at 7 days after the last heat shock. bamΔ86, atg6Δ1 and Fip2003F5 are all null alleles. All images are the same magnification.
(D-F) atg bam double mutant cells contain more Ref(2)P puncta than bam single mutant cells. Homozygous bamΔ86, atg6Δ1 bamΔ86, and Fip2003F5 bamΔ86 mutant cells (GFP− cells outlined by white dotted lines) were analyzed at 7 days after the last heat shock. All images are the same magnification.
(G) Quantification of the mCherry-Atg8a puncta in homozygous bamΔ86, atg6Δ1 bamΔ86, and Fip2003F5 bamΔ86 mutant cells shown in A-C. mCherry-Atg8a puncta were quantified in single confocal microscope focal planes of 20 germaria for each replicate, and three independent replicates were performed for each genotype.
(H, I) Less atg bam double mutant cells are present in the stem cell niche than bam single mutant cells. 100 niches were quantified for each replicate, and three independent replicates were performed for each time point.
In (G-I), data represent mean ± standard deviation, and statistical significance was determined by a two-tailed Student’s t test for two samples assuming unequal variances.
See also Figure S2.
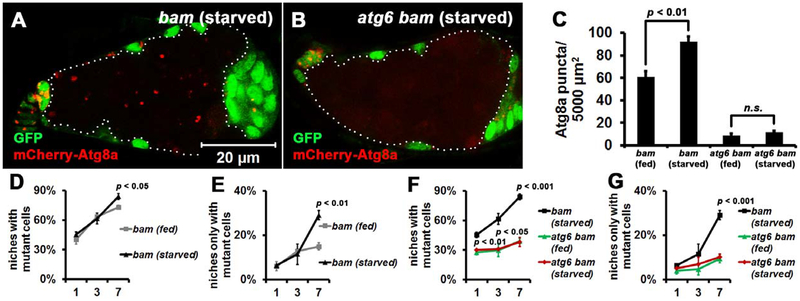
Starvation induces autophagy in Drosophila female germline [31]. Therefore, we investigated if nutrient deprivation influences bam mutant stem cell competition. Because robust germline stem cell loss took place when wild type flies were starved for 14 days, we focused on starvation within 7 days, during which mild stem cell loss was observed (data not shown). Of note, starvation resulted in elevated mCherry-Atg8a puncta in bam mutant stem cells (compare Figure 4A with 3A, statistical data in Figure 4C) compared to wild type stem cells (Figure S3A and B). Significantly, starvation enhanced the competition of bam mutant stem cells at 7 days (Figure 4D and E), while it had no obvious impact on wild type stem cells within the same time frame (Figure S3C and D). To determine whether this enhanced competition depends on autophagy, we examined atg6 bam double mutant cells. As in fed conditions, starved atg6 bam double mutant cells contained very few mCherry-Atg8a puncta (compare Figure 4B with 3B, statistical data in Figure 4C), further supporting that autophagy is blocked in double mutant cells. Importantly, loss of atg6 inhibited the enhanced competition of bam mutant cells that was caused by starvation (compare Figure 4F, G with D, E). Thus, starvation-induced autophagy enhances niche occupancy by bam mutant stem cells.
Figure 4. Starvation-induced Autophagy Enhances Niche Occupancy by bam Mutant Cells.
(A-C) Autophagy reporter mCherry-Atg8a puncta are elevated in bam mutant tumor-like stem cells under nutrient deprivation conditions. (A, B) Images of mCherry-Atg8a autophagy reporter puncta in homozygous bamΔ86 and atg6Δ1 bamΔ86 mutant cells (GFP− cells outlined by white dotted lines) analyzed at 7 days after the last heat shock. Both bamΔ86 and atg6Δ1 are null alleles. All images are the same magnification. (C) Quantification of mCherry-Atg8a puncta in homozygous bamΔ86 and atg6Δ1 bamΔ86 mutant cells shown in A, B. mCherry-Atg8a puncta were quantified in single confocal microscope focal planes of 20 germaria for each replicate, and three independent replicates were performed for each genotype.
(D, E) Starvation influences niche occupancy by bam mutant tumor-like stem cells. 100 niches were quantified for each replicate, and three independent replicates were performed for each time point.
(F, G) Starvation-induced niche occupancy by bam mutant tumor-like stem cells is autophagy-dependent. 100 niches were quantified for each replicate, and three independent replicates were performed for each time point.
In (C-G), data represent mean + standard deviation, and statistical significance was determined by a two-tailed Student’s t test for two samples assuming unequal variances.
See also Figure S3.
Autophagy-defective bam Mutant Stem Cells Attenuate the Cell Cycle
We next investigated how decreased autophagy suppresses niche occupancy by bam mutant stem cells. Previous reports indicated that the elevated niche occupancy by bam mutant stem cells is mediated by upregulation of the niche-stem cell adhesion factor E-Cadherin [11, 32]. Surprisingly, we did not detect a significant difference in E-Cadherin between either wild type, bam single or atg bam double mutant stem cells (Figure S4A–G), indicating that niche occupancy decline by atg bam double mutant cells is not caused by decreased E-Cadherin.
Germline stem cells with increased Myc compete for niche occupancy by elevation of BMP signaling as detected by elevation of phosphorylated Mad [12]. We compared phosphorylated Mad in either neighboring wild type, bam single or atg bam double mutant stem cells, and detected no significant difference (Figure S4H–N). These data indicate that the autophagy/Bam pathway-regulated stem cell competition does not work through BMP signaling.
Our data indicate that niche occupancy favors homogeneous tumor-like stem cells (Figure 1 and S1). Therefore, we investigated if mutations in the autophagy pathway change the fate of bam single mutant cells. Germline stem cells contain round-shaped organelles called spectrosomes, but when stem cells differentiate they become branched structures called fusomes. atg bam double mutant cells contained round-shaped spectrosomes (Figure S5), indicating that they retain stem cell properties.
Autophagy often promotes cell survival [15]. Therefore, we examined whether atg bam double mutant cells are prone to die using the TUNEL assay. Surprisingly, we did not observe any dying bam single or atg bam double mutant germ cells even at 14 days (n > 200 for each genotype), even though dying neighboring follicle cells were frequently detected (Figure 5A–F).
Figure 5. Cell Death Is Not Altered, but the Cell Cycle Is Attenuated in Autophagy-defective bam Mutant Cells.
(A-F) Cell death is not altered in autophagy-defective bam mutant cells. Homozygous bamΔ86, atg6Δ1 bamΔ86, and Fip2003F5 bamΔ86 mutant cells (GFP− cells outlined by white dotted lines) were analyzed at 14 days after the last heat shock. bamΔ86, atg6Δ1 and Fip2003F5 are all null alleles. White arrows denote dying neighboring follicle cells. All images are the same magnification.
(G-J) The cell cycle is attenuated in autophagy-defective bam mutant cells. (G-I) Homozygous bamΔ86, atg6Δ1 bamΔ86 and Fip2003F5 bamΔ86 mutant cells (GFP− cells outlined by white dotted lines) were analyzed at 7 days after the last heat shock. Images are the same magnification. (J) Quantification of BrdU incorporation in homozygous bamΔ86, atg6Δ1 bamΔ86 and Fip2003F5 bamΔ86 mutant cells. The mutant germ cells in single confocal microscope focal plane of 30 germaria were quantified for each replicate, and three independent replicates were performed for each genotype. Data represent mean ± standard deviation, and statistical significance was determined by a two-tailed Student’s t test for two samples assuming unequal variances.
See also Figure S4, S5, S6, and Tables S1 and S2.
Fast-dividing cells appear to outcompete slow-dividing cells during cell competition [33], and slow division rates compromise stem cell competition [3, 11, 13]. Therefore, we compared the cell cycle of bam single mutant cells with that of atg bam double mutant cells by BrdU incorporation. Importantly, BrdU incorporation in atg bam double mutant cells was about half of that of bam single mutant cells (Figure 5G–J and Table S1), indicating that autophagy defect attenuates the cell cycle of bam mutant stem cells. Further investigation of cell cycle phases using the Fly-FUCCI system [34] did not reveal significant differences between bam single and atg bam double mutant cells (Figure S6). In addition, either atg6 bam or Fip200 bam double mutant germ cell clones were smaller than bam single mutant clones (Table S1), further supporting that decreased autophagy compromises cell division and possibly growth of bam mutant stem cells. By contrast, stem cells with single mutations in either atg6 or Fip200 exhibited similar niche competition ability as wild type control stem cells (Figure S2A and B). It is also worth noting that atg6 and Fip200 mutant stem cells had similar BrdU incorporation rates to wild type control stem cells (Table S2).
Regulators of Tissue Growth Influence Niche Occupancy by bam Mutant Stem Cells
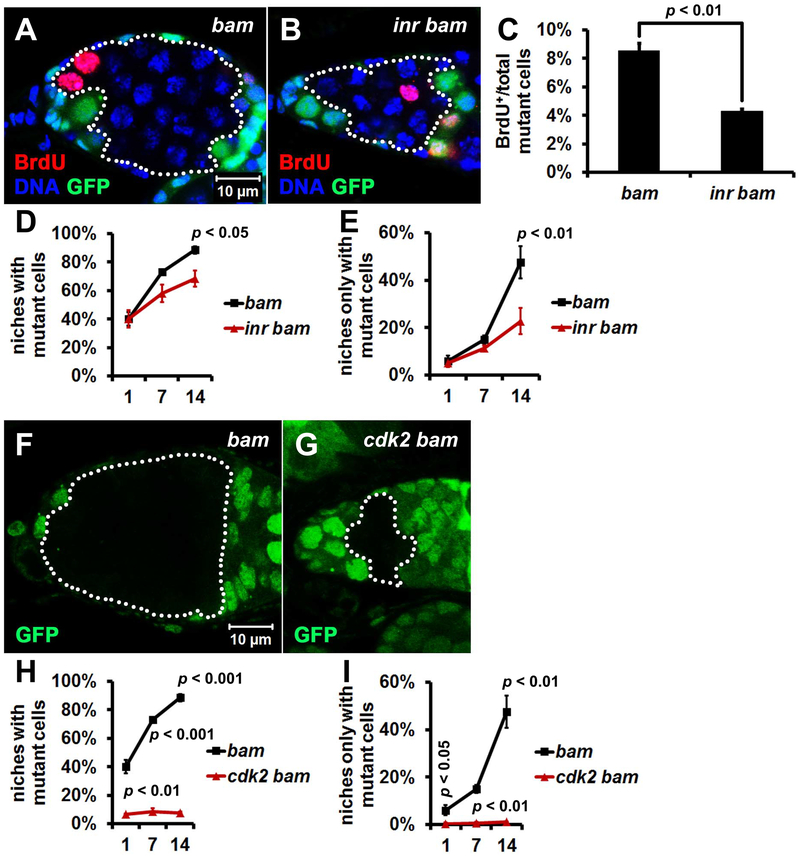
Autophagy has been implicated in cell and tissue growth through association with the nutrient sensing mTOR pathway [35]. Although cell growth and division rates are important factors that may contribute to cell competition [36], the influence of these pathways on stem cell competition for niche occupancy are unclear. Although cells with lower insulin signaling grew more slowly, they were not eliminated by the surrounding faster-growing wild-type epithelial cells [37]. To determine whether this also occurs during bam mutant germline stem cell competition, we examined the influence of insulin/mTOR signaling by generating double mutants of bam and insulin-like receptor (inr), since inr functions as an upstream activator of mTOR [38]. BrdU incorporation was significantly decreased in inr bam double mutant cells compared to control bam single mutant cells (Figure 6A–C and Table S1), indicating that reduced insulin signaling attenuates the cell cycle of bam mutant cells. Importantly, compared with control bam single mutant cells, inr bam double mutant cells had significantly attenuated stem cell niche occupancy (Figure 6D and E). It should be noted that atg mutants have a stronger suppression of niche occupancy by bam mutant stem cells than inr mutants (compared Figure 6D, E, S7 with 3H, I).
Figure 6. Loss of either inr or cdk2 Impairs Niche Occupancy by bam Mutant Cells.
(A-C) The cell cycle is attenuated in inr bam double mutant cells. (A, B) Homozygous bamΔ86 and inr339 bamΔ86 mutant cells (GFP− cells outlined by white dotted lines) were analyzed at 7 days after the last heat shock. Both bamΔ86 and inr339 are null alleles. Images are the same magnification. (C) Quantification of BrdU incorporation in homozygous bamΔ86 and inr339 bamΔ86 mutant cells. The mutant germ cells in single confocal microscope focal plane of 30 germaria were quantified for each replicate, and three independent replicates were performed for each genotype.
(D, E) Fewer inr bam double mutant cells are present in the stem cell niche than bam single mutant cells. 100 niches were quantified for each replicate, and three independent replicates were performed for each time point.
(F, G) bam single mutant clones are bigger than cdk2 bam double mutant clones. bamΔ86 and cdk23 bamΔ86 mutant clones (GFP− cells outlined by white dotted lines) were analyzed at 7 days after the last heat shock. Both bamΔ86 and cdk23 are null alleles. Images are the same magnification.
(H, I) Fewer cdk2 bam double mutant cells are present in the stem cell niche than bam single mutant cells. 100 niches were quantified for each replicate, and three independent replicates were performed for each time point.
In (C, D, E, H, and I), data represent mean ± standard deviation, and statistical significance was determined by a two-tailed Student’s t test for two samples assuming unequal variances.
See also Figure S7 and Tables S1 and S2.
To further interrogate the influence of regulators of tissue growth on niche occupancy by bam mutant stem cells, we generated double mutants of bam and cyclin-dependent kinase 2 (cdk2) that encodes a key G1-S cell cycle regulator. Significantly fewer cdk2 bam double mutant cells were present in the stem cell niche than bam single mutant cells (Figure 6F–I).
Autophagy Promotes the Growth of bam Mutant Ovaries
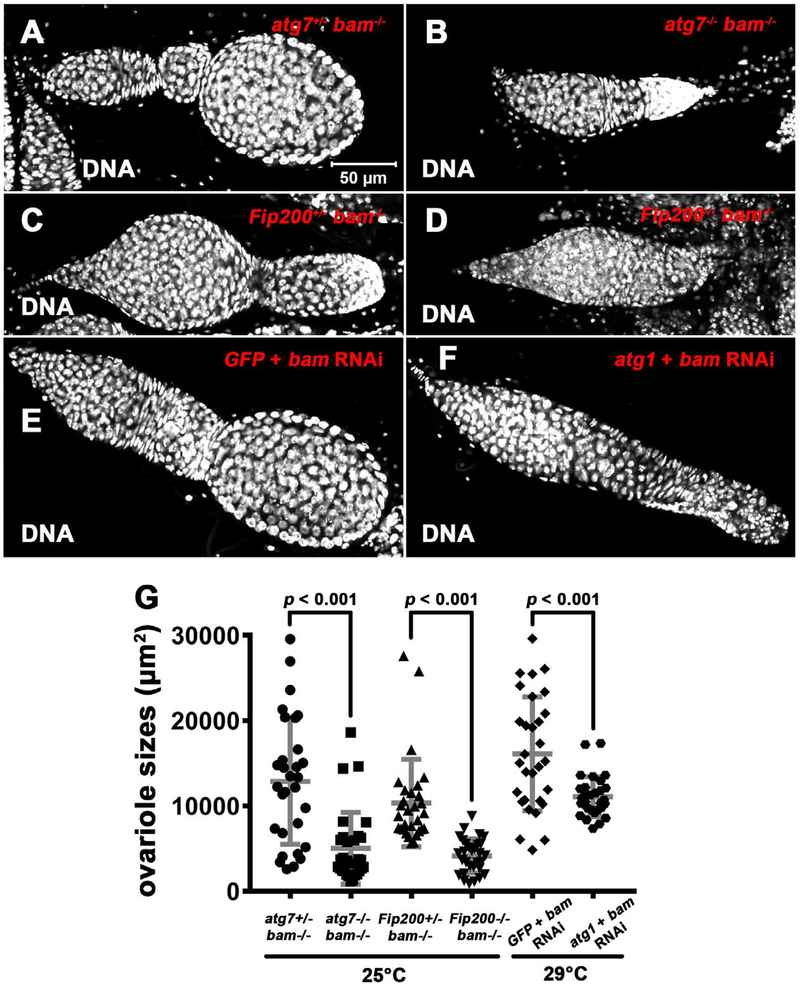
Given the relationship between regulators of tissue growth and tumor-like stem cell niche occupancy, we asked whether autophagy influences the growth of bam mutant ovaries. The ovariole area that is visibly largest in single confocal microscope focal plane was used to analyze the impact of autophagy on bam mutant tissue size. Either atg7 bam or Fip200 bam double mutant ovarioles were smaller than control bam mutant ovarioles with a monoallelic loss of either atg7 or Fip200 (Figure 7A–D and G). In addition, we used RNA interference to knock down genes specifically in germline. Compared with control GFP bam double knock down ovarioles, atg1 bam double knock down ovarioles were smaller (Figure 7E–G). Importantly, atg mutations alone had no impact on ovariole growth and development (data not shown). Thus, these data indicate that autophagy promotes the growth of bam mutant ovaries.
Figure 7. Autophagy Promotes the Growth of bam Mutant Ovaries.
(A–F) Representative images of control (A, C, E) and autophagy defective (B, D, F) ovarioles from animals with decreased bam function. bamBG (null), atg7d4 (null), and Fip2003F5/4G7 (both null) were used as the mutants in A–D. (E) Genotype: UAS-GFP-IR/UAS-dicer2; UAS-bam-IR/nos-GAL4-VP16. (F) Genotype: UAS-atg1-IR/UAS-dicer2; UAS-bam-IR/nos-GAL4-VP16. All images are the same magnification.
(G) Quantification of 7-day-old fly ovariole area of the genotypes shown in A–F. The area from the largest single ovariole confocal plane image per sample was used for quantification, and 30 ovarioles were quantified for each genotype. Statistical significance was determined by a two-tailed Student’s t test for two samples assuming unequal variances.
DISCUSSION
Here we use Drosophila ovarian germline stem cells to study stem cell competition for niche occupancy. Significantly, we find that autophagy promotes niche occupancy by bam mutant stem cells. Autophagy is required for proper cell cycle of bam mutant cells, and regulators of growth influence bam mutant stem cell niche occupancy.
Previous reports indicate that autophagy is required for stem cell maintenance, proper differentiation, and homeostasis in different stem cell systems [39, 40]. By contrast, our data indicate that loss of autophagy does not have a negative impact on Drosophila ovarian germline stem cells (Figure S2A, B and data not shown), consistent with our data indicating that autophagy is low in normal wild type stem cells (Figure 2). Drosophila ovarian germline stem cells are the largest germ cells in germaria [11], and they have a high metabolic rate that is associated with the activation of BMP signaling [41, 42], the expression of Myc that is a key regulator of cell growth and ribosome biogenesis [12], and a high level of rRNA transcription [43]. Therefore, the catabolic autophagy pathway may be dispensable in Drosophila ovarian germline stem cells under normal conditions.
Stem cell renewal is dependent on cell growth and division that is typically regulated by mTOR [38, 44]. In most cell contexts, autophagy is inhibited when mTOR-dependent cell growth is activated. Therefore, it is logical that autophagy levels are low in normal stem cells that are not stressed. By contrast, transformed cells, such as those with activated Ras, have been reported to possess elevated autophagy [45]. Therefore, it seems reasonable that like Ras transformed cells, bam mutant stem cells may depend on autophagy for cell division and ovarian growth. Interestingly, autophagy is required for the proliferation of fast-dividing germline progenitor cells in the C. elegans larval gonad [46]. It is not clear why autophagy may be compatible with and required for tumor-like germline stem cell growth and division. One possibility is that autophagy is functioning to reduce cell stress associated with increased metabolic rate, protein and organelle damage, but if this were the case this would likely be reflected in increased cell death. Since we did not observe an increase in cell death, an alternative explanation is that autophagy is promoting bioenergetic homeostasis that is needed in tumor-like cells.
Unlike previous studies of epithelial cell competition [36], our data indicate that loss of regulators of tissue growth, either the insulin receptor or cyclin-dependent kinase 2 function, influence the competition of tumor-like stem cells for niche occupancy (Figure 6 and Table S1). Stem cells possess properties that distinguish them from epithelial cells in the context of cell competition. First, adult stem cells are usually quiescent while epithelial cell competition often takes place in fast-growing tissues. Second, stem cells compete for niche occupancy while there are no known specialized niches in epithelial cell competition systems. Third, loser stem cells are displaced from the niche and do not die [11, 12], while loser epithelial cells die enabling winners expand during epithelial cell competition [33]. In addition, tumor-like stem cells appear to divide faster than normal adult stem cells. These differences probably make tumor-like stem cells more sensitive to regulators of growth than epithelial cells during competition.
Autophagy can either promote or suppress tumor growth depending on cell and tissue context [18, 47, 48]. Similar phenomena were also observed in different Drosophila tumor-like overgrowth models where autophagy either enhanced or suppressed epithelial overgrowth phenotypes depending on the oncogenic stimulus [49]. Our results indicate that autophagy promotes Drosophila ovarian germline tumor-like growth (Figure 7). Significantly, autophagy promotes niche occupancy by bam mutant tumor-like stem cells (Figure 3, 4, S2 and S3). Of note, our data indicate that the super-competition of bam mutant stem cells largely depends on their proliferative potential (Figure 5, 6 and Table S1). In addition, our data contradicts the current model that the niche-stem cell adhesion factor E-Cadherin plays a vital role [11, 32], as we did not observe a significant difference in E-Cadherin between wild type and bam mutant stem cells (Figure S4A–G). Furthermore, bam mutant stem cells that are out of the niche do not do not possess an E-Cadherin connection with niche cap cells, but they are still more competitive than wild type stem cells for niche occupancy (Figure 1H) [11], further challenging the current model that emphasizes a critical role of E-Cadherin. In addition, although the super-competition of tumor-like stem cells for niche occupancy is proposed to be important for tumor initiation [3, 12–14], it still cannot be excluded that autophagy also contributes to tumor progression in our tumor model system. Importantly, our studies indicate that specifically targeting autophagy in tumor-like stem cells could have potential for cancer therapy.
STAR★METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dr. Eric Baehrecke (eric.baehrecke@umassmed.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Drosophila melanogaster strains used in this study are listed in the key resources table. Flies were reared at 25°C on standard cornmeal/molasses/agar media.
METHOD DETAILS
Ovarian mutant germline clone induction
To make flies developmentally synchronous and growing at a low density, eggs within eight hours after being laid were collected and used for heatshock. The late-Larva 3/early-Pupa animals were heated at 37 °C for two hours each time, twice every day with a three or four-hour interval, and six consecutive days.
Transmission electron microscopy
Ovaries were dissected in 0.1M cacodylate buffer (pH 7.4), fixed for 1 hour at room temperature followed by 48 hours at 4°C in 2.5% glutaraldehyde, 2% paraformaldehyde in 0.1M cacodylate buffer (pH 7.4), washed 4 × 10 minutes with 0.1M cacodylate buffer (pH 7.4) at 4°C, post-fixed for 1 hour in 1% OsO4 at 4°C, washed 4 × 10 minutes with ddH2O at 4°C, stained for 1 hour in 1% aqueous uranyl acetate at 4°C in dark, washed 5 × 5 minutes with ddH2O at 4°C, dehydrated, treated with propylene oxide, and infiltrated for embedding in SPI-pon/Araldite. The serial ultrathin sections were stained with uranyl acetate and lead citrate, and the micrographs were acquired by Philips CM10 TEM with Gatan Erlangshen CCD digital camera and digital micrograph software.
Starvation
Flies were starved at 25°C on protein-free food that is made up of 20% sucrose, 10g/L agar, and Milli-Q water.
BrdU labeling
Ovaries were dissected in Schneider’s insect medium (SIM), incubated with the freshly made BrdU solution (100μg/mL diluted in SIM) for five hours at 25°C, washed with PBS for half an hour, fixed in 4% paraformaldehyde (diluted in PBS) for an hour, washed with PBS for half an hour, incubated with the freshly made RQ1 DNase reaction solution (PROMEGA, Madison, WI, USA) for an hour, washed with PBST (0.3% Triton X-100 diluted in PBS) for half an hour, incubated with mouse anti-BrdU antibody (Sigma) at 1:200 (diluted in PBST) overnight at 4°C, washed with PBST for an hour, incubated with goat anti-mouse 546 at 1:1000 (Molecular Probes of Invitrogen) and 1μM Hoechst 33342 (diluted in PBST) for three hours, washed with PBST for an hour, and then mounted in 70% glycerol.
TUNEL
Ovaries were dissected in PBS, fixed in 4% paraformaldehyde (diluted in PBS) for an hour, washed with PBST for half an hour, incubated with the TUNEL reaction solution (In Situ Cell Death Detection Kit, TMR red, Roche) for three hours at 37°C in dark, washed with PBST for half an hour, incubated with 1μM Hoechst 33342 for half an hour, washed with PBST for half an hour, and then mounted in 70% glycerol.
Immuno-florescent staining and image collection
Ovaries were dissected in PBS, fixed in 4% paraformaldehyde (diluted in PBS) for an hour, washed with PBST for half an hour, incubated with primary antibodies overnight at 4°C, washed with PBST for an hour, incubated with secondary antibodies and 1μM Hoechst 33342 (diluted in PBST) for three hours, washed with PBST for an hour, and then mounted in 70% glycerol. Primary antibodies were used at the following dilutions: mouse anti-α-Spectrin at 1:200 (3A9, DSHB); mouse anti-BrdU at 1:400 (Sigma); rabbit anti-GFP at 1:1000 (O-6381, Molecular Probes of Invitrogen); rabbit anti-pSmad at 1:500 (a gift from Ed Laufer); rabbit anti-Ref(2)P at 1:100 (a gift from Gábor Juhász). Alexa Fluor conjugated secondary antibodies were used at 1:1000 (Molecular Probes of Invitrogen). Florescent images were obtained by using a Zeiss LSM 700 confocal microscope with Zen 2012 imaging software (Carl Zeiss), and processed by Adobe Photoshop CS5 (San Jose, CA, USA).
Ovariole size analyses
The ovariole area that is visibly largest in a single confocal microscope focal plane was used to analyze the ovariole size. Importantly, decreased autophagy gene function did not impact the egg chamber budding process, and enabled the measurement of germaria and egg chambers together to quantify the tumor-like growth.
QUANTIFICATION AND STATISTICAL ANALYSIS
ImageJ (NIH, Bethesda, MD, USA) was used to quantify area size and immunofluorescent intensity. Because there are somatic cells intermingled with germ cells in germaria, the Atg8a puncta numbers in mutant germ cell clones were counted manually, in which the puncta signals co-localized with GFP were not included. Statistical significance was determined by a two-tailed Student’s t test for two samples assuming unequal variances.
Supplementary Material
Highlights.
Autophagy promotes tumor-like stem cell niche occupancy
Autophagy-defective tumor-like stem cells attenuate the cell cycle
Regulators of growth influence tumor-like stem cell niche occupancy
Autophagy promotes the growth of bam mutant ovaries
ACKNOWLEDGEMENTS
We thank M. Buszczak, J. H. Lee, E. T. Ables, Z. Wang, Y. Cai, G. Juhász, E. Laufer, the Baehrecke laboratory, the Bloomington Drosophila Stock Center, the Vienna Drosophila Resource Center, the Kyoto Drosophila Genetic Resource Center, and the Electron Microscopy Core Facility at UMass Medical School for flies, antibodies, advice, and technical support. This work was supported by the Ellison Medical Research Foundation and NIH grant CA159314 to E.H.B..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Stine RR, and Matunis EL (2013). Stem cell competition: finding balance in the niche. Trends Cell Biol 23, 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Navascues J, Perdigoto CN, Bian Y, Schneider MH, Bardin AJ, Martinez-Arias A, and Simons BD (2012). Drosophila midgut homeostasis involves neutral competition between symmetrically dividing intestinal stem cells. EMBO J 31, 2473–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amoyel M, Simons BD, and Bach EA (2014). Neutral competition of stem cells is skewed by proliferative changes downstream of Hh and Hpo. EMBO J 33, 2295–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kronen MR, Schoenfelder KP, Klein AM, and Nystul TG (2014). Basolateral junction proteins regulate competition for the follicle stem cell niche in the Drosophila ovary. PLoS One 9, e101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clayton E, Doupe DP, Klein AM, Winton DJ, Simons BD, and Jones PH (2007). A single type of progenitor cell maintains normal epidermis. Nature 446, 185–189. [DOI] [PubMed] [Google Scholar]
- 6.Doupe DP, Klein AM, Simons BD, and Jones PH (2010). The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev Cell 18, 317–323. [DOI] [PubMed] [Google Scholar]
- 7.Doupe DP, Alcolea MP, Roshan A, Zhang G, Klein AM, Simons BD, and Jones PH (2012). A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science 337, 1091–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Garcia C, Klein AM, Simons BD, and Winton DJ (2010). Intestinal stem cell replacement follows a pattern of neutral drift. Science 330, 822–825. [DOI] [PubMed] [Google Scholar]
- 9.Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, Barker N, Klein AM, van Rheenen J, Simons BD, et al. (2010). Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144. [DOI] [PubMed] [Google Scholar]
- 10.Klein AM, Nakagawa T, Ichikawa R, Yoshida S, and Simons BD (2010). Mouse germ line stem cells undergo rapid and stochastic turnover. Cell Stem Cell 7, 214–224. [DOI] [PubMed] [Google Scholar]
- 11.Jin Z, Kirilly D, Weng C, Kawase E, Song X, Smith S, Schwartz J, and Xie T (2008). Differentiation-defective stem cells outcompete normal stem cells for niche occupancy in the Drosophila ovary. Cell Stem Cell 2, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhiner C, Diaz B, Portela M, Poyatos JF, Fernandez-Ruiz I, Lopez-Gay JM, Gerlitz O, and Moreno E (2009). Persistent competition among stem cells and their daughters in the Drosophila ovary germline niche. Development 136, 995–1006. [DOI] [PubMed] [Google Scholar]
- 13.Snippert HJ, Schepers AG, van Es JH, Simons BD, and Clevers H (2014). Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep 15, 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vermeulen L, Morrissey E, van der Heijden M, Nicholson AM, Sottoriva A, Buczacki S, Kemp R, Tavare S, and Winton DJ (2013). Defining stem cell dynamics in models of intestinal tumor initiation. Science 342, 995–998. [DOI] [PubMed] [Google Scholar]
- 15.Das G, Shravage BV, and Baehrecke EH (2012). Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizushima N, and Komatsu M (2011). Autophagy: renovation of cells and tissues. Cell 147, 728–741. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Yu DD, Yan F, Jing YY, Han ZP, Sun K, Liang L, Hou J, and Wei LX (2015). The role of autophagy induced by tumor microenvironment in different cells and stages of cancer. Cell Biosci 5, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White E (2015). The role for autophagy in cancer. J Clin Invest 125, 42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie T, and Spradling AC (2000). A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328–330. [DOI] [PubMed] [Google Scholar]
- 20.Losick VP, Morris LX, Fox DT, and Spradling A (2011). Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell 21, 159–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu T, and Rubin GM (1993). Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117, 1223–1237. [DOI] [PubMed] [Google Scholar]
- 22.McKearin D, and Ohlstein B (1995). A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development 121, 2937–2947. [DOI] [PubMed] [Google Scholar]
- 23.Lavoie CA, Ohlstein B, and McKearin DM (1999). Localization and function of Bam protein require the benign gonial cell neoplasm gene product. Dev Biol 212, 405–413. [DOI] [PubMed] [Google Scholar]
- 24.Schulz C, Wood CG, Jones DL, Tazuke SI, and Fuller MT (2002). Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development 129, 4523–4534. [DOI] [PubMed] [Google Scholar]
- 25.Liu M, Lim TM, and Cai Y (2010). The Drosophila female germline stem cell lineage acts to spatially restrict DPP function within the niche. Sci Signal 3, ra57. [DOI] [PubMed] [Google Scholar]
- 26.Harris RE, Pargett M, Sutcliffe C, Umulis D, and Ashe HL (2011). Brat promotes stem cell differentiation via control of a bistable switch that restricts BMP signaling. Dev Cell 20, 72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denton D, Chang TK, Nicolson S, Shravage B, Simin R, Baehrecke EH, and Kumar S (2012). Relationship between growth arrest and autophagy in midgut programmed cell death in Drosophila. Cell Death Differ 19, 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M, Park HL, Park HW, Ro SH, Nam SG, Reed JM, Guan JL, and Lee JH (2013). Drosophila Fip200 is an essential regulator of autophagy that attenuates both growth and aging. Autophagy 9, 1201–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shravage BV, Hill JH, Powers CM, Wu L, and Baehrecke EH (2013). Atg6 is required for multiple vesicle trafficking pathways and hematopoiesis in Drosophila. Development 140, 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nezis IP, Simonsen A, Sagona AP, Finley K, Gaumer S, Contamine D, Rusten TE, Stenmark H, and Brech A (2008). Ref(2)P, the Drosophila melanogaster homologue of mammalian p62, is required for the formation of protein aggregates in adult brain. J Cell Biol 180, 1065–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barth JM, Szabad J, Hafen E, and Kohler K (2011). Autophagy in Drosophila ovaries is induced by starvation and is required for oogenesis. Cell Death Differ 18, 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen R, Weng C, Yu J, and Xie T (2009). eIF4A controls germline stem cell self-renewal by directly inhibiting BAM function in the Drosophila ovary. Proc Natl Acad Sci U S A 106, 11623–11628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claveria C, and Torres M (2016). Cell Competition: Mechanisms and Physiological Roles. Annu Rev Cell Dev Biol 32, 411–439. [DOI] [PubMed] [Google Scholar]
- 34.Zielke N, Korzelius J, van Straaten M, Bender K, Schuhknecht GF, Dutta D, Xiang J, and Edgar BA (2014). Fly-FUCCI: A versatile tool for studying cell proliferation in complex tissues. Cell Rep 7, 588–598. [DOI] [PubMed] [Google Scholar]
- 35.Scott RC, Schuldiner O, and Neufeld TP (2004). Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell 7, 167–178. [DOI] [PubMed] [Google Scholar]
- 36.Di Gregorio A, Bowling S, and Rodriguez TA (2016). Cell Competition and Its Role in the Regulation of Cell Fitness from Development to Cancer. Dev Cell 38, 621–634. [DOI] [PubMed] [Google Scholar]
- 37.Böhni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, and Hafen E (1999). Autonomous Control of Cell and Organ Size by CHICO, a Drosophila Homolog of Vertebrate IRS1–4. Cell 97, 865–875. [DOI] [PubMed] [Google Scholar]
- 38.Saxton RA, and Sabatini DM (2017). mTOR Signaling in Growth, Metabolism, and Disease. Cell 168, 960–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan JL, Simon AK, Prescott M, Menendez JA, Liu F, Wang F, Wang C, Wolvetang E, Vazquez-Martin A, and Zhang J (2013). Autophagy in stem cells. Autophagy 9, 830–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodolfo C, Di Bartolomeo S, and Cecconi F (2016). Autophagy in stem and progenitor cells. Cell Mol Life Sci 73, 475–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kai T, and Spradling A (2003). An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci U S A 100, 4633–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, and Xie T (2004). Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131, 1353–1364. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q, Shalaby NA, and Buszczak M (2014). Changes in rRNA transcription influence proliferation and cell fate within a stem cell lineage. Science 343, 298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaFever L, Feoktistov A, Hsu HJ, and Drummond-Barbosa D (2010). Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development 137, 2117–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo JY, Chen HY, Mathew R, Fan J, Strohecker AM, Karsli-Uzunbas G, Kamphorst JJ, Chen G, Lemons JM, Karantza V, et al. (2011). Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes Dev 25, 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ames K, Da Cunha DS, Gonzalez B, Konta M, Lin F, Shechter G, Starikov L, Wong S, Bulow HE, and Melendez A (2017). A Non-Cell-Autonomous Role of BEC-1/BECN1/Beclin1 in Coordinating Cell-Cycle Progression and Stem Cell Proliferation during Germline Development. Curr Biol 27, 905–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhi X, and Zhong Q (2015). Autophagy in cancer. F1000Prime Rep 7, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Towers CG, and Thorburn A (2016). Therapeutic Targeting of Autophagy. EBioMedicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez E, Das G, Bergmann A, and Baehrecke EH (2015). Autophagy regulates tissue overgrowth in a context-dependent manner. Oncogene 34, 3369–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.