Abstract
Background:
Timely pre-exposure prophylaxis (PrEP) initiation is critical in at-risk populations given that HIV acquisition risk persists during delays. Time to treatment initiation, a key metric in HIV care, has not been explored among PrEP users. Interventions that reduce time to PrEP initiation could prevent HIV infections.
Setting:
Individuals initiating PrEP in a large, primary care health network of 15 clinics, the San Francisco Primary Care Clinics (SFPCC) from July 2012-July 2017 (N=411).
Methods:
We examined factors associated with time from first PrEP discussion with a provider to PrEP initiation date using an adjusted Cox proportional-hazards model, with hazard ratios (HR) >1 indicating earlier initiation. We also examined the relationship between delayed PrEP initiation and PrEP persistence (staying on PrEP) in an adjusted Cox proportional-hazards model.
Results:
PrEP users initiated PrEP after a median of only 7 days. However, there were notable outliers, with 29% waiting >30 days and 12% waiting >90 days. In an adjusted proportional-hazards model, a panel management and patient navigation intervention was associated with earlier PrEP initiation (HR: 1.5; 95% CI: 1.1–2.0), while only other race/ethnicity compared to White race was associated with delayed PrEP initiation (HR:0.7; 0.5–1.0). Delayed PrEP initiation >30 days was associated with shorter PrEP persistence in an adjusted proportional-hazards model (HR: 1.3; 1.0–1.7).
Conclusion:
PrEP initiation within a week is feasible in a primary care safety-net health system. Setting a goal of rapid PrEP initiation, with the support of panel management and patient navigation, could address delays in at-risk groups.
Keywords: Pre-exposure prophylaxis, PrEP, panel management, patient navigation, rapid treatment initiation, delayed PrEP initiation
Introduction:
Timely pre-exposure prophylaxis (PrEP) initiation is critical in at-risk populations given that HIV acquisition risk persists during delays. In PROUD, a pragmatic trial which randomized participants to immediate or delayed PrEP, HIV incidence in the delayed arm was 9 per 100 person-years compared to 1 in the immediate group.1 Patients who ask to initiate PrEP may do so at times of elevated HIV risk.2 Furthermore, factors motivating providers to offer PrEP, such as a sexually transmitted infection (STI) diagnosis, reflect proximate HIV risk.3 Delays in PrEP initiation should be avoided as they represent missed opportunities to prevent HIV acquisition during risk intervals.
Initiating PrEP quickly may have indirect benefits that encourage PrEP persistence (staying on PrEP) as has been seen in HIV treatment.4–7 Individuals who have streamlined PrEP initiation may experience enhanced self-efficacy, encouraging continued engagement.8,9 Furthermore, individuals with delayed initiation may experience “temporal discounting,” a behavioral economics concept which describes the tendency to discount benefits in the future compared to the present, particularly when the future benefit requires overcoming immediate barriers.10
Panel management is a population-based care approach that proactively focuses on the health of the population of patients assigned to a clinic. We previously found that panel management interventions were associated with greater completion of PrEP lab monitoring.11 We examined factors associated with earlier PrEP initiation in a safety-net health care system of 15 clinics, the San Francisco Public Health Primary Care Clinics (SFPCC). We hypothesized that initiating PrEP with panel management/patient navigation, which utilizes a PrEP registry to generate visit reminders, allows standing laboratory orders, and provides access to a navigator, would be associated with earlier PrEP initiation. As a secondary outcome, we measured if delays in PrEP initiation >30 days in the SFPCC were associated with shorter PrEP persistence.
Methods:
We analyzed data for patients prescribed PrEP in the SFPCC from July 1st 2012 until July 31st, 2017 (n=411). We could not capture individuals who were interested but weren’t prescribed PrEP. We obtained demographic, visit data, and PrEP prescriptions via chart abstraction and e-prescription databases. PrEP indication was classified by the following descending hierarchy: sero-different relationship, men having sex with men (MSM), transgender women having sex with men (TGWSM), people who inject drugs (PWID), or at-risk heterosexual. A panel management/patient navigation intervention, initiated in 2015 in 4 of 15 clinics in response to seroconversions at higher-volume PrEP clinics, included creating PrEP patient registries using a shared spreadsheet, routinizing follow-up/lab reminders, making a pharmacist available for follow-up visits, and providing patient navigators for both in-person and text messaging (SMS) assistance. The navigators set a goal of <72 hours for PrEP initiation, or one week if there were insurance or other barriers.
We determined the date of initial PrEP discussion by reviewing all visits in this cohort in which PrEP or HIV prevention medication was discussed. We collected PrEP start/stop dates as documented by the provider. For the fewer than 20% of records in which a start date was not available, we used the date a PrEP prescription was sent. The primary outcome was time from first documented PrEP discussion to initiation. We used time to PrEP initiation rather than time to PrEP prescription to best approximate the at-risk period in which the patient requested or the provider recommended PrEP but PrEP was not yet of benefit. As a secondary outcome, we assessed PrEP persistence defined by time between PrEP start/stop dates, with discontinuation defined as >90 days without restarting PrEP. For the less than one-fifth of records in which a stop date was unavailable, discontinuation was defined as 90 days not covered by an active prescription including refills.
Using a Cox-proportional hazards model, we evaluated the association of factors including demographics, PrEP indication, provider panel size, total prescription duration (e.g. 30 pills with 3 refills was coded as >90 days), year, and insurance status, with time from PrEP discussion to initiation. The panel management/navigation variable was coded at the clinic level as a time-dependent covariate, based on when the program started at each clinic. Hazard ratios greater than 1 indicate earlier PrEP initiation. We tested for violations of the proportional hazards assumption by examining correlation between time and scaled Schoenfeld residuals.
To explore incomplete documentation of PrEP initiation date’s effect on the outcome of delayed initiation, we performed a sensitivity analysis using an alternate outcome of time from PrEP discussion to first prescription. We performed a second sensitivity analysis controlling for clinic sites receiving the panel management intervention to explore if clinic-related factors besides the intervention could account for the relationship between panel management and time to PrEP initiation. As a final sensitivity analysis, we re-estimated the primary model substituting indicators for each PrEP indication (allowing more than one to be positive) for the original hierarchical classification.
To evaluate the impact of delayed PrEP initiation (defined as >30 days in this specific analysis) on later PrEP persistence, we used a Cox-proportional hazards model, controlling for patient age, sex at birth, race/ethnicity, PrEP indication, year of initiation, and total PrEP prescription duration, including refills.
Results:
During the study period, 411 patients across SFPCC received PrEP. Most (85%) were male sex at birth, with a median age of 34 (Table). The cohort was racially/ethnically diverse: 13% were African-American, 8% Asian, 27% Latino, 17% other, and 36% White. Overall, 16% had a primary PrEP indication of sero-different relationship, 65% as an MSM, 1% PWID, 13% TGWSM, and 5% at-risk heterosexual. PrEP users received panel management/patient navigation support for 27% of starts in 4 clinics beginning in 2015 (Table). Navigators estimated that all PrEP initiators in the post-intervention intervals received some component of the intervention, and no SFPCC PrEP users were missing from the PrEP registries. There were three HIV seroconversions among the 411: one prescribed but who had not yet started taking PrEP due to side effects concerns, the second was using intermittent PrEP at non-recommended frequencies, and the third had discontinued PrEP due to self-perceived low risk.
Table:
Characteristics of San Francisco Primary Care Clinics pre-exposure prophylaxis (PrEP) users and factors predictive of earlier PrEP initiation in a Cox proportional-hazards model
| Characteristic (N=1411) | Overall | Adjusted HR for Earlier PrEP Initiation (95% CI) | p-value | |
|---|---|---|---|---|
| Age, median; IQR | 34; 28–46 | 1.00 (0.99–1.00) | 0.34 | |
| Male Sex at Birth | 85% | 0.91 (0.65–1.30) | 0.62 | |
| Race/ethnicity: | White | 36% | Ref. | - |
| Asian | 8% | 1.09 (0.70–1.60) | 0.78 | |
| Black | 13% | 0.75 (0.54–1.04) | 0.09 | |
| Latino | 27% | 0.98 (0.74–1.30) | 0.89 | |
| other | 17% | 0.71 (0.53–0.96) | 0.03 | |
| PrEP patients per provider: | 1 | 19% | Ref. | - |
| 2–4 | 26% | 0.95 (0.69–1.31) | 0.75 | |
| 5+ | 56% | 0.90 (0.68–1.20) | 0.48 | |
| Year of PrEP Initiation : | 2012–14 | 17% | Ref. | - |
| 2015 | 29% | 0.97 (0.71–1.32) | 0.85 | |
| 2016 | 42% | 0.82 (0.59–1.14) | 0.25 | |
| 2017 (until 7/31) | 13% | 0.75 (0.49–1.14) | 0.18 | |
| Indication for PrEP: | MSM | 65% | Ref. | - |
| At-risk heterosexual | 5% | 1.42 (0.82–2.47) | 0.21 | |
| PWID | 1% | 1.24 (0.49–3.15) | 0.65 | |
| Sero-different couple | 16% | 1.11 (0.82–1.52) | 0.49 | |
| TGWSM | 13% | 1.04 (0.72–1.49) | 0.83 | |
| Insurance: | uninsured | 14% | Ref. | - |
| public | 79% | 1.00 (0.61–1.65) | 0.30 | |
| private | 7% | 0.82 (0.54–1.24) | 0.34 | |
| Panel management/patient navigation support | 27% | 1.48 (1.10–2.00) | 0.01 | |
HR: Hazard Ratio; PWID: people who inject drugs; MSM: men having sex with men; TGWSM: transgender women having sex with men
Delays in PrEP Initiation
Median time from documented PrEP discussion to initiation was 7 days. However, a substantial minority of individuals experienced delayed PrEP initiation. Overall, 29% waited >30 days to initiate PrEP and 12% waited >90 days. Reasons for delay >30 days included: 49% provider [i.e. delaying to review labs/adherence at future visits or deferring to the primary care provider (PCP)]; 33% patient factors or concerns (including 12% no show visits, 10% concern if at risk, 9% concern about side effects); and 18% insurance or pharmacy issues.
The only factor associated with earlier PrEP initiation in the adjusted model was panel management/navigation (HR:1.5; 95% Confidence Interval (95%CI):1.1–2.0), while other race/ethnicity was associated with later PrEP initiation (HR:0.7; 95% CI:0.5–1.0), with a trend towards later PrEP initiation in African-Americans (HR 0.8; 95% CI:0.5–1.0; both vs. White race; Table). The association of panel management/patient navigation with earlier PrEP initiation did not differ among sub-groups of race/ethnicity (overall heterogeneity p=0.17).
Overall, median PrEP persistence was 6 months. In adjusted models, PrEP initiation delays >30 days were associated with higher PrEP discontinuation (HR:1.3; 95% CI:1.0–1.7).
In our first sensitivity analysis using time to PrEP prescription rather than documented PrEP initiation date, the median time to PrEP prescription was 6 days, and the estimated effect of panel management was slightly stronger (HR:1.6; 95%CI:1.2–2.1); results were otherwise unchanged. The second analysis revealed no confounding of panel management/navigation’s effect by fixed characteristics of the four sites that introduced it (HR remained 1.5; 95%CI:1.1–2.2). In the third sensitivity analysis which allowed multiple PrEP indications rather than a hierarchy, the effect of panel management did not change (HR: 1.5; 95% CI:1.1–2.0)
Discussions:
In a diverse sample of 411 PrEP users at 15 clinics within an urban safety-net system, the median delay to PrEP initiation was one week. Given challenges around access that publicly-insured and uninsured individuals face, this achievement is remarkable. Our data indicate that even within the primary care safety-net, PrEP initiation in a week is feasible, a timeframe which should be emulated in other settings.
In spite of this achievement, nearly a third of patients waited >30 days to start PrEP, with 12% waiting >90 days. Patients with delayed PrEP initiation may be at risk of HIV infection, and, indeed, one SFPCC client seroconverted during delayed PrEP initiation.1 Although we observed a trend towards delayed PrEP initiation among African-Americans, this was not statistically significant in the adjusted model, and the mechanism of delay in those with other race/ethnicity is unclear. Outcomes in PrEP users should be examined by race/ethnicity given observed disparities in PrEP uptake among African Americans in other studies.12
Approximately one-third of delays were related to patient concerns or contemplation. Providers must navigate tensions between respect for patient autonomy vs. expedient initiation based on the known efficacy and safety of PrEP. This period of patient doubt has been termed “PrEP contemplation without initiation,” and may be amenable to provider or navigator counseling or other interventions.13 Finally, the majority of delays were related to systems issues including waiting for a follow-up visit to review labs, deferring PrEP initiation to the PCP, or prior authorization or coverage gaps. As patient willingness to initiate PrEP may wax and wane over time, we should take advantage of opportunities to initiate PrEP when patient interest is high and provide mechanisms to address visit availability, insurance, pharmacy issues, or patient concerns expeditiously.13
Panel management approaches to PrEP delivery have been implemented in specialty PrEP programs and STD clinics.14–16 Our analysis is the first to examine factors associated with PrEP initiation in a real-world setting and to identify that a panel management/patient navigation program is associated with earlier PrEP initiation. Panel management/patient navigation may have led to earlier PrEP initiation by addressing insurance barriers, providing automatic reminders to providers to send PrEP prescriptions, and responding to patient concerns expeditiously by SMS messaging with navigators. Panel management approaches may be well-suited to PrEP roll-out in primary care settings where PrEP experience among providers may vary.17
Recent and ongoing HIV research has supported rapid antiretroviral initiation given high acceptance, improved care retention, and increased rates of viral suppression, with community prevention benefits.4–7 Analogously, setting a goal of PrEP initiation within a week can decrease HIV risk intervals, and may facilitate PrEP persistence, although this association requires additional study. Use of same-day HIV testing and follow-up of initial labs, support of navigators to negotiate insurance and other barriers, and using panel management staff to identify missing labs, prescriptions, or follow-up visits could facilitate this approach. Rapid fourth-generation HIV-testing and point-of-care creatinine and hepatitis B surface antigen testing could allow for provision of the first dose of PrEP on the day of discussion.18 Health systems should track delayed initiation, which could be automated via ICD-10 codes and structured medical record fields, to identify disparities in initiation time by subgroup and areas for improvement.
Limitations of the study include inability to analyze undocumented data or to distinguish which components of the panel management/navigation intervention were associated with earlier initiation. Moreover, our data has limited generalizability to populations not within a primary care, safety-net setting in a Medicaid expansion state. Although it is possible that we underestimated time to PrEP initiation due to incomplete documentation, we would predict that incomplete documentation on average would bias panel management/navigation’s association with earlier PrEP initiation towards the null given that PrEP users who received the intervention had increased healthcare contact and PrEP documentation.
Although we identified delays in PrEP initiation in a minority of PrEP users in a safety-net primary care clinic system, the median time to PrEP initiation in a diverse population was just one week. Moreover, our data suggests that PrEP panel management/navigation could assist the near one-third of our sample who experienced delays greater than a month. Interventions that reduce delays in PrEP initiation are critical because they can potentially reduce HIV seroconversion risk. Further research should examine if same day or early PrEP initiation, potentially with the support of panel management and patient navigation, could improve PrEP outcomes such as adherence and persistence in a randomized clinical trial.
Figure:
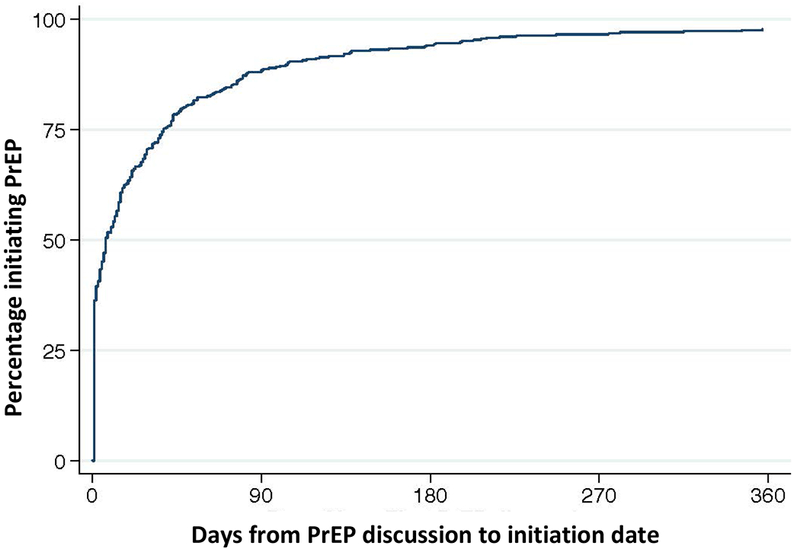
The Kaplan-Meier curve depicts time from the first discussion of pre-exposure prophylaxis (PrEP) between a patient and provider to the reported PrEP initiation date in patients who were prescribed PrEP in the San Francisco Primary Care Clinics (SFPCC) from July 2017 to July 2017.
Acknowledgements:
We would like to acknowledge Hali Hammer and Catherine James for programmatic support of the PrEP registry, Anne Hirozawa and Patricia von Felten for assistance with data collection and management, Miranda Nordell for her description and feedback on the patient navigation program, and Elvin Geng for review of the manuscript.
Footnotes
Conflicts of Interest and Sources of Funding: The authors have no conflicts of interest to report. This work was supported by the National Institute of Mental Health at the National Institute of Health [R01MH109320].
References:
- 1.McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z, Lau JTF, Fang Y, Ip M, Gross DL. Prevalence of actual uptake and willingness to use pre-exposure prophylaxis to prevent HIV acquisition among men who have sex with men in Hong Kong, China. PLoS One. 2018;13(2):e0191671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castel AD, Feaster DJ, Tang W, et al. Understanding HIV Care Provider Attitudes Regarding Intentions to Prescribe PrEP. J Acquir Immune Defic Syndr. 2015;70(5):520–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koenig SP, Dorvil N, Devieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomized unblinded trial. PLoS Med. 2017;14(7):e1002357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amanyire G, Semitala FC, Namusobya J, et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: a stepped-wedge cluster-randomised trial. Lancet HIV. 2016;3(11):e539–e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilcher CD, Ospina-Norvell C, Dasgupta A, et al. The Effect of Same-Day Observed Initiation of Antiretroviral Therapy on HIV Viral Load and Treatment Outcomes in a US Public Health Setting. J Acquir Immune Defic Syndr. 2017;74(1):44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosen S, Maskew M, Fox MP, et al. Initiating Antiretroviral Therapy for HIV at a Patient’s First Clinic Visit: The RapIT Randomized Controlled Trial. PLoS Med. 2016;13(5):e1002015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jackson T, Huang A, Chen H, Gao X, Zhang Y, Zhong X. Predictors of willingness to use HIV pre-exposure prophylaxis among female sex workers in Southwest China. AIDS Care. 2013;25(5):601–605. [DOI] [PubMed] [Google Scholar]
- 9.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Story GW, Vlaev I, Seymour B, Darzi A, Dolan RJ. Does temporal discounting explain unhealthy behavior? A systematic review and reinforcement learning perspective. Front Behav Neurosci. 2014;8:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spinelli MA, Scott HM, Vittinghoff E, et al. Provider Adherence to Pre-exposure Prophylaxis Monitoring Guidelines in a Large Primary Care Network. Open Forum Infect Dis. 2018;5(6):ofy099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott H, Hirozawa A, James C, Hammer H, Scheer S, Buchbinder SB. Disparities in PrEP uptake among primary care patients screened for HIV/STIs in SF [#1015]. Paper presented at: Conference on Retroviruses and Opportunistic Infections2018; Boston. [Google Scholar]
- 13.Serota DP, Rosenberg ES, Lockard AM, et al. Beyond the Biomedical: PrEP Failures in a Cohort of Young Black Men who have Sex with Men in Atlanta, GA. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan PA, Mena L, Patel R, et al. Retention in care outcomes for HIV pre-exposure prophylaxis implementation programmes among men who have sex with men in three US cities. J Int AIDS Soc. 2016;19(1):20903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volk JE, Marcus JL, Phengrasamy T, et al. No New HIV Infections With Increasing Use of HIV Preexposure Prophylaxis in a Clinical Practice Setting. Clin Infect Dis. 2015;61(10):1601–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hojilla JC, Vlahov D, Crouch PC, Dawson-Rose C, Freeborn K, Carrico A. HIV Pre-exposure Prophylaxis (PrEP) Uptake and Retention Among Men Who Have Sex with Men in a Community-Based Sexual Health Clinic. AIDS Behav. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marcus JL, Volk JE, Pinder J, et al. Successful Implementation of HIV Preexposure Prophylaxis: Lessons Learned From Three Clinical Settings. Curr HIV/AIDS Rep. 2016;13(2):116–124. [DOI] [PubMed] [Google Scholar]
- 18.Scott H HIV testing and linakge: the gateway to treatment and prevention [#61]. Paper presented at: Conference on Retroviruses and Opportunistic Infections2018; Boston. [Google Scholar]


