Abstract
Convection Enhanced Delivery (CED) infuses therapeutic agents directly into the intracranial area continuously under pressure. The convection improves the distribution of therapeutics such as those aimed at brain tumors. Although CED successfully delivers small therapeutic agents, this technique fails to effectively deliver cells largely due to cell sedimentation during delivery. To overcome this limitation, we have developed a low viscosity hydrogel (LVHydrogel), which is capable of retaining cells in suspension. In this study, we evaluated whether LVHydrogel can effectively act as a carrier for the CED of tumor-specific chimeric antigen receptor (CAR) T cells. CAR T cells were resuspended in saline or LVHydrogel carriers, loaded into syringes, and passed through the CED system for 5 hours. CAR T cells submitted to CED were counted and the efficiency of delivery was determined. In addition to delivery, the ability of CAR T cells to migrate and induce cytotoxicity was evaluated. Our studies demonstrate that LVHydrogel is a superior carrier for CED in comparison to saline The efficiency of cell delivery in saline carrier was only ~3–5% of the total cells whereas delivery by the LVHydrogel carrier was much higher, reaching ~45–75%. Migration and Cytotoxicity was similar in both carriers in non-infused samples but we found superior cytotoxicity in LVHydrogel group postinfusion. We demonstrate that LVHydrogel, a biodegradable biomaterial which does not cause acute toxicity on preclinical animal models, prevents cellular sedimentation during CED and presents itself as a superior carrier to the current carrier, saline, for the CED of CAR T cells.
Keywords: glioblastoma, Convection Enhanced Delivery, CED, immunotherapy, chimeric antigen receptor, CARs
Introduction
Glioblastoma is the most common primary malignant brain tumor. Standard of care (SOC) for glioblastoma consists of surgery followed by chemotherapy with alkylating agents and the median survival remains less than ~20 months [1]. SOC drugs for glioblastoma are also non-specific and engender toxicity to normal brain and systemic tissue [2]. Furthermore, due to the presence of the Blood Brain Barrier (BBB), therapeutics applied systemically have limited intracranial effect [3]. In order to successfully treat glioblastoma, novel approaches are needed to increase the delivery of therapeutics to the tumor and to enhance the tumor-specificity of therapy.
One novel approach for glioblastoma addresses the issue of the localized intratumoral delivery. The delivery of therapeutic agents directly next to malignant cells via intracranial infusion has been evaluated and is widely used to deliver chemotherapeutics. An optimal version of intracranial delivery is convection enhanced delivery (CED), in which an intracranial catheter is placed into the tumor that infuses the agent with positive-pressure over time. This positive-pressure improves the delivery over time as well as the distribution of the therapeutic agent being delivered [4]. Previous studies with CED have demonstrated that infusate can permeate to adjacent tissue and the distribution of an agent can be up to 8-times greater than delivery from an intracranial single-direct injection technique [5]. Therefore, evaluation of CED for the treatment of glioblastoma remains of paramount interest since it promises to increase the potency and efficacy of therapeutic agents.
While CED can both bypass the BBB and increase the delivery of agents to the tumor, current chemotherapeutics still have the drawback of being non-specific to the tumor and are limited in dose and efficacy even if directly applied due to toxicity [6]. Chimeric antigen receptor (CAR) T cells are artificially engineered effector T lymphocytes capable of specifically attacking one or more targets on tumor cells. CAR T cells have been extensively investigated in our laboratory and shown to effectively treat glioblastoma when using EGFRvIII antigen as a glioblastomaspecific target [7–10]. Although the systemic application of these cells is a promising technique, only a portion of the cells pass the BBB to attack tumor in the intracranial area [11, 12]. Therefore, direct intracranial infusion via single or repeated injections has been investigated as a means to enhance CAR T cell delivery and the subsequent efficacy of therapy [13–15].
While CED has the potential to increase the delivery efficiency of CAR T cells into the intracranial tumor area, our data shows that the traditional CED carrier, saline, results in low numbers of delivered cells. Our studies demonstrate that this inefficient delivery is due to sedimentation of the CAR T cells within the CED system, likely due to the extensive delivery time required for CED (4–6 hours). To solve this purely technical shortcoming, our laboratory has developed a compatible, non-toxic, biodegradable, low viscosity, hyaluronic acid based LVHydrogel carrier (LVHydrogel) that prevents cellular sedimentation and maintains viable, homogenously distributed CAR T cells during CED. We demonstrate that CAR T cells resuspended in our novel carrier are continuously delivered over time at a much higher delivery yield than when used in saline. Additionally, a high number of functional LVHydrogel-delivered cells were capable of successfully migrating out of the LVHydrogel and mediating tumor-specific cytotoxicity when compared to those resuspended in saline. Therefore, we propose the use of LVHydrogel as a carrier of cellular therapeutics by CED in patients with glioblastoma.
Materials and Methods
Tumor Lines
The human glioma cell line U87MG, as well as the Epidermal Growth Factor Receptor variant III (EGFRvIII) expressing subline, U87MG.ΔEGFR, have been previously described [16].
Carrier preparation and Optimization
Injectable, low viscosity LVHydrogel is comprised of two ingredients, thiolized hyaluronic acid and gelatin (denatured collagen) from (Ascendance Biotech©, MA). Both elements were used in rodent brain without any toxicity[17] and currently under development for human subjects (Ascendance Biotech Clinical Trial). These two ingredients were mixed with degassed water and processed a certain way which cannot be revealed here due to the being a corporate secret. After reaching the proper viscosity level, CAR T cells were resuspended at the indicated concentration in LVHydrogel at room temperature. To verify correct viscosity level, number of thiol groups must be evaluated with a fluorescence thiol assay.
Rheology
Rheological testing was performed on a Kinexus Pro Rheometer (Malvern, Malvern, UK) with an 8 mm diameter parallel plate attachment at 25°C. Gels were loaded onto the bottom platen and the top platen was lowered to a gap of 1 mm. Samples were then trimmed to the proper geometry. The linear viscoelastic region was determined by performing a strain sweep from 0.1 to 10% strain at an oscillation frequency of 1 Hz. The elastic modulus (G'), viscous modulus (G''), and viscosity were calculated by averaging the values across this linear range (0.8–5% strain). Measurements were done in triplicate.
Human Pump System
The devices used in CED are a MedFusion 3010a syringe pump (Smiths Medical©, MN), 20 ml syringes (Becton-Dickinson©, NJ), and 20 cm long coiled silicon extension tube (SOPHYSA4, France, model PIT400). The system was placed on a flat surface for the horizontal application. In Vertical Infusion setup, injection pump-syringe and extension tube was placed on a shelf while tip of the syringe looking downward and extension tube is connected to an Eppendorf tube to collect infusate. Infusion rate was adjusted to 500 µl/hr. All infusions were performed at room temperature for five hours to replicate the clinical setup.
Calculating Number of Cells Post infusion
20 µl sample was taken from final infusate and mixed with 180 µl trypan blue to make 1/10 dilution. After mixing gently 10 µl sample was transferred to hemocytometer and counted under microscope to evaluate number of live CAR T cells. Following formula was used to calculate true number of cells
Total Number of Viable Cells/mL = (Average cell count from each 16 corner squares) × (Dilution Factor) × 10.000
Cytotoxicity Assay
5.0×106 U87MG and U87MG.ΔEGFR glioma cells were radioactively labelled with 100 µCi of Chromium51 at 37°C for 90 minutes. Cells were washed three times, co-incubated with saline and LVHydrogel carriers containing EGFRvIII-specific CAR T cells at a 10:1 ratio (CAR T cells: tumor cells), and incubated at 37°C for a minimum of four hours in a total of 200 µls. After incubation, supernatant was harvested and radioactivity was measured to determine the release of chromium 51 and thereby the effective lysis of the glioma cells by the CAR T cells. Percentage of tumor specific cytotoxicity was calculated using the following equation:
Tumor specific cytotoxicity (%) = ((sample lysis ‒ spontaneous)/(maximum lysis ‒ spontaneous)) × 100.
Migration Assay
The lower chamber of 24-well transwell plates (Corning©, NY) was loaded with 600 µl RPMI (without serum). The human chemokine CXCL10, responsible for the migration of T cells [18, 19], was added to each chamber at 1 ng/ml, 10ng/ml, 100 ng/ml, 500 ng/ml and 1000 ng/ml. The upper chamber of each well was seeded with serum starved 6 × 105 EGFRvIII-specific CAR T cells in either saline or LVHydrogel carriers. Transwell plates were then incubated at 37°C for 12 hours. Plates were removed from the incubator and the cells in the lower chamber of the transwell were counted in a hemocytometer using trypan blue staining. Percentage of migration by CAR T cells was calculated by the following formula:
CAR T cell migration (%) = ((Total number of cells that migrated to lower chamber)/(Total number of cells loaded in upper chamber)) × 100
Safety and Toxicity Testing
Toxicity studies were done in non-tumor bearing 6–8 week old, C57BL/6 mice. Groups of 20 mice (10 males and 10 females per group) received 24 µL saline or LVHydrogel into the caudate nucleus utilizing a loaded 250-µl syringe (Hamilton, Reno, NV) with an attached 25-gauge needle. The tip of the needle was positioned at bregma and 2 mm to the right of the cranial midline suture and 4 mm below the surface of the cranium using a stereotactic frame (David Kopf Instruments, Tujunga, CA). The mice were weighed weekly and observed for six weeks, after which, they were sacrificed and the brains were harvested, formalin-fixed and paraffin-embedded. 5 µm sections near the injection site were stained for myelin with Luxol Fast Blue and counter stained with Hematoxylin and Eosin.
Statistical Analysis
The difference in CAR T cell yield and viability between saline and LVHydrogel at each hour was assessed using a t-test with Bonferroni correction. A linear regression model including a quadratic term for log-transformed CXCL10 concentration and corresponding interaction terms was used to assess the difference in CAR T cell migration between saline and LVHydrogel. A 2-way ANOVA with interaction was used to assess tumor-specific cytotoxicity among T cell carriers, and differences in weight of mice injected intracranially with saline or LVHydrogel. Significance was determined at the level of p < 0.05. Values of p > 0.05 were Non-Significant (NS).
Results
Comparison of saline and LVHydrogel physical properties
While viscosity values for normal saline as well as water exist in the literature [20, 21], we utilized dynamic mechanical testing to evaluate the rheological properties for the LVHydrogel. In these measurements, we found the average dynamic viscosity of the LVHydrogel to be 2360 ± 330 centipoise, roughly 2000-fold greater than saline or water alone (Table 1). It should be noted that the estimated viscosity for saline in this reference is for a 1M salt concentration, whereas standard isotonic saline contains 154 mM NaCl. However, saline’s viscosity increases with salt concentration [20], indicating that the LVHydrogel viscosity is still well above that of the carrier saline solution. We could identify the storage (G') and loss (G'') moduli from these data as 14.85 ± 2.09 and 0.37 ± 0.06 Pa, respectively. While values for water and saline are unavailable, they would be expected to be significantly lower than those established for the LVHydrogel. Furthermore, as the storage modulus is greater than the loss modulus for the LVHydrogel, we can confidently state that we have formed a gel during the synthesis of our hyaluronic acid based LVHydrogel carrier [22].
Table 1.
Comparison of Dynamic Mechanical Testing Parameters (at 25°C)
| Hydrogel | Saline (1 M) | Water | |
|---|---|---|---|
| Viscosity (cP) | 2360 ± 330 | 0.9742 | 0.89031 |
| Storage Modulus, G' (Pa) | 14.85 ± 2.09 | N/A | N/A |
| Loss Modulus, G" (Pa) | 0.37 ± 0.06 | N/A | N/A |
Korson, L., W. Drost-Hansen, and F.J. Millero, Visocsity of water at various temperatures. J Phys Chem, 1969. 73(1): p. 34–39.
Aleksandrov, A.A., E.V. Dzhuraeva, V.F. Utenkov, Viscosity of aqueous solutions of sodium chloride. High Temp, 2012. 50(3): p. 354–358.
Saline as a carrier for cell-based CED fails to deliver CAR T cells
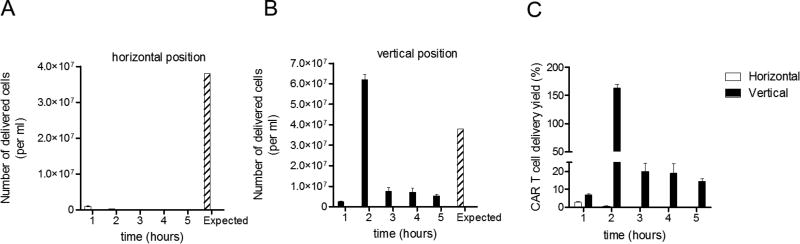
Saline has been the carrier of choice for small chemotherapeutic agent delivery via CED. In light of our interest in delivering therapeutic cells to the brain, and the known advantages of increased infusion dose and distribution that CED offers, we decided to evaluate the efficiency of saline as a carrier for the delivery of CAR T cells. We positioned the infusion pump and extension tube system in a horizontal orientation as it is commonplace in the clinic. To our surprise, a minor fraction of the total CAR T cells resuspended in saline were capable of being delivered. While 3.8 × 107 cells/ml were expected to be delivered per hour, less than ~1.0 × 106 cells/ml were delivered per hour (Figure 1A). In order to overcome this problem, we evaluated the impact of rotating the infusion system to a vertical position; however, an erratic rather than continuous delivery was observed, where the number of cells delivered ranged between ~2.5×106 – 6.0×107 cells/ml in each hour (Figure 1B). This vertical position created large clots that intermittently blocked cellular flow and failed to provide uniform delivery. When the efficiency of T cell delivery per hour was calculated, only 2–4% of the T cells were effectively delivered when the pump was positioned horizontally, and a much more variable efficiency, 7–150%, was obtained when the pump was in a vertical position (Figure 1C). Altogether, our data demonstrate that saline is not a suitable carrier for CED of CAR T cells, regardless of pump positioning. The failure of saline as a carrier for CED was largely due to the sedimentation of CAR T cells.
Figure 1. Saline as a carrier for cell-based CED fails to deliver CAR T cells.
MedFusion 3010a syringe clinical pumps were loaded with a 20cc syringe containing a saline solution of 3.8 × 107 T cells/ml. This was connected to a 20 cm long coiled silicon extension tube. These pumps were placed either horizontally (A) or vertically (B) and the infusion rate was adjusted to 500 µl/hr. All infusion procedures were performed for 5 hours at room temperature to replicate a clinical environment. Collected infusates after CED were transferred to a 1.7 ml eppendorf tube and counted using trypan blue exclusion method. The Expected value indicates the theoretical yield of 100% delivery efficiency. C) Comparison of the efficiency of CAR T cell delivery (yield) from horizontally and vertically positioned infusion pumps. CAR T cell delivery yield (%) was calculated: (CAR T cells counted in infusate per ml per hour/Expected (3.8 × 107 T cells/ml per hour)) × 100.
Hyaluronic acid based LVHydrogel increases the CAR T cell delivery rate of CED
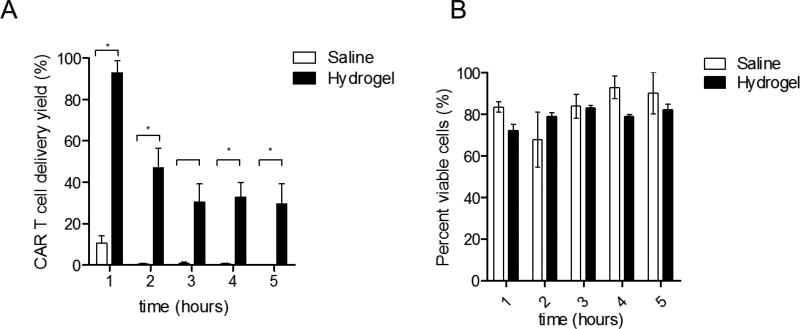
In order to solve the sedimentation of CAR T cells in saline and to generate both a high yield and a homogenous distribution of delivered CAR T cells, we developed a non-toxic, biodegradable low viscosity LVHydrogel carrier. To evaluate the delivery capacity of LVHydrogel, T cells were resuspended in LVHydrogel or control saline for 5-hour CED with the pump placed in a horizontal position. The efficiency of cell delivery in saline was dismal with less than ~10% delivered in the first hour, and ~0.5% delivered at later hours (Figure 2A, *p < 0.05). In contrast, LVHydrogel efficiency of delivery was significantly higher. During the first hour the efficiency of CAR T cell delivery in LVHydrogel reached over ~90%, while at the later hours the delivery seen was ~40% (Figure 2A). Of note, viability of the delivered T cells was not compromised during the CED procedure in either saline or LVHydrogel (Figure 2B, p > 0.23 NS). These data demonstrate that LVHydrogel as a carrier increases the efficiency of continuously delivered CAR T cells during the CED procedure, making this an ideal carrier in the context of CED.
Figure 2. Hyaluronic acid based LVHydrogel increases the CAR T cell delivery rate of CED.
MedFusion 3010a syringe clinical pumps were loaded with a 20cc syringe containing either a saline solution or LVHydrogel containing 1.2×107–1.7×107 CAR T cells/ml, and submitted to a 5 hour infusion procedure at room temperature. Collected infusates after CED were transferred to a 1.7 ml eppendorf tube and counted using trypan blue exclusion method. A) The efficiency of CAR T cell delivery (yield) in different carriers was calculated: CAR T cell delivery yield (%) was calculated: (CAR T cells counted in infusate per ml per hour/Expected value assuming 100% delivery efficiency. The Expected value was the concentration of CAR T cells at the time of infusion per hour)) × 100. B) Viability of the CAR T cells present in infusate was determined using the trypan blue exclusion method. Percent viable cells was calculated: (Live cells/(Live+Dead cells)) × 100. Statistical analysis was performed using the Bonferroni-corrected t-test. Significance was determined at the level of *p < 0.05.
Effective migration and tumor specific killing can be achieved with CED of CAR T cells in LVHydrogel
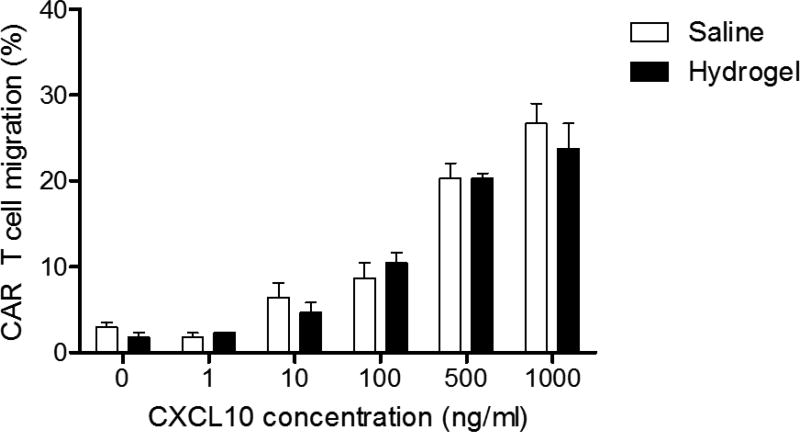
In order for CAR T cells to effectively mediate tumor killing, these cells need to be able to migrate out of the LVHydrogel carrier matrix. To evaluate the impact of LVHydrogel on CAR T cell migration, CAR T cells were resuspended in LVHydrogel or control saline and submitted to in vitro migration. CAR T cells in LVHydrogel displayed an equivalent migration capacity compared to those in saline carrier (Figure 3, p = 0.5431 NS). These data demonstrate that CAR T cells can effectively migrate out of the LVHydrogel.
Figure 3. Effective Migration Assay of Human CAR T cells in LVHydrogel.
The lower chambers of 24-well transwell plates were loaded with media containing the human chemokine CXCL10 at the indicated concentrations: 1 ng/ml, 10ng/ml, 100 ng/ml, 500 ng/ml and 1000 ng/ml. The upper chamber of each well was seeded with serum starved 6 × 105 CAR T cells in either saline or LVHydrogel carriers. Transwell plates were then incubated at 37°C for 12 hours. Plates were then removed from the incubator and the cells in the lower chamber of the transwell were counted in a hemocytometer using trypan blue exclusion counting method. Percentage of migration by CAR T cells was calculated by the following formula: CAR T cell migration (%) = ((Total number of cells that migrated to lower chamber)/(Total number of cells loaded in upper chamber)) × 100. Statistical analysis was performed using the quadratic interaction term between log-CXCL concentration and group in the regression model. Significance was determined at the level of *p < 0.05.
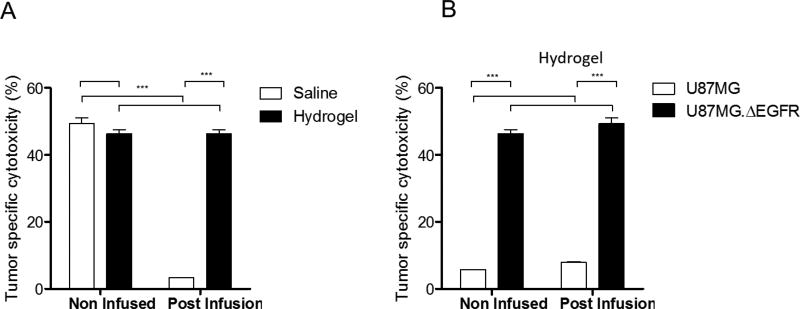
Having demonstrated that CAR T cells have the capacity to migrate out of the LVHydrogel to the same extent as saline, we next investigated if CAR T cells delivered via CED in LVHydrogel or saline can effectively mediate tumor cell killing. After 5-hour CED, CAR T cells from LVHydrogel or saline infusate were incubated with chromium labeled glioma cells bearing the CAR tumor-target in a standard cytotoxicity assay. Cytotoxicity of fresh cells prior to CED was also evaluated as a control. Prior to CED, the cytotoxicity of all CAR T cells was equivalent as expected. However, after CED, CAR T cells from LVHydrogel exhibited significantly superior tumor-specific killing of glioma cells versus saline (Figure 4A,***p < 0.0001). Furthermore, the cytotoxicity of CAR T cells from LVHydrogel after CED was similar to the fresh non-infused CAR T cells in LVHydrogel, demonstrating that the CED process of delivery did not impair CAR T cell cytotoxicity. In contrast, the cytotoxicity of the infused CAR T cells resuspended in saline was much lower than the fresh CAR T cells in saline. This significant reduction was due to the drastic decrease of CAR T cell delivery observed when saline was used as a carrier. Of note, while the recognition and killing of the tumor by CAR T cells delivered in LVHydrogel still required target-specific engagement of the CAR with the tumor, glioma cells that did not express the CAR target EGFRvIII were not killed either before or after CED in LVHydrogel (Figure 4B, p = 0.7176 NS). Altogether, these data demonstrate that CAR T cells delivered in LVHydrogel retain tumor-specific recognition and cytotoxicity to effectively kill tumor.
Figure 4. Tumor specific killing can be achieved with CED of CAR T cells in LVHydrogel.
A cytotoxicity assay was utilized to evaluate the killing capacity of CAR T cells resuspended in saline or LVHydrogel. A) CAR T cells were submitted to the CED procedure and the infusate was collected at the end of the 5 hours infusion procedure and cocultured with Chromiun51 labelled U87MG.ΔEGFR glioma cells expressing the CAR T cell target antigen. CAR T cells resuspended in Fresh saline or LVHydrogel were prepared right before coculture with the glioma tumor cells. B) To determine whether the tumor cell killing seen in the LVHydrogel group retain CAR T cell specificity U87MG glioma cells lacking the EGFRvIII antigen were utilized and compared to those containing U87MG.ΔEGFR. For both cytotoxicity assays CAR T cells and glioma tumor cells were cocultured at a 10:1 ratio for at 4 hours at 37°C. Supernatant was collected and Chromiun51 was measure. Tumor specific cytotoxicity (%) = ((sample lysis ‒ spontaneous)/ (maximum lysis ‒ spontaneous)) × 100. Statistical analysis was performed using 2-way ANOVA model. Significance was determined at the level of *p < 0.05, ***p< 0.0001.
LVHdrogel has no acute toxicity on brain parenchyme when compared to saline
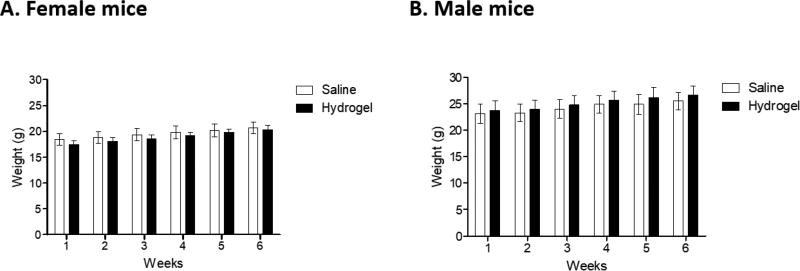
In order to evaluate the safety and potential toxicity associated with intracranial infusion of LVHydrogel into brain parenchyma, 6–8 week old, C57 BL6 mice received 24 µL saline or LVHydrogel into the caudate nucleus. There was no difference in the weight gained in the males or females in either test group during the six week observation period (Figure 5). The only histopathology seen was a small collection of lymphocytes that was seen in both the saline control (4/10 males, 0/10 females) and the hydrogel (2/10 males, 0/10 females) treatment groups. In the female saline group there was one possible hemorrhage and a possible unilateral focus of demyelination in the corpus collosum. No other pathology or demyelination was noted after pathology examination. Therefore, administration of LVHydrogel could be an advantageous delivery matrix over standard saline for CED without compromising safety.
Figure 5. Mice receiving LVHydrogel or saline display equal weight overtime.
Non-tumor bearing 6–8 week old-mice (n=20 mice; 10 females (A) and 10 males (B) per group) received 24 µL saline or LVHydrogel into the caudate nucleus. The mice were weighed weekly and observed for six weeks. No significant differences in weight were observed between mice receiving LVHydrogel or saline. Statistical analysis was performed using 2-way ANOVA model. Significance was determined at the level of *p < 0.05.
Discussion
In this study we have demonstrated that LVHydrogel enables the continuous infusion of CAR T cells with high efficiency and no negative effect to therapeutic function. We found the CED process is greatly affected by the sedimentation of the cells in saline, which is compounded by the inner diameter of the syringes, extension tubes, infusion time, infusion rate and pump position. We tried to correct the sedimentation problem by rotating the CED pump into a vertical position and thus theoretically preventing sedimentation inside the syringe. However, our experiments demonstrated that the cells continued to sediment inside the extension tubing and syringe over 5 hours of infusion period. Most of the cells were irregularly infused within the first and second hour of CED, and some portions of the infusate contained cell clots which eventually disrupted the infusion and required flushing of the extension tubing. Conversely, sedimentation of cells in LVHydrogel was minimum. Our data indicates that regardless of the syringe and extension tube used in the CED system, LVHydrogel provides much higher yield during infusion without damaging migration capacity or cytotoxicity of CAR T cells.
Previously used injectable LVHydrogels were based on the premise of in situ gelation post injection [23, 24], which had two major technical drawbacks for cellular delivery of therapeutic cells into brain tumors. First, gelation might cause a mass effect in the intracranial area, which presents a safety risk due to damage to the eloquent brain. Second, the gelation process will result in a viscous LVHydrogel that limits the ability of the CAR T cells to migrate out of the carrier matrix to the parenchyma thereby minimizing their cytotoxic effect [25]. LVHydrogel overcomes these limitations by phasing into a less viscous shape at body temperature thereby letting CAR T cells escape from the scaffold. Therefore, utilization of LVHydrogel as a carrier for CED could very well be applicable for CAR T cell therapy, as well as other cellular therapies which might require migration out of the scaffold in order to mediate their therapeutic function.
Furthermore, the chemical components of LVHydrogel are compatible with human tissues, including the brain, the potential adverse effects are minimal and CED of cellular therapeutics has the potential to improve brain tumor therapy through the localized delivery of highly functional tumor-specific effectors. This safety profile and the existence of Good Manufacture Practices grade LVHydrogels could theoretically allow for the rapid implementation of LVHydrogel as a cell-carrier for CED in patients with glioblastoma.
Highlights.
Convection Enhanced Delivery of CAR T cells was not possible due to technical difficulties such as sedimentation, clogging of tubes, low viability rate.
Low viscosity hydrogel is a hyaluronic based material that can be degraded by macrophages inside the brain and it is similar to brain extracellular matrix
We successfully increased the delivery rate of CAR T cells x20 by using Low Viscosity Hydrogel with Convection Enhanced Delivery system
Cytotoxicity capacity of CAR T cells are not negatively affected by hydrogel after infusion, also migration capacity of CAR T cells also preserved.
This hydrogel showed no toxicity to brain and was shown to be fully digested in preclinical rodent models 2 weeks after injection.
Acknowledgments
Financial support: This work was supported by funding from the National Institutes of Health: 5R01-NS085412-04 (J.H. Sampson), 5R01-CA177476-04 (J.H. Sampson) 5R01-NS086943-03 (J.H. Sampson), 4P01-CA154291-05 (D.D. Bigner and J.H. Sampson), 5U01-NS090284-02 (J.H. Sampson), 5R25-NS065731-08 (J.H. Sampson), and 5P50-CA190991-02 (J.H. Sampson). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kanu OO, Mehta A, Di C, Lin N, Bortoff K, Bigner DD, et al. Glioblastoma multiforme: a review of therapeutic targets. Expert Opin Ther Targets. 2009;13:701–18. doi: 10.1517/14728220902942348. [DOI] [PubMed] [Google Scholar]
- 2.Imperato JP, Paleologos NA, Vick NA. Effects of treatment on long-term survivors with malignant astrocytomas. Annals of neurology. 1990;28:818–22. doi: 10.1002/ana.410280614. [DOI] [PubMed] [Google Scholar]
- 3.Misra A, Ganesh S, Shahiwala A, Shah SP. Drug delivery to the central nervous system: a review. Journal of pharmacy & pharmaceutical sciences : a publication of the Canadian Society for Pharmaceutical Sciences, Societe canadienne des sciences pharmaceutiques. 2003;6:252–73. [PubMed] [Google Scholar]
- 4.Raghavan R, Brady ML, Rodriguez-Ponce MI, Hartlep A, Pedain C, Sampson JH. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurgical focus. 2006;20:E12. doi: 10.3171/foc.2006.20.4.7. [DOI] [PubMed] [Google Scholar]
- 5.Chaichana KL, Pinheiro L, Brem H. Delivery of local therapeutics to the brain: working toward advancing treatment for malignant gliomas. Ther Deliv. 2015;6:353–69. doi: 10.4155/tde.14.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashby LS, Smith KA, Stea B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol. 2016;14:225. doi: 10.1186/s12957-016-0975-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Riccione K, Suryadevara CM, Snyder D, Cui X, Sampson JH, Sanchez-Perez L. Generation of CAR T cells for adoptive therapy in the context of glioblastoma standard of care. J Vis Exp. 2015 doi: 10.3791/52397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miao H, Choi BD, Suryadevara CM, Sanchez-Perez L, Yang S, De Leon G, et al. EGFRvIII-specific chimeric antigen receptor T cells migrate to and kill tumor deposits infiltrating the brain parenchyma in an invasive xenograft model of glioblastoma. PLoS One. 2014;9:e94281. doi: 10.1371/journal.pone.0094281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampson JH, Choi BD, Sanchez-Perez L, Suryadevara CM, Snyder DJ, Flores CT, et al. EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res. 2014;20:972–84. doi: 10.1158/1078-0432.CCR-13-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi BD, Suryadevara CM, Gedeon PC, Herndon JE, 2nd, Sanchez-Perez L, Bigner DD, et al. Intracerebral delivery of a third generation EGFRvIII-specific chimeric antigen receptor is efficacious against human glioma. J Clin Neurosci. 2014;21:189–90. doi: 10.1016/j.jocn.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prins RM, Shu CJ, Radu CG, Vo DD, Khan-Farooqi H, Soto H, et al. Anti-tumor activity and trafficking of self, tumor-specific T cells against tumors located in the brain. Cancer Immunol Immunother. 2008;57:1279–89. doi: 10.1007/s00262-008-0461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL, et al. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989;7:250–61. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- 13.Balyasnikova IV, Wainwright DA, Solomaha E, Lee G, Han Y, Thaci B, et al. Characterization and immunotherapeutic implications for a novel antibody targeting interleukin (IL)-13 receptor alpha2. J Biol Chem. 2012;287:30215–27. doi: 10.1074/jbc.M112.370015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, et al. CAR-Engineered NK Cells Targeting Wild-Type EGFR and EGFRvIII Enhance Killing of Glioblastoma and Patient-Derived Glioblastoma Stem Cells. Sci Rep. 2015;5:11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med. 2016;375:2561–9. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A. 1994;91:7727–31. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kauer TM, Figueiredo JL, Hingtgen S, Shah K. Encapsulated therapeutic stem cells implanted in the tumor resection cavity induce cell death in gliomas. Nat Neurosci. 2011;15:197–204. doi: 10.1038/nn.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–81. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 19.Muller M, Carter S, Hofer MJ, Campbell IL. Review: The chemokine receptor CXCR3 and its ligands CXCL9, CXCL10 and CXCL11 in neuroimmunity--a tale of conflict and conundrum. Neuropathol Appl Neurobiol. 2010;36:368–87. doi: 10.1111/j.1365-2990.2010.01089.x. [DOI] [PubMed] [Google Scholar]
- 20.Aleksandrov AA, Dzhuraeva EV, Utenkov VF. Viscosity of aqueous solutions of sodium chloride. High Temp+ 2012;50:354–8. [Google Scholar]
- 21.Korson L, Drost-Hansen W, Millero FJ. Viscosity of water at various temperatures. The Journal of Physical Chemistry. 1969;73:34–9. [Google Scholar]
- 22.Almdal K, Dyre J, Hvidt S, Kramer O. Towards a phenomenological definition of the term ‘gel’. Polymer Gels and Networks. 1993;1:5–17. [Google Scholar]
- 23.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217–27. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu L, Ding J. Injectable hydrogels as unique biomedical materials. Chemical Society reviews. 2008;37:1473–81. doi: 10.1039/b713009k. [DOI] [PubMed] [Google Scholar]
- 25.Tsao CT, Kievit FM, Ravanpay A, Erickson AE, Jensen MC, Ellenbogen RG, et al. Thermoreversible poly(ethylene glycol)-g-chitosan hydrogel as a therapeutic T lymphocyte depot for localized glioblastoma immunotherapy. Biomacromolecules. 2014;15:2656–62. doi: 10.1021/bm500502n. [DOI] [PMC free article] [PubMed] [Google Scholar]