Abstract
Objective
The prevalence and importance of early right ventricular (RV) dysfunction and pulmonary hypertension (PH) in pediatric acute respiratory distress syndrome (PARDS) are unknown. We aimed to describe the prevalence of RV dysfunction and PH within 24 hours of PARDS diagnosis and their associations with outcomes.
Design
Retrospective, single-center cohort study.
Setting
Tertiary care, university-affiliated PICU.
Patients
Children who had echocardiograms performed within 24 hours of PARDS diagnosis.
Interventions
None
Measurements and Main Results
Between July 1, 2012 and June 30, 2016, 103 children met inclusion criteria. Echocardiograms were analyzed using established indices of RV and left ventricular (LV) systolic function and for evidence of PH. Echocardiographic abnormalities were common: 26% had low RV fractional area change, 65% had low tricuspid annular plane systolic excursion, 30% had low LV fractional shortening, and 21% had evidence of PH. Abnormal RV global longitudinal strain (GLS) and abnormal RV free wall strain (FWS) were present in 35% and 40% of patients, respectively. No echocardiographic parameters differed between or across PARDS severity. In multivariable analyses, RV GLS was independently associated with PICU mortality (OR 3.57 [1.33–9.60], p=0.01) while RV GLS, RV FWS, and the presence of PH were independently associated with lower probability of extubation (subdistribution hazard ratio [SHR] 0.46, [0.26–0.83], p=0.01; SHR 0.58 [0.35–0.98], p=0.04; and SHR 0.49 [0.26–0.92], p=0.03, respectively).
Conclusions
Early ventricular dysfunction and PH were detectable, prevalent, and independent of lung injury severity in children with PARDS. RV dysfunction was associated with PICU mortality, while RV dysfunction and PH were associated with lower probability of extubation.
Keywords: acute respiratory distress syndrome, pediatrics, echocardiography, right ventricular dysfunction, pulmonary hypertension, left ventricular dysfunction
INTRODUCTION
Pediatric acute respiratory distress syndrome (PARDS) is characterized by acute hypoxemic respiratory failure with both morbidity and mortality primarily related to the development of multiple organ dysfunction syndrome (MODS) (1–6). Acute right ventricular (RV) systolic dysfunction and/or pulmonary hypertension (PH) can worsen oxygenation via pulmonary ventilation-perfusion mismatching and may contribute to nonpulmonary organ failure.
In adult ARDS, a spectrum of acute RV dysfunction severity is recognized with a prevalence anywhere from 20–50% during the course of ARDS (7–13), which increases the risk for systemic circulatory failure, shock (8,9), and mortality (9, 11–13). Pulmonary hypertension, a risk factor for RV dysfunction, has been described in pediatric patients with severe acute hypoxemic respiratory failure (14, 15) and, when present beyond 36 hours of diagnosis, is associated with mortality (14).
Echocardiography is a non-invasive, readily available bedside tool that can detect the presence of PH and measure ventricular systolic function. While RV systolic function can be assessed by various qualitative and quantitative parameters, the use of speckle tracking echocardiography (STE) has emerged in recent years as an insonation angle-independent measure of RV systolic function (16–19). STE allows for the assessment of longitudinal RV deformation as a percentage of myocardial fiber shortening (more negative values reflect better systolic function) (17–19) and may allow for earlier, more accurate detection of RV dysfunction (19–21).
The prevalence and importance of early RV dysfunction and PH in PARDS are unknown and the role of echocardiography in these patients is ill-defined. This retrospective cohort study aimed to assess the prevalence of RV dysfunction and PH within 24 hours of PARDS diagnosis. We hypothesized that early RV dysfunction and PH would be associated with greater PARDS severity, higher mortality, and longer duration of mechanical ventilation.
MATERIALS AND METHODS
Study Design and Patient Selection
This retrospective cohort study of an ongoing prospective study was approved by the Children’s Hospital of Philadelphia’s (CHOP) Institutional Review Board with a waiver of the need for informed consent. The cohort was derived from a previously described PARDS database (2) of prospectively enrolled intubated children admitted to the CHOP pediatric intensive care unit (PICU) between July 1, 2012 and June 30, 2016. The American-European Consensus Conference (AECC) criteria (22) was used for database enrollment but all subjects also met Berlin (23) and PALICC criteria (24). Patients were included if they had a clinically performed echocardiogram within 24 hours of PARDS diagnosis with recorded images of sufficient quality for RV strain analyses.
Data Collection
Data that was prospectively collected following inclusion in the PARDS database has been previously described (2). Briefly, these data included demographics, measures of oxygenation at various time points, use of ancillary therapies within 72 hours, any use of extracorporeal membrane oxygenation (ECMO), Pediatric Risk of Mortality (PRISM) III score at 12 hours, and patient outcomes. Charts were retrospectively reviewed to determine inhaled nitric oxide (iNO) use, vasopressor/inotrope use, and ventilator settings at the time of echocardiogram as well as plasma B-type natriuretic peptide (BNP) concentrations measured within 24 hours of echocardiogram.
Echocardiographic Measurements
Echocardiograms were analyzed by a trained cardiologist (Y.W.) blinded to patient history and outcome. Echocardiographic indices of RV systolic function included: qualitative assessment (normal or mildly, moderately, or severely diminished), fractional area change (FAC), tricuspid annular plane systolic excursion (TAPSE), and myocardial performance index (MPI). STE analyses included RV peak systolic global longitudinal strain (GLS) and strain rate as well as the RV free wall peak systolic longitudinal strain (FWS) and strain rate. LV systolic function was assessed using qualitative assessment (see RV above) and LV fractional shortening (LV FS). PH was assessed using ventricular septal position, tricuspid regurgitant velocity, pulmonary artery acceleration time (PAAT) and RV ejection time (RVET). RV dilation was assessed qualitatively. TAPSE was either measured via M-mode interrogation of the lateral portion of the tricuspid valve annulus or, if M-mode imaging was not available, by offline measurement of the displacement of the tricuspid valve from the apical four-chamber view images (25). Strain analyses were performed using post-processing software (TomTec; Cardiac Performance Analysis, Munich, Germany). The apical four-chamber view was used to measure the strain and strain rate values. Control values for RV strain and strain rate data were obtained from 60 echocardiograms performed on healthy pediatric patients of representative age groups identified in the CHOP Echolab digital system.
Definitions
Abnormal TAPSE was defined as a value <−2 standard deviations (SD) below published pediatric normal values for age (26). Absolute TAPSE z-scores were calculated with an online calculator (parameterz.blogspot.com). Abnormal FAC was defined as < 35%. Evidence of PH was defined as abnormal septal position given that only about 5% of the studies had images of sufficient quality for accurate measurement of TR jet and PAAT. Abnormal GLS and FWS were defined as <−2 SD below the internal controls: for GLS, this was considered a value >−18.2%; for FWS, this was considered a value >−21.4%. Abnormal LVFS was defined as < 28%. OI was defined as mPaw × FiO2 × 100/PaO2. PARDS severity was defined using worst OI in the first 24 hours of PARDS (2) according to PALICC categories (mild OI 4 to < 8; moderate OI 8 to < 16; severe OI ≥ 16) (24). A patient was identified as “immunocompromised” in the presence active immunosuppressive therapy or a congenital immunodeficiency (27). All mention of “ventilation” indicates invasive ventilation; non-invasive ventilation was not counted. “Day 1” was initiation of invasive ventilation. Liberation from invasive ventilation ≥ 24 hours defined duration of ventilation. Patients requiring re-initiation of invasive ventilation had the extra days counted towards total ventilator days. VFD was determined by subtracting total ventilator days from 28 in survivors. Patients with ≥ 28 ventilator days and PICU non-survivors were assigned VFD = 0.
Data Analysis
Statistical analyses were conducted with R (www.r-project.com) and Stata 14.2 SE (StataCorp, College Station, TX). Data are expressed as median values (interquartile range, IQR) or percentages. Categorical data are compared by the Fisher exact test. Continuous data are compared using the Wilcoxon rank-sum test or Kruskal-Wallis test by ranks. Cuzick non-parametric test of trend is used to assess for presence of monotonic trend across worsening PARDS severity categories. For univariate correlation analyses, Spearman’s rank correlation coefficient test was used. Intra-rater reliability for the echocardiogram interpretations was determined with repeated measurements in 20 subjects greater than 2 months after initial interpretation. Intra-class correlation coefficients (ICCs) were used to assess continuous measurements while weighted kappa was used for categorical measurements. Parameter reproducibility was graded as acceptable (R >0.6), good (R >0.7), or excellent (R >0.8).
To test for independent association between echocardiography parameters and PICU mortality, logistic regression was performed upon each parameter, adjusting for PRISM III and worst OI in the first 24 hours. We have previously demonstrated the association between PRISM III and worst OI and outcomes in PARDS (2, 28–29). As there were a limited number of deaths, we avoided fitting additional variables for concern of over-fitting. Immunocompromised status was also assessed but did not improve fit of any model by likelihood ratio testing, and so was not included in the final model. The final fit of each model was assessed using the Hosmer-Lemeshow test.
To assess the independent association with probability of extubation, we performed competing risk regression, using successful extubation as the primary outcome, and death as the competing risk. Observations were censored at 28 days, making this outcome comparable to VFD at 28 days. Fine and Gray (30) competing risk regression calculates a subdistribution hazard ratio (SHR) for risk of extubation, accounting for the competing risk of death. The SHR is affected by the probability of extubation (primary outcome), time to extubation, and probability of death (competing event). As for mortality, SHR are adjusted for PRISM III and worst OI. The proportional hazards assumption was assessed by testing for interaction with a time-dependent covariate.
RESULTS
One hundred and three patients met inclusion criteria, which represents 29% of all patients with PARDS during the study time period; 14% met criteria for mild, 35% for moderate, and 51% for severe PARDS. Patients who met inclusion criteria had more severe illness, a higher rate of immunocompromised comorbidities, and higher iNO utilization compared to database patients that were excluded (Table 1, Supplemental Table 1). Infectious causes of PARDS, defined as infectious pneumonia and sepsis, accounted for 73% of the diagnoses. Pre-existent PH was present in 7 patients (7%) with 3 patients actively receiving pre-illness sildenafil therapy. Invasive mechanical ventilation support characteristics at the time of echocardiogram were available in a subset of patients (89%) and notable for a lower OI at the time of echocardiogram compared to the worst OI in 24 hours (p<0.001, Supplemental Table 2). PICU mortality was 27% (Table 1).
Table 1.
Patient characteristics of study cohort (n=103 unless otherwise noted).
| Variable | Values* |
|---|---|
|
| |
| Age (yr) | 4.6 (1.7, 10.8) |
|
| |
| Female/Male (%/%) | 44 (43%)/59 (57%) |
|
| |
| PRISM III at 12 hours | 14.0 (8.0, 23.0) |
|
| |
| Worst OI in the first 24 hours | 16.7 (10.0, 28.9) |
|
| |
| Worst P/F in the first 24 hours | 110 (74, 165) |
|
| |
| OI at 24 hours | 8.0 (5.2, 12.6) |
|
| |
| P/F at 24 hours | 207 (145, 282) |
|
| |
| Comorbidities, n (%) | 59 (57%) |
| Pre-existent PH | 7 (7%) |
| Genetic Syndrome | 16 (15%) |
| Prematurity | 10 (10%) |
| Immunocompromised | 30 (29%) |
| Stem cell transplant | 10 (10%) |
|
| |
| Cause of PARDS, n (%) | |
| Infectious Pneumonia | 48 (47%) |
| Aspiration Pneumonia | 10 (10%) |
| Sepsis | 27 (26%) |
| Trauma | 4 (4%) |
| Other | 14 (13%) |
|
| |
| Ancillary therapies in 72h, n (%) | |
| APRV/HFPV/HFOV | 7 (7%)/13 (13%)/18 (17%) |
| Continuous NMB | 55 (53%) |
| iNO | 53 (51%) |
| Surfactant | 3 (3%) |
| Prone positioning | 1 (1%) |
|
| |
| Characteristics at the time of Echocardiogram: | |
| Time from diagnosis to first echo (hours) | 13.0 (9.1, 15.6) |
| NIPPV, HFNC, no respiratory support, n (%) | 10 (10%) |
| ECMO, n (%) | 1 (1%) |
| Invasive mechanical ventilation, n (%) | 92 (89%) |
| iNO use, n (%) | 31 (30%) |
| Inotrope/Vasopressor use, n (%) | 57 (55%) |
| Vasopressor score | 14 (8,23) |
|
| |
| Outcomes | |
| ECMO, n (%) | 6 (6%) |
| Duration of MV (days) | 10.0 (6.0, 18.5) |
| Duration of PICU Stay (days) | 14.0 (8.0, 24.0) |
| VFD at 28 days (days) | 11.0 (0,19.0) |
| PICU Mortality, nonsurvivors (n, %) | 28 (27%) |
|
| |
| Closest BNP to time of echo, (pg/mL), n=55 | 370 (108,1904) |
| Time to Closest BNP measurement (hrs), n=55 | 0.2 (−2.6, 3.7) |
IQR = interquartile range; PRISM = Pediatric Risk of Mortality; OI = oxygenation index; PARDS = pediatric acute respiratory syndrome; APRV = airway pressure release ventilation; HFPV = high frequency percussive ventilation; HFOV = high frequency oscillatory ventilation; NMB = neuromuscular blockade; iNO = inhaled nitric oxide; NIPPV = non-invasive positive pressure ventilation; HFNC = high flow nasal cannula; ECMO = extracorporeal membrane oxygenation; MV = mechanical ventilation; PICU = pediatric intensive care unit; VFD = ventilator free days; BNP = B-type natriuretic peptide
All continuous variables are reported as median (interquartile range, IQR).
Prevalence of Echocardiography Measurements
At the time of echocardiogram, 31 patients (30%) were receiving iNO, which was more common among patients with severe PARDS compared to those categorized as mild or moderate PARDS (Fisher exact p=0.002). Fifty-seven patients (55%) were on inotropic and/or vasopressor support at the time of echocardiogram and this was not different between or across PARDS severity (Fisher exact p=0.79, Cuzick p=0.66). The overall rates of FAC < 35% was 26%, evidence of PH was 21%, and LV FS < 28% was 30%. Abnormal RV GLS and FWS were observed in 35% and 40%, respectively. The median TAPSE z-score for the entire cohort was −2.97 (IQR −5.53, −1.12) with 65% having TAPSE z-score <−2 SD for age. There were no significant differences between various echocardiography measures of RV and LV systolic function, PH, or qualitative RV dilation between or across severity of PARDS when characterized by worst OI in the first 24 hours following PARDS diagnosis or when stratified by iNO exposure at the time of echocardiogram (Table 2, Supplemental Table 3). There was a trend toward longer duration of MV and reduced VFD at 28 days with worse PARDS severity and with iNO exposure at the time of echocardiogram (Supplemental Tables 4 and 5). ICC values for continuous echocardiographic parameters were all excellent (range 0.93 to 0.99). Weighted kappa values for categorical parameters were all > 0.91 with the exception of qualitative RV dilation (κ=0.76).
Table 2.
Echocardiography measurements stratified by PARDS severity (worst OI in 24 hours). n=103 unless otherwise noted.
| Echo Parameter | Mild† (n=14) | Moderate† (n=36) | Severe† (n=53) | Total† (n=103) | p-value* | p-value for trend** |
|---|---|---|---|---|---|---|
| TAPSE (cm), n=102 | 1.3 (0.7, 1.9) | 1.4 (1.0, 1.6) | 1.2 (0.9, 1.6) | 1.3 (0.9, 1.7) | 0.89 | 0.50 |
| TAPSE z-score, n=102 | −2.83 (−5.57, −0.64) | −3.40 (−5.25, −1.42) | −2.93 (−5.51, −1.34) | −2.97 (−5.53, −1.12) | 0.15 | 0.94 |
| TAPSE z-score < −2 SD, n (%), n=102 | 7 (50%) | 25 (69%) | 35 (66%) | 67 (65%) | 0.33 | 0.38 |
| RV FAC (%), n=102 | 39.4 (31.7, 44.1) | 43.6 (36.9, 48.1) | 42.9 (32.2, 41.5) | 43.0 (33.8, 48.2) | 0.38 | 0.53 |
| RV FAC < 35%, n=102 | 5 (36%) | 5 (14%) | 17 (32%) | 27 (26%) | 0.06 | 0.60 |
| RV Qualitative Systolic Dysfunction, n (%) | 6 (43%) | 7 (19%) | 17 (32%) | 30 (29%) | 0.21 | 0.91 |
| RV GLS (%) | −22.3 (−24.9, −17.2) | −19.3 (−24.2, −17.1) | −19.9 (−25.2, −15.7) | −20.0 (−24.7, −16.8) | 0.18 | 0.68 |
| RV GLS < −2 SD, n (%) | 4 (29%) | 12 (33%) | 20 (38%) | 36 (35%) | 0.88 | 0.49 |
| RV peak systolic longitudinal global strain rate (sec−1) | −1.3 (−1.6, −1.0) | −1.2 (−1.6, −1.0) | −1.2 (−1.6, −1.0) | −1.2 (−1.6, −1.3) | 0.97 | 0.82 |
| RV FWS (%) | −24.3 (−26.1, −18.6) | −23.0 (−26.0, −19.7) | −22.6 (−28.5, −17.7) | −22.7 (−27.5, −18.9) | 0.92 | 0.76 |
| RV FWS < −2 SD, n (%) | 5 (36%) | 13 (36%) | 23 (43%) | 41 (40%) | 0.78 | 0.49 |
| RV peak systolic longitudinal free wall strain rate (sec−1) | −1.5 (−1.9, −1.1) | −1.4 (−1.7, −1.2) | −1.4 (−1.7, −1.1) | −1.4 (−1.7, −1.2) | 0.99 | 0.92 |
| Echo Evidence of PH, n (%), n=94 | 3/12 (25%) | 9/36 (25%) | 8/46 (17%) | 20/94 (21%) | 0.68 | 0.42 |
| LV FS (%), n=88 | 30.3 (26.6, 33.6) | 33.3 (26.0, 40.2) | 35.7 (23.0, 40.0) | 33.3 (24.2, 40.0) | 0.37 | 0.44 |
| LV FS < 28%, n (%), n=88 | 3/11 (27%) | 10/36 (28%) | 13/43 (30%) | 26/88 (30%) | 1 | 0.92 |
| RV qualitative dilation, n (%) | 3 (21%) | 6 (17%) | 10 (19%) | 20 (18%) | 0.83 | 0.95 |
SD = standard deviation; RV = right ventricle; FAC = fractional area change; GLS = global longitudinal strain; FWS = free wall strain; TAPSE = tricuspid annular peak systolic excursion; PH = pulmonary hypertension, LV = left ventricle; FS = fractional shortening
Kruskal-Wallis test or Fisher exact test comparing mild, moderate, severe PARDS categories
Cuzick non-parametric test of trend across mild, moderate, severe PARDS categories
All continuous variables are reported as median (interquartile range, IQR).
Associations of Patient Outcomes
Abnormal RV GLS within the first 24 hours was independently associated with PICU mortality, both in the overall cohort and when restricted to those not exposed to iNO at the time of echocardiogram. Other echocardiogram parameters of RV systolic function, LV systolic function, or PH were not associated with mortality (Tables 3 and 4).
Table 3.
Association between ventricular function and pulmonary hypertension with PICU mortality and probability of extubation. Both models adjusted for Worst OI in the first 24 hours after PARDS diagnosis and PRISM III score at 12 hours (n=103).
| Echo findings within 24 hours of PARDS diagnosis | PICU mortality | Probability of extubation | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | SHR (95% CI) | p-value | |
| RV FAC < 35% | 0.59 (0.19 to 1.82) | 0.356 | 0.89 (0.50 to 1.57) | 0.677 |
| RV GLS < −2 SD | 3.57 (1.33 to 9.60) | 0.012 | 0.46 (0.26 to 0.83) | 0.009 |
| RV FWS < −2 SD | 1.33 (0.51 to 3.41) | 0.559 | 0.58 (0.35 to 0.98) | 0.041 |
| TAPSE z-score < −2SD | 1.17 (0.42 to 3.30) | 0.764 | 0.85 (0.51 to 1.44) | 0.546 |
| Evidence of PH | 1.15 (0.33 to 4.06) | 0.824 | 0.49 (0.26 to 0.92) | 0.027 |
| LV FS < 28% | 2.58 (0.86 to 7.78) | 0.091 | 0.76 (0.37 to 1.53) | 0.440 |
Table 4.
Association between ventricular function and pulmonary hypertension with PICU mortality and probability of extubation for patients not exposed to iNO at the time of echocardiogram. Both models adjusted for Worst OI in the first 24 hours after PARDS diagnosis and PRISM III score at 12 hours (n=72).
| Echo findings within 24 hours of PARDS diagnosis | PICU mortality | Probability of extubation | ||
|---|---|---|---|---|
| OR (95% CI) | p value | SHR (95% CI) | p value | |
| RV FAC < 35% | 0.94 (0.21 to 04.21) | 0.940 | 0.97 (0.49 to 1.93) | 0.939 |
| RV GLS < −2SD | 4.11 (1.10 to 15.30) | 0.035 | 0.45 (0.23 to 0.88) | 0.020 |
| RV FWS < −2SD | 2.00 (0.56 to 7.10) | 0.285 | 0.63 (0.34 to 1.14) | 0.128 |
| TAPSE z-score < −2SD | 1.27 (0.33 to 4.79) | 0.729 | 0.77 (0.43 to 1.37) | 0.378 |
| Evidence of PH | 1.30 (0.29 to 5.78) | 0.735 | 0.49 (0.27 to 0.99) | 0.047 |
| LV FS < 28% | 1.76 (0.47 to 6.66) | 0.404 | 0.78 (0.35 to 1.77) | 0.557 |
OR = odds ratio; SHR: Subdistribution hazard ratio; SD = standard deviation; RV = right ventricle; FAC = fractional area change; GLS = global longitudinal strain; FWS = free wall strain; TAPSE = tricuspid annular peak systolic excursion; PH = pulmonary hypertension, LV = left ventricle; FS = fractional shortening
Abnormal RV GLS, abnormal RV FWS, and PH were independently and inversely associated with the probability of successful extubation (Table 3, Figure 1). When restricted to the subgroup of patients not exposed to iNO, RV GLS and PH (but not RV FWS) were independently and inversely associated with the probability of successful extubation (Table 4).
Figure 1.
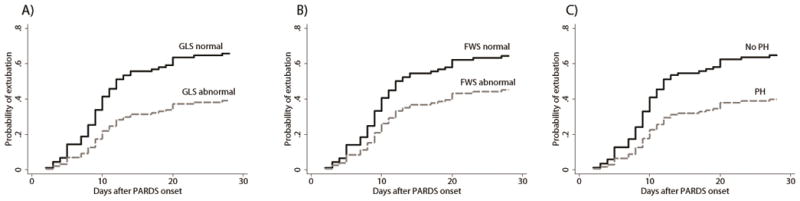
Probability of successful extubation accounting for the competing risk of death after adjustment for Pediatric Risk of Mortality III and worst oxygenation index within the first 24 hours following PARDS diagnosis for (A) abnormal versus normal RV global longitudinal strain (GLS) (n=103), (B) abnormal versus normal RV free wall strain (FWS) (n=103), and (C) echocardiographic evidence of pulmonary hypertension (PH) (n=94). Abnormal RV GLS and RV FWS were defined as < −2 SD compared to healthy internal controls: for GLS, this was considered a value > −18.2%; for FWS, this was considered a value > −21.4%
Subgroup multivariate analyses excluding patients with pre-existing PH and for the subgroups of patients with conventional ventilator parameters at the time of echocardiogram showed similar results with respect to RV GLS and PH associations with outcomes (Supplementary Tables 6–10).
BNP Correlations
BNP was available for a subset of patients (Table 1). BNP was inversely and moderately correlated with LV FS (ρ=−0.52) but only modestly correlated with measures of RV systolic function (RV FAC ρ=−0.29, RV GLS ρ=0.41, RV FWS ρ=0.38, TAPSE z-score ρ=−0.27). There was no association between BNP level and PICU mortality, ventilator days, VFD, or need for ECMO.
DISCUSSION
This study demonstrated that echocardiographic evidence of RV and LV systolic dysfunction and PH were prevalent within 24 hours of PARDS diagnosis. RV GLS was independently associated with PICU mortality, while RV GLS, RV FWS, and the presence of PH were independently associated with lower probability of extubation in PARDS patients. Abnormalities in RV and LV function appeared to be independent of PARDS severity in this group of patients.
We found that up to 40% of patients had echocardiographic evidence of RV systolic dysfunction and 21% had PH early in their course of PARDS, even with use of iNO and/or inotropes/vasopressors in a significant proportion of patients. These results are similar to those reported in adult studies (7–13).
In adult ARDS, RV dysfunction can vary from mild, with modest changes in TAPSE, to severe cases of acute cor pulmonale (ACP) (7–8, 32). Moreover, TAPSE has been shown to correlate with severity of ARDS and increased 30-day mortality (12). Contrary to these findings, we found no correlation between early TAPSE z-scores with PARDS severity (Table 2), or with mortality or probability of extubation (Tables 3 and 4). One of the limitations of TAPSE is that it can be affected by LV contractility (33). Indeed, in our study, TAPSE z-scores correlated just as well with LV FS (ρ=0.54, p<0.0001) as with RV GLS (ρ=−0.53, p<0.0001), RV FWS (ρ=−0.51, p<0.0001) and less so with RV FAC (ρ=0.46, p<0.0001). This, along with the fact that the 30% of our population had abnormal LV systolic function, suggests that early TAPSE z-scores may not discriminate degrees of RV dysfunction in PARDS patients.
Strain echocardiography has allowed for earlier and more accurate detection of acute RV dysfunction in various populations (19–21). In our study, we found abnormal strain to be more commonly detected than low FAC. We found that diminished RV GLS was the only echocardiographic parameter of ventricular function that was associated with increased PICU mortality; diminished RV GLS and RV FWS were both associated with lower probability of extubation. Despite the fact that RV dysfunction is prevalent in pediatric and adult ARDS, no studies to our knowledge have examined strain as a measure of function that is potentially superior to other standard measures, such as TAPSE and FAC. There is very likely a spectrum of RV dysfunction in PARDS patients that changes with time and future research should focus on better defining this spectrum based on echocardiographic parameters, including TAPSE and strain echocardiography, as well as other patient characteristics and biomarkers.
B-type natriuretic peptide (BNP), and its precursor N-terminal prohormone (NT-proBNP), are well-studied plasma biomarkers of cardiac dysfunction (33). However, these substances may also be expressed in and released from inflamed lung (33). There is conflicting data in adult ARDS about the utility of BNP as a marker of RV dysfunction and in predicting mortality (33–36). Contrary to the only report of BNP levels in children with acute lung injury (37), we found that BNP concentration within the first 24 hours of PARDS diagnosis was not associated with patient outcomes and correlated best with LV FS. Despite its wide clinical use, BNP may not accurately reflect RV dysfunction in the setting of acute non-cardiac organ damage and inflammation such as in PARDS.
The presence and persistence of PH in pediatric non-survivors of pediatric patients with acute hypoxemic respiratory failure was demonstrated when pulmonary artery catheters were routinely used and prior to current PARDS management strategies (14–15). Acute RV dysfunction in PARDS can have multifactorial etiologies, including PH, systemic inflammatory and other neurohumoral processes, or disease-related primary LV dysfunction with systemic hypotension that can compromise RV function (38–39). In addition, acute RV dysfunction can cause LV dysfunction by ventricular interdependence mechanisms (38). As morbidity and mortality in PARDS is driven by the development of MODS (1–6), future clinical and translational research should address interventions and therapies that identify (i.e. echocardiography) and treat early modifiable hemodynamic risk factors (including PH, RV and LV dysfunction) that may prevent or mitigate the development or progression of MODS.
This single center, retrospective cohort study has several limitations. Inherent in any retrospective study, causality cannot be determined and associations that we found to patient outcomes are limited by lack of defined criteria for parameters such as inotrope/vasopressor use and extubation. Our institutional practice is to initiate iNO at 20 ppm for hypoxemia associated with PARDS. However, it is difficult to know exactly from the medical records if or when iNO therapy was used solely to treat PH. For example, 6 of 7 patients with history of PH were started on iNO prior to an echocardiogram being performed and only 4 had severe PARDS. The included patients had higher disease severity and were a subset of PARDS patients admitted over the study time period (see Supplemental Table 1). We characterized PARDS severity by worst OI in 24 hours and, for many patients including subjects in this study, OI will improve by 24 hours (Table 1). While OI at 24 hours may better discriminate mortality in isolation (2), we characterizing PARDS severity by worst OI in 24 hours so as to mitigate effects of patient management decisions made after the echocardiogram was performed, including initiation of iNO if PH or RV dysfunction was observed. Echocardiogram images were obtained for clinical purposes using undefined criteria. These factors likely introduce confounding by indication since there was likely a clinical reason for obtaining an echocardiogram for most patients. Patients did not undergo a standard image acquisition protocol and, subsequently, accurate RVPE measures were only obtained in a small minority of patients. In addition, assessment of the RV by echocardiography is often challenging, especially in patients with significant lung disease. We only included echocardiograms with apical 4-chamber windows that were of sufficient quality to accurately perform RV strain analyses which introduces some selection bias. Pre-PARDS echocardiograms were not evaluated in this study as none existed in the vast majority. Finally, the high use of iNO and inotropes/vasopressors may have treated some PH, RV dysfunction, or LV dysfunction that otherwise would have been detected with echocardiography and therefore would have biased our result toward the null.
CONCLUSIONS
In conclusion, our data suggests that RV and LV systolic dysfunction and PH are prevalent in PARDS and detectable by echocardiography within 24 hours of PARDS diagnosis. These abnormalities are independent of PARDS severity. Early abnormal RV GLS is associated with PICU mortality while early abnormal RV GLS, abnormal RV FWS, and PH are associated with decreased probability of extubation. Our results should inform future studies for targeted detection and treatment of ventricular dysfunction and PH in PARDS patients with the potential for patient stratification based on early and evolving hemodynamic profiles.
OR = odds ratio; SHR: Subdistribution hazard ratio; SD = standard deviation; RV = right ventricle; FAC = fractional area change; GLS = global longitudinal strain; FWS = free wall strain; TAPSE = tricuspid annular peak systolic excursion; PH = pulmonary hypertension, LV = left ventricle; FS = fractional shortening
Supplementary Material
Acknowledgments
Financial support used for the study was from the Department of Anesthesiology and Critical Care Medicine at the Children’s Hospital of Philadelphia
Footnotes
Data from this manuscript was presented as a STAR Research Presentation at the 47th Critical Care Congress of the Society of Critical Care Medicine on February 25, 2018.
Reprints: Reprints will not be ordered for this manuscript. The address for reprints is the same as the Corresponding Author.
Conflicts of Interest and Sources of Funding: Dr. Yehya is currently receiving a grant (K23 HL-136688) from the NIH/NHLBI. Dr. Mercer-Rosa is currently receiving grants from the NIH (K01 HL-125521) and from the Pulmonary Hypertension Association. The remaining authors have disclosed that they do not have any conflicts of interest.
Copyright form disclosure: Dr. Himebauch received funding from the Society of Critical Care Medicine (honoraria and travel costs). Dr. Yehya’s institution received funding from the National Institutes of Health (NIH)/ National Heart, Lung, and Blood Institute (NHLBI). Drs. Yehya and Mercer-Rosa received support for article research from the NIH. Dr. McGowan received funding from Merck. Dr. Mercer-Rosa’s institution received funding from NIH/NHLBI grant K01HL125521. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Schouten LR, Veltkamp F, Bos AP, et al. Incidence and mortality of acute respiratory distress syndrome in children: a systematic review and meta-analysis. Crit Care Med. 2016;44(4):819–829. doi: 10.1097/CCM.0000000000001388. [DOI] [PubMed] [Google Scholar]
- 2.Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med. 2015;43(5):937–946. doi: 10.1097/CCM.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 3.Leclerc F, Duhamel A, Deken V, et al. Nonrespiratory Pediatric Logistic Organ Dysfunction-2 Score is a good predictor of mortality in children with acute respiratory failure. Pediatr Crit Care Med. 2014;5:590–593. doi: 10.1097/PCC.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Fernandez Y, Azagra AM, de la Oliva P, et al. Pediatric acute lung injury epidemiology and natural history study: incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med. 2012;40:3238–3245. doi: 10.1097/CCM.0b013e318260caa3. [DOI] [PubMed] [Google Scholar]
- 5.Erickson S, Schibler A, Numa A, et al. Paediatric Study Group; Australian and New Zealand Intensive Care Society: acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8:317–323. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 6.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 7.Zochias V, Parhar K, Tunnicliffe W, et al. The right ventricle in ARDS. Chest. 2017;152(1):181–193. doi: 10.1016/j.chest.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Repesse X, Charron C, Vieillard-Baron A. Acute cor pulmonale in ARDS: rationale for protecting the right ventricle. Chest. 2015;147(1):259–265. doi: 10.1378/chest.14-0877. [DOI] [PubMed] [Google Scholar]
- 9.Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725–1733. doi: 10.1007/s00134-013-2941-9. [DOI] [PubMed] [Google Scholar]
- 10.Lhéritier G, Legras A, Caille A, et al. Prevalence and prognostic value of acute cor pulmonale and patent foramen ovale in ventilated patients with early acute respiratory distress syndrome: a multicenter study. Intensive Care Med. 2013;39(10):1734–1742. doi: 10.1007/s00134-013-3017-6. [DOI] [PubMed] [Google Scholar]
- 11.Osman D, Monnet X, Castelain V, et al. French Pulmonary Artery Catheter Study Group. Incidence and prognostic value of right ventricular failure in acute respiratory distress syndrome. Intensive Care Med. 2009;35(1):69–76. doi: 10.1007/s00134-008-1307-1. [DOI] [PubMed] [Google Scholar]
- 12.Shah TG, Wadia SK, Kovach J, et al. Echocardiographic parameters of right ventricular function predict mortality in acute respiratory distress syndrome: a pilot study. Pulm Circ. 2016;6(2):155–160. doi: 10.1086/685549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wadia SK, Shah TG, Hedstrom G, Kovach JA, Tandon R. Early detection of right ventricular dysfunction using transthoracic echocardiography in ARDS: a more objective approach. Echocardiography. 2016;33(12):1874–1879. doi: 10.1111/echo.13350. [DOI] [PubMed] [Google Scholar]
- 14.Katz R, Pollack M, Spady D. Cardiopulmonary abnormalities in severe acute respiratory failure. J Pediatr. 1984;104:357–364. doi: 10.1016/s0022-3476(84)81095-1. [DOI] [PubMed] [Google Scholar]
- 15.DeBruin W, Notterman DA, Magid M, et al. Acute hypoxemic respiratory failure in infants and children: clinical and pathological characteristics. Crit Care Med. 1992;20(9):1223–1234. doi: 10.1097/00003246-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 16.DiLorenzo MP, Bhatt SM, Mercer-Rosa L. How best to assess right ventricular function by echocardiography. Cardiol Young. 2015;25(8):1473–1481. doi: 10.1017/S1047951115002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Urheim S, Edvardsen T, Torp H, et al. Myocardial strain by Doppler echocardiography: validation of a new method to quantify regional myocardial function. Circulation. 2000;102:1158–1164. doi: 10.1161/01.cir.102.10.1158. [DOI] [PubMed] [Google Scholar]
- 18.Jamal F, Bergerot C, Argaud L, et al. Longitudinal strain quantitates regional right ventricular contractile function. Am J Physiol Heart Circ Physiol. 2003;285:H2842–H2847. doi: 10.1152/ajpheart.00218.2003. [DOI] [PubMed] [Google Scholar]
- 19.Vitarelli A, Barilla F, Capotosto L, et al. Right ventricular function in acute pulmonary embolism: a combined assessment by three-dimensional and speckle-tracking echocardiography. J Am Soc Echocardiogr. 2014;27:329–338. doi: 10.1016/j.echo.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Wright L, Dwyer N, Power J, et al. Right ventricular systolic function responses to acute and chronic pulmonary hypertension: assessment with myocardial deformation. J Am Soc Echocardiogr. 2016;29:259–266. doi: 10.1016/j.echo.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Werther Evaldsson A, Ingvarsson A, Waktare J, et al. Right ventricular speckle tracking assessment for differentiation of pressure-versus volume-overloaded right ventricle. Clin Physiol Funct Imaging. 2017 Oct 26; doi: 10.1111/cpf.12477. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Hernu R, Wallet F, Thiollière F, et al. An attempt to validate the modification of the American-European consensus definition of acute lung injury/acute respiratory distress syndrome by the Berlin definition in a university hospital. Intensive Care Med. 2013;39:2161–2170. doi: 10.1007/s00134-013-3122-6. [DOI] [PubMed] [Google Scholar]
- 23.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 24.The Pediatric Acute Lung Injury Consensus Conference Group. Pediatric acute respiratory distress syndrome: consensus recommendations from the pediatric acute lung injury consensus conference. Pediatr Crit Care Med. 2015;16(5):428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qureshi MY, Eidem BW, Reece CL, O’Leary PW. Two-dimensional measurement of tricuspid annular plane systolic excursion in children: can it substitute for an m-mode assessment? Echocardiography. 2015;32(3):528–534. doi: 10.1111/echo.12687. [DOI] [PubMed] [Google Scholar]
- 26.Koestenberger M, Ravekes W, Everett AD, et al. Right ventricular function in infants, children, and adolescents: reference values of the tricuspid annular plane systolic excursion (TAPSE) in 640 healthy patients and calculation of z score values. J Am Soc Echocardiogr. 2009;22:715–719. doi: 10.1016/j.echo.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Yehya N, Topjian A, Thomas N, et al. Improved oxygenation 24 hours after transition to airway pressure release ventilation or high-frequency oscillatory ventilation accurately discriminates survival in immunocompromised pediatric patients with acute respiratory distress syndrome. Pediatr Crit Care Med. 2014;15:e147–156. doi: 10.1097/PCC.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yehya N, Servaes S, Thomas NJ, et al. Corticosteroid exposure in pediatric acute respiratory distress syndrome. Intensive Care Med. 2015;41(9):1658–1666. doi: 10.1007/s00134-015-3953-4. [DOI] [PubMed] [Google Scholar]
- 29.Yehya N, Thomas NJ. Dissociating lung mechanics and oxygenation in pediatric acute respiratory distress syndrome. Crit Care Med. 2017;45(7):1232–1239. doi: 10.1097/CCM.0000000000002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 31.Lazzeri C, Peris A. The spectrum of changes in the right ventricle in ARDS: dilatation, dysfunction, and acute cor pulmonale. Chest. 2017;152(1):214. doi: 10.1016/j.chest.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 32.López-Candales A, Rajagopalan N, Saxena N, et al. Right ventricular systolic function is not the sole determinant of tricuspid annular motion. Am J Cardiol. 2006;98:973–977. doi: 10.1016/j.amjcard.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 33.Phua J, Lim TK, Lee KH. B-type natriuretic peptide: issues for the intensivist and pulmonologist. Crit Care Med. 2005;33(9):2094–2103. doi: 10.1097/01.ccm.0000178351.03327.9f. [DOI] [PubMed] [Google Scholar]
- 34.Bayes-Genis A, Santalo-Bel M, Zapico-Muniz E, et al. N-terminal probrain natriuretic peptide (NT-proBNP) in the emergency diagnosis of patients with dyspnea and ventricular dysfunction. Eur J Heart Fail. 2004;6:301–308. doi: 10.1016/j.ejheart.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 35.Cepkova M, Kapur V, Ren X, et al. Clinical significance of elevated B-type natriuretic peptide in patients with acute lung injury with or without right ventricular dilatation: an observational cohort study. Annals of Intensive Care. 2011;1:18. doi: 10.1186/2110-5820-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park BH, Kim YS, Chang J, et al. N-terminal pro-brain natriuretic peptide as a marker of right ventricular dysfunction after open-lung approach in patients with acute lung injury/acute respiratory distress syndrome. J Crit Care. 2011;26:241–248. doi: 10.1016/j.jcrc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Reel B, Oishi PE, Hsu J-H, et al. Early elevations in B-type natriuretic peptide levels are associated with poor clinical outcomes in pediatric acute lung injury. Pediatr Pulmonol. 1999;44:1118–1124. doi: 10.1002/ppul.21111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinsky MR. The right ventricle: interaction with the pulmonary circulation. Critical Care. 2016;20:266. doi: 10.1186/s13054-016-1440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodard JC, Chow E, Farrar DJ. Isolated ventricular systolic interaction during transient reductions in left ventricular pressure. Circ Res. 1992;70:944–951. doi: 10.1161/01.res.70.5.944. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


