Abstract
Multiple myeloma (MM) is an age-related haematological malignancy with an estimated 30,000 new cases and 13,000 deaths per year. A disease of antibody-secreting malignant plasma B-cells that grow primarily in the bone marrow (BM), MM causes debilitating fractures, anaemia, renal failure, and hypercalcemia. In addition to the abnormal genetic profile of MM cells, the permissive BM microenvironment (BMM) supports MM pathogenesis. Although advances in treatment options have significantly enhanced survival in MM patients, transient perfusion of small molecule drugs in the BM does not provide sufficient residence to enhance MM cell-drug interaction, thus allowing some myeloma cells to escape the first line of treatment. As such, there remains a crucial need to develop advanced drug delivery systems that can navigate the complex BMM and effectively reach the myeloma cells. The high vascular density and spongy nature of bone structure suggest that nanoparticles can serve as smart drug-delivery systems capable of extravasation and retention in various BM compartments to exert a durable therapeutic effect. In this focus article, we first summarize the pathophysiology of MM, emphasizing how the BM niche presents serious challenges for effective treatment of MM with small molecule drugs. We then pivot to current efforts to develop nanoparticle-based drug carriers and intrinsically therapeutic nanotherapeutics. The article concludes with a brief perspective on the opportunities and challenges in developing and translating nanotherapeutics to improve the treatment outcomes of MM patients.
Graphical abstract
Targeting and eradicating multiple myeloma cells with nanotherapeutics: Nanoparticles can serve as smart drug-delivery systems capable of extravasation and retention in various bone marrow compartments to exert a durable therapeutic effect.
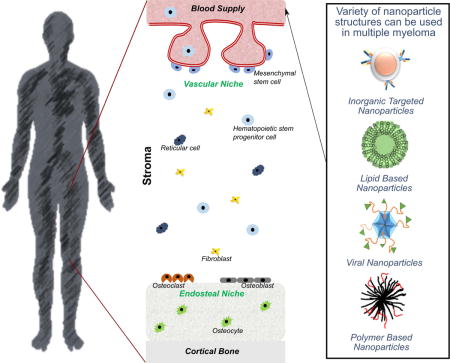
PATHOGENESIS OF MULTIPLE MYELOMA
Multiple myeloma (MM) is a hematologic malignancy of antibody-secreting malignant plasma B-cells that grow primarily in the bone marrow (BM) (Bergin, McQuilten, Moore, Wood, & Spencer, 2017; Landau, Carter, Getz, & Wu, 2014; Nishihori & Shain, 2017; Raffaghello, Vacca, Pistoia, & Ribatti, 2015; Ramdass, Chowdhary, & Koka, 2013; Rollig, Knop, & Bornhauser, 2015; San Miguel, 2014; Tamura, 2018; Terpos, Ntanasis-Stathopoulos, Gavriatopoulou, & Dimopoulos, 2018). BM is the anatomical site of haematopoiesis located in the trabeculae of vascularized bones (Anthony & Link, 2014; Ghobrial, Detappe, Anderson, & Steensma, 2018; Mu et al., 2018; Rankin, Narla, Park, Lin, & Sakamoto, 2015). The cellularity of BM is quite diverse and is generally organized into vascular, reticular, and endosteal niches, depending on the proximity of vasculature and osteoblasts (Figure 1) (Manier, Kawano, Bianchi, Roccaro, & Ghobrial, 2016; Ribatti, Nico, & Vacca, 2015; Tenreiro, Correia, & Brito, 2017). Hematopoietic cells localized in the BM interact extensively with the bone marrow stromal cells, such as fibroblasts and pericytes, and the cells that regulate bone morphology - osteoclasts, osteoblasts, and osteocytes (Furukawa & Kikuchi, 2015; Morrison & Scadden, 2014). Together with the extracellular matrix proteins, these cells comprise the bone marrow microenvironment (BMM) (Kawano et al., 2015).
Figure 1.
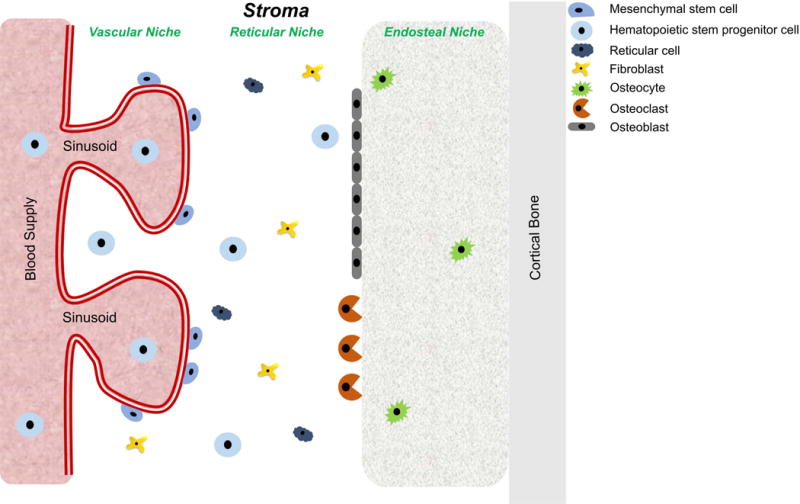
Schematic representation of bone marrow niches and cell types comprising the stroma.
Normally, the immature myeloid and lymphoid progenitor cells interact extensively with the BMM compartments that provide pro-survival and maturation signals (Papayannopoulou & Scadden, 2008; Park, Sykes, & Scadden, 2012; Yu & Scadden, 2016). Similarly, MM plasma cells exhibit high degree of BMM dependence. Changes in BMM that are advantageous to MM development and survival typically accompany or precede the appearance of bone lesions (Bhowmick et al., 2004; Hanahan & Weinberg, 2011; Kaplan et al., 2005; Valastyan & Weinberg, 2011). Therefore, the importance of MM interaction with the BMM cannot be overestimated. MM arises when mutations in the antibody coding genes lead to a clonal expansion of malignant plasma cells (Cowan et al., 2015; Hansmann, Han, Penter, Liedtke, & Davis, 2017; Hosen, 2013; Morgan, Walker, & Davies, 2012; Narayanan, Duan, Butcher, Mazumder, & Narayanan, 2014; Ribourtout & Zandecki, 2015). These clones are usually genetically diverse and exhibit dominance-based hierarchy. Clinically, the disease progresses from monoclonal gammopathy of undetermined significance to anaemia, myelosuppression, and osteolysis, which are often accompanied by renal failure (Barley & Chari, 2016; Kumar et al., 2017; Muchtar, Kumar, Magen, & Gertz, 2018). Within this range, the appearance of individual symptoms is quite often uniquely individual and the disease may persist without any clear symptoms until uncovered through a routine screening. Epidemiologically, MM is predominantly associated with the older population, with a median age of 69 years at diagnosis (Rollig et al., 2015).
CHALLENGES WITH SMALL MOLECULE DRUG OR BIOLOGIC THERAPIES FOR MULTIPLE MYELOMA
Despite major advances in treatment, MM is still mostly a fatal disease (Gay et al., 2018; Usmani, Rodriguez-Otero, Bhutani, Mateos, & Miguel, 2015). Current treatments usually include some combinations of autologous stem cell transplantation and pharmacologic agents (van de Donk & Lokhorst, 2013). The pharmacological agents are administered in various combinations of immunomodulatory drugs (IMiD) (Thalidomide, Lenalidomide, Pomalidomide), corticosteroids (Dexamethasone), proteasome inhibitors (Bortezomib, Carfilzomib, Ixazomib), deacetylase inhibitors (Panobinostat), and monoclonal antibodies (Elotuzumab, Daratumumab) (Dimopoulos, Richardson, Moreau, & Anderson, 2015; Kohler et al., 2018; Larocca, Mina, Gay, Bringhen, & Boccadoro, 2017).
IMiD agents exert their therapeutic effects through a variety of mechanisms that include the inhibition of anti-proliferative and pro-apoptotic signals, modulation of BMM, and upregulation of anti-tumour cellular immunity (Rios-Tamayo et al., 2017). A combination of IMiD and corticosteroids has improved the overall response rate and decreased the time to progression (Rajkumar et al., 2008). However, all IMiDs in the clinic are structural analogues of Thalidomide, and therefore could be considered teratogenic. Additionally, thrombotic events, anaemia, and peripheral neuropathy were among common side effects of IMiD use (Ghobrial & Rajkumar, 2003; Lyman, 2014; Palumbo et al., 2008). These clinical findings stimulated interest in the development of alternative drugs for MM.
Studies have shown that proteasome inhibitors impede 26S proteasome pathway, resulting in the downregulation of MM survival, growth, and drug resistance (Hideshima & Anderson, 2012). Although proteasome inhibitors are capable of inducing therapeutic response when used alone, combination with other drugs has improved MM response to treatment (Alsina et al., 2012; Chauhan et al., 2011; Moreau et al., 2015; Siegel et al., 2012). Unfortunately, proteasome inhibitors are also associated with clinical complications such as peripheral neuropathy, anaemia, thrombocytopenia, and hepatic toxicity (Kumar, Bensinger, et al., 2014; Kumar, Berdeja, et al., 2014; Richardson et al., 2014).
With advances in the development of biologics for cancer therapy, several monoclonal antibodies targeting MM pathology have been evaluated for their therapeutic efficacy with varying outcomes. These biological molecules bind to their target receptors with exceptionally high specificity. For example, the CD38-targeting antibody, Daratumumab, showed an objective, durable response and survival advantage among responding patients and patients with stable disease or minimal response (Usmani et al., 2016). Although clinical studies with anti-CS1 antibody (Elotuzumab) did not demonstrate objective responses, the drug was well tolerated and exhibited minimal side effects (Zonder et al., 2012). As with many other cancer therapies, combination of elotuzumab with lenalidomide and dexamethasone showed improved efficacy (Dimopoulos et al., 2017).
Overall, many small molecule drugs and biologics remain the mainstay of MM therapy today. They are more effective when used as combination drugs. However, the complex BMM, together with clonal evolution and cancer stem cell survival, allows a significant number of MM cells to escape therapy, leading to constant relapse and eventual death. Moreover, the serious toxic effects of these drugs diminish enthusiasm for their routine use, especially for non-responding patients. Further, the prohibitive costs of the newer biologics in clinics today confine immunotherapy to few patients and clinical centres. Nanoparticles (NP) could address some of these challenges.
NANOPARTICLE PLATFORMS FOR EFFICIENT TARGETING DRUG DELIVERY
The multidimensional functionality and high loading capacity of NP are attractive for developing new delivery and treatment strategies to improve outcomes with minimal side effects. Most chemotherapeutics are often poorly soluble, requiring the use of excipients to formulate them for in vivo applications. In addition, the reliance on stochastic events to deliver small molecule drugs to tumours further diminishes their effectiveness either through rapid clearance from blood or inadequate concentration in cancer cells (Figure 2) (Dilnawaz, Acharya, & Sahoo, 2018; Hu & Hu, 2018; Izzedine & Perazella, 2017; Mateos, Ocio, et al., 2015; Mateos, Oriol, et al., 2015; Sanna, Pala, & Sechi, 2014).
Figure 2.
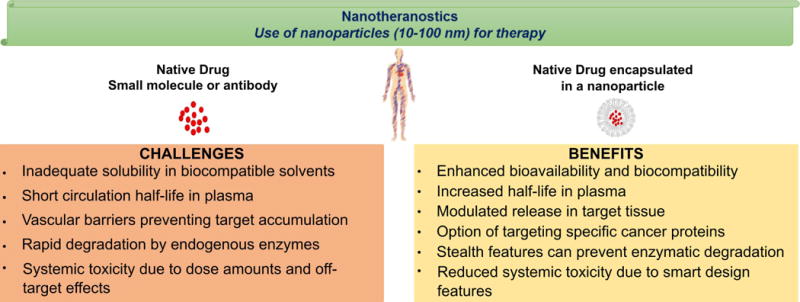
Advantages of nanoparticles over the use of small molecule drugs such as peptides or biologics.
A viable solution to overcome these challenges is to encapsulate the drugs in NP to control drug release, prolong circulation in blood, increase payload in tumours, and minimize off-target toxicity (Ahmadzada, Reid, & McKenzie, 2018; Casals, Gusta, Cobaleda-Siles, Garcia-Sanz, & Puntes, 2017; Dilnawaz et al., 2018; Lacombe, Porcel, & Scifoni, 2017; Lu, Wang, & Ling, 2018). Because different drugs can be encapsulated in NP at the same time, this platform provides a synergistic strategy to deliver combination drugs to the same tissue. Some nanomaterials are themselves therapeutic agents. These NPs can leverage the strengths of nanoconstructs to directly or indirectly induce a therapeutic response. Generally, classification of NP can be based on either the material used for their manufacture or the mechanisms for targeted delivery to tumours (Figure 3) (Bahia, 2016). Among the materials used to manufacture the NP are lipids (micelles, liposomes, stealth liposomes), polymers (polymeric micelles, dendrimers), inorganic materials (metal core, silica), drug conjugates (antibody-drug conjugates, polymer-drug conjugates, polymer-protein conjugates), and viral NP (Tran, DeGiovanni, Piel, & Rai, 2017). Additionally, development of hybrid nanoplatforms such as nanoscale metal-organic frameworks (NMOF), polysilsesquioxane (PSQ) nanoparticles (He & Lin, 2015), semiconducting polymer nanoparticles (J. Li, Rao, & Pu, 2018), and nanobody based platforms (Bannas, Hambach, & Koch-Nolte, 2017) is in progress.
Figure 3.
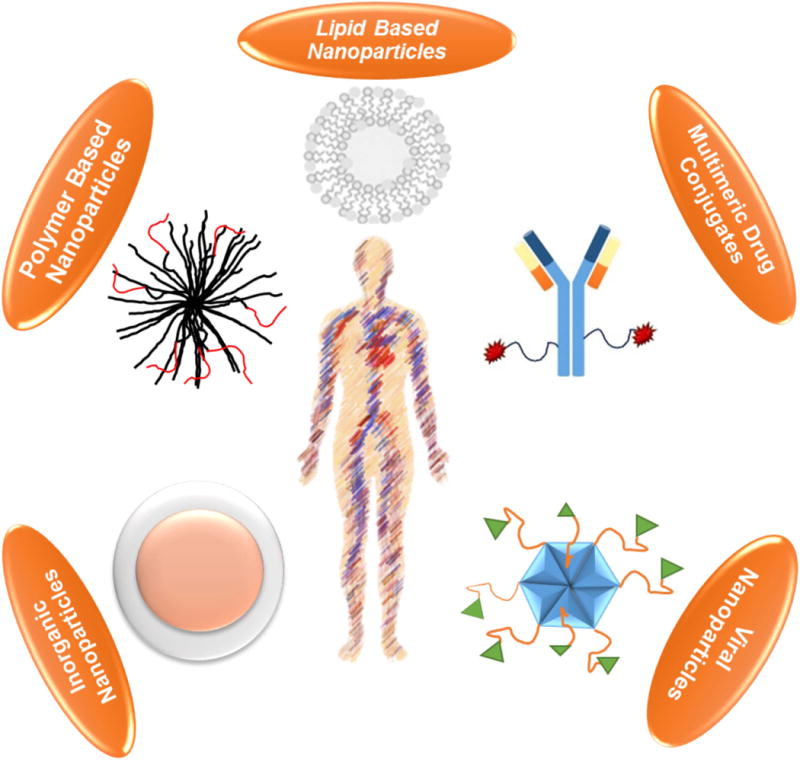
Various nanoparticle platforms that can be utilized for targeting multiple myeloma in vivo.
NON-CANCER TARGETED NANOPARTICLES FOR MULTIPLE MYELOMA THERAPY
A variety of methods have been explored to deliver drug-carrying NP to MM, including non-targeted, actively targeted, or triggered release approaches. Non-targeted delivery relies on the passive diffusion of NP through the compromised vascular endothelial barrier of tumour vasculature into the interstitial space, a process termed enhanced permeability and retention (Azzopardi, Ferguson, & Thomas, 2013; Kobayashi, Watanabe, & Choyke, 2013; Maeda, Tsukigawa, & Fang, 2016). This approach is best suited for NP with long blood circulation time, such as liposomes, allowing significant accumulation and retention at tumour sites, where they are able to exert a sustainable therapeutic effect.
Liposomes
Liposomes come in different sizes. Many of these (liposome) NP approved for human use are micron-sized materials, but stable nano-sized analogues have been developed. A notable use of this technology is the delivery of bortezomib, a proteasome inhibitor used as frontline therapy for newly diagnosed MM patients. Systemic administration of free bortezomib is usually associated with significant toxicity, which can be obviated by encapsulating the drug inside liposomes for safer delivery to the target (Fonseca, 2012; Lok et al., 2014; Richardson et al., 2010). Most new cancer therapies involve combinations of two to three drugs to provide a synergistic effect and improved outcome (Eldar-Boock, Polyak, Scomparin, & Satchi-Fainaro, 2013; Yhee, Son, Lee, & Kim, 2015). In MM, the combination of bortezomib and doxorubicin, an anthracycline antibiotic, has been studied extensively and demonstrated a synergistic effect in suppression of disease progression (Du et al., 2012; Munshi & Anderson, 2013). A recent study encapsulated both carfilzomib, a second-generation proteasome inhibitor, and doxorubicin in a liposomal formulation for MM therapy (Ashley et al., 2016). After testing different ratios of carfilzomib and doxorubicin to evaluate the cytotoxic effect in NCI-H929 and MM.1S myeloma lines, carfilzomib-to-doxorubicin ratios of 1:1 and 2:1 were shown to be highly synergistic. This platform maximized the synergistic effect by loading drug ratio to optimize therapeutic effect. Although similar combination therapies are routinely used, the disparate distribution of each drug can alter the delivery and co-localization of the drugs after sequential or joint systemic administration. Although these NP demonstrated the potential of using liposomal combination drug formulations for drug delivery, the subcutaneous tumour implantation model used in this study did not address the ongoing controversy regarding the delivery of large NP to the BMM (Mu et al., 2018).
Polymeric Nanoplatforms
A variety of polymeric nanomaterials, especially those utilizing N,N,N-trimethyl chitosan, have received significant attention for drug delivery to MM tissue. This approach was elegantly demonstrated by encapsulating a plant alkaloid with anti-cancer properties, camptothesin, in N,N,N-trimethyl chitosan NP (Z. Li et al., 2012). Camptothesin is a specific inhibitor of DNA topoisomerase I extracted from the bark of Camptotheca acuminate tree. Administration of this drug alone significantly inhibited the proliferation of MPC-11 murine MM in vitro. Whereas the camptothecin-chitosan formulation did not enhance the in vitro effect, it significantly prolonged the survival of MPC-11 bearing animals. This finding suggests that several factors could mediate drug delivery and release in vivo, including the extended blood circulation time, shear forces created in the complex BMM, and timely release of the drug for durable therapeutic effect. Similar to the model used in the liposome NP, it would be interesting to explore whether the chitosan encapsulated Camptothecin will be effective in a disseminated/medullary MM model.
In addition to small organic drugs, the excitement of using nucleic acid-based drugs to treat MM has not been achieved because of the rapid degradation of these drugs by nucleases. A recent study combined chitosan with poly(D,L-lactide-co-glycolide) to encapsulate tumour suppressing microRNA-34a (miR-34a) (Cosco et al., 2015). MiR-34a is known to arrest tumour cells in the G1 phase leading to apoptosis (Wu et al., 2016). The mechanism of miR-34a in MM cells involves the induction of apoptosis by downregulating the transforming growth interaction factor 2 (Wu et al., 2016). This formulation was effective in RNase A protection assay up to 48 hours. In vitro, the chitosan-poly(D,L-lactide-co-glycolide)-miR-34a nanoplatforms inhibited the proliferation of RPMI-8226 (60%) and SKMM1 (50%) MM cells at 48 hours. When the formulation was used on SKMM1 xenograft model, tumour progression was specifically and significantly inhibited compared to either non-miR-34a particles or particles loaded with a scrambled sequence miRNA-34a. Ex vivo analysis of tumours from miRNA-34a-NP treated animals demonstrated increased miRNA-34a expression and downregulation of BCL2 protein compared to the animals from the control groups. Although the NP-induced a good therapeutic response in vitro and in vivo, selectively targeting the drug to MM could further improve the outcome and accelerate clinical translation.
Another gene-based NP therapy approach employed a polyethylene nanoconstruct SNS01. The NP contains a B cell-specific plasmid encoding a mutant of eIF5A eukaryotic translation initiation factor that is resistant to hypusination and a small interfering RNA to inhibit endogenous hypusinated eIF5A (Taylor et al., 2012). Interference with eIF5A level leads to downregulation of ERK MAPK and NF-κB with resulting tumour suppression. When SNS01 NP were tested in KAS-6/1 and RPMI 8226 MM xenografts, the formulation inhibited tumour progression by 95% (KAS-6/1) and 59% (RPMI 8226) compared to untreated controls. This system was particularly attractive since the NP carries not only the genetic material to inhibit the synthesis of MM-promoting translation factor, but also the genetic material to block the endogenous hypusinated translation factor. Overall, the genetic modulation approach has been shown to be effective in suppressing disease progression, provided that the genetic material is delivered to the tumour cells efficiently and safely.
TARGETED NANOPARTICLES FOR MULTIPLE MYELOMA THERAPY
Although the non-targeted NP can passively deliver drugs to tumours based on pathophysiological factors, the addition of targeting molecules to the surface of the NP can enhance the selective accumulation of drugs in cancer cells (Wicki, Witzigmann, Balasubramanian, & Huwyler, 2015). Targeting moieties such as antibodies, antibody fragments, and other proteins or small molecules are selected for inclusion in NP formulation based on their ability to bind strongly to tumour-associated biomarkers, especially over-expressed cell surface receptors. Theoretically, this approach should significantly reduce the off-target toxicity encountered with non-targeted NP. Practically, the observed enhancement in tumours is a result of both active and passive targeting properties of the NPs. Depending on the nature of the tumour microenvironment, passive targeting via enhanced permeability and retention pathway can dominate tumour uptake, irrespective of whether targeted or non-targeted NPs are used.
A variety of NP have been designed to target several cell surface receptors overexpressed on the cell surface of MM cells. One example is very late antigen-4 (VLA-4), also known as α4ß1, which is a member of the integrin family of cell adhesion receptors. Whereas VLA-4 is normally expressed by myeloid cells, studies have shown that this receptor is overexpressed by hematopoietic cancers such as leukaemia, lymphoma, and MM (Ley, Rivera-Nieves, Sandborn, & Shattil, 2016; Schlesinger & Bendas, 2015). As a result, VLA-4 has received extensive attention as a target for NP-based drug delivery to MM. Many small molecule ligands for VLA-4 have been identified and incorporated into NPs. For example, the VLA-4 antagonist cyclic peptide, Tyr-Cys-Asp-Pro-Cys, was coupled to lipid micelle core decorated with PEG2000-NH2 (Kiziltepe et al., 2012). Doxorubicin was then conjugated to the micelle through an acid-labile bond to ensure the release of active drug in the lysosomal compartment. The optimal number of peptide molecules on the micelle surface was optimized to maximize VLA-4 binding. The resulting formulation significantly inhibited in vivo progression of NCI-H929 myeloma xenografts compared to either the untreated group or the cohort treated with non-targeted micelles. Because this antagonist binds both VLA-4 and inhibits VLA-4 adhesion to the extracellular matrix, the therapeutic response could be attributed to the effects of both the cytotoxic doxorubicin-induced MM cell death and the reduced adhesion of MM to BM stroma needed for its survival and expansion. Increasing the hydrophilicity of the peptide by adding three lysine residues in conjunction with ethylene glycol 6 (EG6) short PEG linker improved liposome internalization by myeloma cells up to 80-fold (Stefanick, Ashley, & Bilgicer, 2013). When ethylene glycol 18 (EG18) linker was combined with the lysine-modified VLA-4 peptide, internalization of the micelles increased by up to 27-fold. Further assessment in disseminated MM models is needed to determine the potential impact and translatability of these NPs to MM patients.
The rapid degradation of peptides by lytic enzymes in the blood remains an endemic problem. One approach to combat this problem is to design peptidomimetic analogues of the peptide. This approach was recently demonstrated by incorporating a peptidomimetic with picomolar affinity for activated VLA-4 (Peng et al., 2006) into nanomicelles via conjugation to a phospholipid (Soodgupta et al., 2015). The VLA-4 targeted nanomicelle was loaded with a prodrug that inhibits the MYC-MAX oncogene pathway. The MYC inhibitor pro-drug was incorporated into the lipid layer to protect it from degradation that usually occurs during systemic administration. The study showed that the 20 nm nanomicelle demonstrated a better survival advantage compared to identically targeted 200 nm liposomes carrying the same pro-drug in an in vivo orthotopic 5TGM1 mouse MM model. This therapy relied on the “contact-facilitated drug delivery” mechanism, a process where the interaction of the ligand with its receptor positions the nanomicelle into very close proximity to the cell membrane, resulting in the transfer of the pro-drug to the outer leaflet of the plasma membrane (Pan et al., 2016). Subsequent intracellular activation of the prodrug by cellular phospholipases converts it into the active form. Here, the researchers employed a disseminated allograft mouse model for the assessment of the therapy, assuring that the crucial BMM – MM interaction was accounted for in the overall evaluation.
Apart from VLA-4, other MM targeting approaches have been explored. Anti-CD38 antibody conjugated to bortezomib was recently loaded in polymeric chitosan NP using streptavidin-biotin chemistry (de la Puente et al., 2017). CD38 is normally expressed at low levels on lymphocytes and some other tissues, but its expression is increased on proliferating plasma cells (Lin, Owens, Tricot, & Wilson, 2004; Shallis, Terry, & Lim, 2017; van de Donk et al., 2016). The antibody-driven targeting allowed for accurate delivery to MM and internalization of the NP, while efficient bortezomib loading improved the proteasome inhibition, thus improving therapeutic potential and reducing off-target side effects. Encapsulated bortezomib demonstrated higher efficiency of killing in vitro when the MM interaction with stromal cells was taken into account by placing MM cells in a 3D culture together with bone marrow stromal cells. Bortezomib release was attributed to the low pH of tumor microenvironment. In vivo, CD-38 targeted NP were significantly more effective in tumour suppression than either the non-targeted NP or bortezomib alone, while limiting the weight loss to 10%. Combined with the proven low toxicity of chitosan (Kean & Thanou, 2010), this approach represents another promising targeted NP drug delivery strategy to improve the treatment of MM.
PERSPECTIVES AND CONCLUSIONS
Small molecule drugs can be rapidly removed from circulation or via the lymphatic system, effectively reducing their dwell times in the tumour vicinity. For small drugs that exert their toxic effects intracellularly, processes required for internalization would benefit from longer retention time in the tumour environment. Although the high vascularity and enhanced fenestrations in the BMM allow adequate delivery and extravasation of both small molecule drugs and NP, a combination of larger size, shape, and charge favour the longer retention of NP in this tissue for the treatment of MM. Disruption of normal physiology by the presence of lesions caused during MM creates additional factors to selectively anchor NP in this environment. The versatility of NP design enhances the flexibility in the choice of materials, structures, and functionality for a specific application. Either cancer-targeted or non-targeted NP have been shown to treat MM. These NPs can serve as a drug delivery agent, solubilizing medium, and protection agent against drug inactivation. Some studies have also demonstrated the use of NP to deliver combination therapies, especially in situations where the pharmacokinetic profiles of each drug are vastly different.
Inasmuch as NP can improve drug delivery to tumours, only a small fraction of the drug will reach the target. By relying on intrinsic processes to release drugs from NP, it is expected that similar events will occur in non-tumour tissue, including the major excretion organs such as the liver and the kidneys, leading to off-target toxicity. One way to decrease systemic toxicity is to load NP with inactive drugs (pro-drug) and stimulate the release at the target sites via specific stimuli such as the acidic microenvironment in tumours, increased levels of reactive oxygen species, high protease activity, or other factors (Hatakeyama, 2017). However, optimizing externally triggered drug release method could overcome this potential obstacle to human translation of NP. An interesting report utilizing this approach demonstrated the successful use of ultrasound to locally release of epirubicin targeted to ABCG2 transporter molecule expressed by MM cancer stem cells (Pol et al., 2015; Shi et al., 2017). Epirubicin, an anthracycline clinically used to treat MM, was loaded into lipid microbubbles decorated with an antibody against the ABCG2. CD138-/CD34- MM cancer stem cells were used to initiate subcutaneous tumours in severely immune-compromised mice. The drug was released by applying ultrasound (US) burst with a mechanical index of 0.4 for 3 minutes. The tumour volumes of animals treated with US-released epirubicin were 10-fold smaller when compared with the tumour volumes of untreated animals and 3-5-fold smaller than those from either free epirubicin or the epirubicin microbubbles that were not activated with US. The dual targeting approach, first with NP and then US-mediated drug release provides an excellent spatiotemporal accuracy that minimizes systemic toxicity. Although this approach could be used to treat extramedullary MM, the limited penetration of US across bone tissue could dampen the application of this technique for MM treatment in human patients.
An alternative spatiotemporal triggered therapy utilized radionuclides to potentiate the effects of photosensitizers in solid tumours, a process termed Čerenkov radiation-induced therapy (CRIT) (Kotagiri, Sudlow, Akers, & Achilefu, 2015). The radionuclide generated Čerenkov radiation (CR), which in turn increased the level of cytotoxic reactive oxygen species in cancer cells to allow a tissue depth independent cancer therapy. In a way, CRIT was inspired by the limitations of light-based therapies such as photodynamic therapy. Photodynamic therapy combines light with photosensitizers to produce high levels of reactive oxygen species that eventually lead to cell death. It has been used successfully to treat a variety of topically localized infections and cancers accessible by an external light source (Abo-Neima, 2017; Chitgupi, Qin, & Lovell, 2017; J. Li et al., 2018; Nesi-Reis et al., 2017; Rundle, 2017; Youssef et al., 2017). Replacement of external light with radionuclides extends the potential use of light-sensitive drugs to treat previously inaccessible tumours such as MM and metastatic breast cancer (Kotagiri et al., 2018). This approach relied on the delivery of a photosensitizer (titanocene) encapsulated in a VLA-4 targeted nanomicelle and the radiopharmaceutical, 2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG) as the source of CR. The orthogonal delivery of nanomicelle via VLA4 and 18F-FDG via GLUT1 glucose transporter prevented competition between the photosensitizer and the radiopharmaceutical for the same target. CRIT-treated human MM tumour (MM1.S) bearing mice exhibited a significant decrease in tumour burden throughout the study compared to all control groups. The therapy also provided a significant survival benefit compared to the controls. This work demonstrated the advantage of orthogonal delivery strategy in minimizing the off-target toxicity and reducing the active amounts of drugs involved in the therapy, while utilizing the more realistic disseminated models of MM. Subpopulations of MM cells with markedly decreased expression of activated VLA-4 avoided destruction by minimizing the nanomicelle uptake.
Despite the enormous advances in nanomedicine, microparticles such as liposomes and iron oxide particles have dominated clinical use today. Several factors account for the slow translation of the myriad of NPs that have shown efficacy in small animals models to human patients. Compared to liposomes, NP designed for cancer therapy are relatively new. Moreover, concerns over reproducibility, large-scale manufacturing, efficacy, and long-term toxicity require extensive regulatory oversight before approval for human use. From a business perspective, the incidence of MM is relatively lower than most solid tumours such as the breast, lung, and prostate cancers but the research and development, as well as regulatory approval processes, are similar. As such, valuable resources are used to develop therapies and delivery vehicles that can treat major cancer types.
However, developing NP for MM has many advantages. MM is a bone cancer and many cancer types such as breast and prostate metastasize to the bone. As such, successful translation of NP for MM will open new opportunities to apply them to metastatic bone lesions, which is the major cause of morbidity and death for many patients. If diagnosed early, MM is confined to bone tissue, making it possible to harness the wealth of information available on bone pathophysiology to selectively target these lesions.
SIDE BAR TEXT.
Defining myeloma disease spectrum: Overt multiple myeloma (MM) is preceded by a precursor condition called monoclonal gammopathy of undetermined significance (MGUS), while a fraction of patients manifest an intermediate condition (between MGUS and MM) called smouldering multiple myeloma (SMM). Historically, diagnosis of MM required evidence of end-organ damage referred to as CRAB (hypercalcemia, renal failure, anaemia, and osteolytic bone lesions) features. The revised International Myeloma Working Group diagnostic criteria for MM recommends three additional specific biomarkers (≥60% of clonal plasma cells in BM, serum involved/uninvolved free light chain ratio ≥100, and more than one focal lesions identified by magnetic resonance imaging ≥5 mm) to define MM (Rajkumar et al., 2014). The presence of at least one of these markers would identify MM, regardless of full CRAB. Here, for clarity, we used MM as the target disease.
Acknowledgments
The authors were funded in part by grants from the National Institutes of Health (U54 CA199092, R01 EB021048, R01 CA171651, P50 CA094056, P30 CA091842, S10 OD016237, S10 RR031625, and S10 OD020129), the Department of Defense Breast Cancer Research Program (W81XWH-16-1-0286), and the Alvin J. Siteman Cancer Research Fund (11-FY16-01). We thank Deep Hathi for help with formatting of figures.
Footnotes
ORCID: 0000-0001-8114-9584
ORCID: 0000-0002-3133-6717
Conflict of Interest: NONE
Contributor Information
Alexander Zheleznyak, Department of Radiology, Washington University, St. Louis, MO, USA.
Monica Shokeen, Departments of Radiology, and Biomedical Engineering, Washington University, St. Louis, MO, USA.
Samuel Achilefu, Departments of Radiology, Biomedical Engineering, and Biochemistry & Molecular Biophysics, Washington University, St. Louis, MO, USA.
References
- Abo-Neima SE. Treatment of cancer by low intensity laser radiation therapy. Prog Biophys Mol Biol. 2017 doi: 10.1016/j.pbiomolbio.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Ahmadzada T, Reid G, McKenzie DR. Fundamentals of siRNA and miRNA therapeutics and a review of targeted nanoparticle delivery systems in breast cancer. Biophys Rev. 2018 doi: 10.1007/s12551-017-0392-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsina M, Trudel S, Furman RR, Rosen PJ, O’Connor OA, Comenzo RL, Goy A. A phase I single-agent study of twice-weekly consecutive-day dosing of the proteasome inhibitor carfilzomib in patients with relapsed or refractory multiple myeloma or lymphoma. Clin Cancer Res. 2012;18(17):4830–4840. doi: 10.1158/1078-0432.CCR-11-3007. [DOI] [PubMed] [Google Scholar]
- Anthony BA, Link DC. Regulation of hematopoietic stem cells by bone marrow stromal cells. Trends Immunol. 2014;35(1):32–37. doi: 10.1016/j.it.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley JD, Quinlan CJ, Schroeder VA, Suckow MA, Pizzuti VJ, Kiziltepe T, Bilgicer B. Dual Carfilzomib and Doxorubicin-Loaded Liposomal Nanoparticles for Synergistic Efficacy in Multiple Myeloma. Mol Cancer Ther. 2016;15(7):1452–1459. doi: 10.1158/1535-7163.MCT-15-0867. [DOI] [PubMed] [Google Scholar]
- Azzopardi EA, Ferguson EL, Thomas DW. The enhanced permeability retention effect: a new paradigm for drug targeting in infection. J Antimicrob Chemother. 2013;68(2):257–274. doi: 10.1093/jac/dks379. [DOI] [PubMed] [Google Scholar]
- Bahia S. Nanoparticles Types, Classification, Characterization, Fabrication Methods, and Drug Delivery Applications. Springer International Publishing; Switzerland: 2016. [Google Scholar]
- Bannas P, Hambach J, Koch-Nolte F. Nanobodies and Nanobody-Based Human Heavy Chain Antibodies As Antitumor Therapeutics. Front Immunol. 2017;8:1603. doi: 10.3389/fimmu.2017.01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barley K, Chari A. Diagnostic Advances in Multiple Myeloma. Curr Hematol Malig Rep. 2016;11(2):111–117. doi: 10.1007/s11899-016-0314-5. [DOI] [PubMed] [Google Scholar]
- Bergin K, McQuilten Z, Moore E, Wood E, Spencer A. Myeloma in the Real World: What Is Really Happening? Clin Lymphoma Myeloma Leuk. 2017;17(3):133–144 e131. doi: 10.1016/j.clml.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Moses HL. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303(5659):848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Casals E, Gusta MF, Cobaleda-Siles M, Garcia-Sanz A, Puntes VF. Cancer resistance to treatment and antiresistance tools offered by multimodal multifunctional nanoparticles. Cancer Nanotechnol. 2017;8(1):7. doi: 10.1186/s12645-017-0030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan D, Tian Z, Zhou B, Kuhn D, Orlowski R, Raje N, Anderson KC. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin Cancer Res. 2011;17(16):5311–5321. doi: 10.1158/1078-0432.CCR-11-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitgupi U, Qin Y, Lovell JF. Targeted Nanomaterials for Phototherapy. Nanotheranostics. 2017;1(1):38–58. doi: 10.7150/ntno.17694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosco D, Cilurzo F, Maiuolo J, Federico C, Di Martino MT, Cristiano MC, Paolino D. Delivery of miR-34a by chitosan/PLGA nanoplexes for the anticancer treatment of multiple myeloma. Sci Rep. 2015;5:17579. doi: 10.1038/srep17579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan G, Weston-Bell NJ, Bryant D, Seckinger A, Hose D, Zojer N, Sahota SS. Massive parallel IGHV gene sequencing reveals a germinal center pathway in origins of human multiple myeloma. Oncotarget. 2015;6(15):13229–13240. doi: 10.18632/oncotarget.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Puente P, Luderer MJ, Federico C, Jin A, Gilson RC, Egbulefu C, Azab AK. Enhancing proteasome-inhibitory activity and specificity of bortezomib by CD38 targeted nanoparticles in multiple myeloma. J Control Release. 2017;270:158–176. doi: 10.1016/j.jconrel.2017.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilnawaz F, Acharya S, Sahoo SK. Recent trends of nanomedicinal approaches in clinics. Int J Pharm. 2018 doi: 10.1016/j.ijpharm.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Dimopoulos MA, Lonial S, White D, Moreau P, Palumbo A, San-Miguel J, Richardson P. Elotuzumab plus lenalidomide/dexamethasone for relapsed or refractory multiple myeloma: ELOQUENT-2 follow-up and post-hoc analyses on progression-free survival and tumour growth. [Clinical Trial, Phase III Randomized Controlled Trial Research Support, Non-U.S. Gov’t] Br J Haematol. 2017;178(6):896–905. doi: 10.1111/bjh.14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimopoulos MA, Richardson PG, Moreau P, Anderson KC. Current treatment landscape for relapsed and/or refractory multiple myeloma. Nat Rev Clin Oncol. 2015;12(1):42–54. doi: 10.1038/nrclinonc.2014.200. [DOI] [PubMed] [Google Scholar]
- Du BY, Song W, Bai L, Shen Y, Miao SY, Wang LF. Synergistic effects of combination treatment with bortezomib and doxorubicin in human neuroblastoma cell lines. Chemotherapy. 2012;58(1):44–51. doi: 10.1159/000335603. [DOI] [PubMed] [Google Scholar]
- Eldar-Boock A, Polyak D, Scomparin A, Satchi-Fainaro R. Nano-sized polymers and liposomes designed to deliver combination therapy for cancer. Curr Opin Biotechnol. 2013;24(4):682–689. doi: 10.1016/j.copbio.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Fonseca R. Frontline treatment of multiple myeloma. Hematology. 2012;17(Suppl 1):S101–104. doi: 10.1179/102453312X13336169156096. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Kikuchi J. Molecular pathogenesis of multiple myeloma. Int J Clin Oncol. 2015;20(3):413–422. doi: 10.1007/s10147-015-0837-0. [DOI] [PubMed] [Google Scholar]
- Gay F, Engelhardt M, Terpos E, Wasch R, Giaccone L, Auner HW, Sonneveld P. From transplant to novel cellular therapies in multiple myeloma: European Myeloma Network guidelines and future perspectives. Haematologica. 2018;103(2):197–211. doi: 10.3324/haematol.2017.174573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghobrial IM, Detappe A, Anderson KC, Steensma DP. The bone-marrow niche in MDS and MGUS: implications for AML and MM. Nat Rev Clin Oncol. 2018 doi: 10.1038/nrclinonc.2017.197. [DOI] [PubMed] [Google Scholar]
- Ghobrial IM, Rajkumar SV. Management of thalidomide toxicity. J Support Oncol. 2003;1(3):194–205. [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hansmann L, Han A, Penter L, Liedtke M, Davis MM. Clonal Expansion and Interrelatedness of Distinct B-Lineage Compartments in Multiple Myeloma Bone Marrow. Cancer Immunol Res. 2017;5(9):744–754. doi: 10.1158/2326-6066.CIR-17-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama H. Recent Advances in Endogenous and Exogenous Stimuli-Responsive Nanocarriers for Drug Delivery and Therapeutics. Chem Pharm Bull (Tokyo) 2017;65(7):612–617. doi: 10.1248/cpb.c17-00068. [DOI] [PubMed] [Google Scholar]
- He C, Lin W. Hybrid nanoparticles for cancer imaging and therapy. Cancer Treat Res. 2015;166:173–192. doi: 10.1007/978-3-319-16555-4_8. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Anderson KC. Biologic impact of proteasome inhibition in multiple myeloma cells–from the aspects of preclinical studies. Semin Hematol. 2012;49(3):223–227. doi: 10.1053/j.seminhematol.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosen N. Multiple myeloma-initiating cells. Int J Hematol. 2013;97(3):306–312. doi: 10.1007/s12185-013-1293-0. [DOI] [PubMed] [Google Scholar]
- Hu J, Hu WX. Targeting signaling pathways in multiple myeloma: Pathogenesis and implication for treatments. Cancer Lett. 2018;414:214–221. doi: 10.1016/j.canlet.2017.11.020. [DOI] [PubMed] [Google Scholar]
- Izzedine H, Perazella MA. Anticancer Drug-Induced Acute Kidney Injury. Kidney Int Rep. 2017;2(4):504–514. doi: 10.1016/j.ekir.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Moschetta M, Manier S, Glavey S, Gorgun GT, Roccaro AM, Ghobrial IM. Targeting the bone marrow microenvironment in multiple myeloma. Immunol Rev. 2015;263(1):160–172. doi: 10.1111/imr.12233. [DOI] [PubMed] [Google Scholar]
- Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62(1):3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Kiziltepe T, Ashley JD, Stefanick JF, Qi YM, Alves NJ, Handlogten MW, Bilgicer B. Rationally engineered nanoparticles target multiple myeloma cells, overcome cell-adhesion-mediated drug resistance, and show enhanced efficacy in vivo. Blood Cancer J. 2012;2(4):e64. doi: 10.1038/bcj.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Watanabe R, Choyke PL. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics. 2013;4(1):81–89. doi: 10.7150/thno.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler M, Greil C, Hudecek M, Lonial S, Raje N, Wasch R, Engelhardt M. Current developments in immunotherapy in the treatment of multiple myeloma. [Review] Cancer. 2018 doi: 10.1002/cncr.31243. [DOI] [PubMed] [Google Scholar]
- Kotagiri N, Cooper ML, Rettig M, Egbulefu C, Prior J, Cui G, Achilefu S. Radionuclides transform chemotherapeutics into phototherapeutics for precise treatment of disseminated cancer. Nat Commun. 2018;9(1):275. doi: 10.1038/s41467-017-02758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nanotechnol. 2015;10(4):370–379. doi: 10.1038/nnano.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SK, Bensinger WI, Zimmerman TM, Reeder CB, Berenson JR, Berg D, Niesvizky R. Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma. Blood. 2014;124(7):1047–1055. doi: 10.1182/blood-2014-01-548941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Laubach JP, Hamadani M, Richardson PG. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15(13):1503–1512. doi: 10.1016/S1470-2045(14)71125-8. [DOI] [PubMed] [Google Scholar]
- Kumar SK, Rajkumar V, Kyle RA, van Duin M, Sonneveld P, Mateos MV, Anderson KC. Multiple myeloma. Nat Rev Dis Primers. 2017;3:17046. doi: 10.1038/nrdp.2017.46. [DOI] [PubMed] [Google Scholar]
- Lacombe S, Porcel E, Scifoni E. Particle therapy and nanomedicine: state of art and research perspectives. Cancer Nanotechnol. 2017;8(1):9. doi: 10.1186/s12645-017-0029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau DA, Carter SL, Getz G, Wu CJ. Clonal evolution in hematological malignancies and therapeutic implications. Leukemia. 2014;28(1):34–43. doi: 10.1038/leu.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca A, Mina R, Gay F, Bringhen S, Boccadoro M. Emerging drugs and combinations to treat multiple myeloma. Oncotarget. 2017;8(36):60656–60672. doi: 10.18632/oncotarget.19269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley K, Rivera-Nieves J, Sandborn WJ, Shattil S. Integrin-based therapeutics: biological basis, clinical use and new drugs. Nat Rev Drug Discov. 2016;15(3):173–183. doi: 10.1038/nrd.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Rao J, Pu K. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials. 2018;155:217–235. doi: 10.1016/j.biomaterials.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li X, Cao Z, Xu Y, Lin H, Zhao Y, Qian Z. Camptothecin nanocolloids based on N,N,N-trimethyl chitosan: efficient suppression of growth of multiple myeloma in a murine model. Oncol Rep. 2012;27(4):1035–1040. doi: 10.3892/or.2012.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004;121(4):482–488. doi: 10.1309/74R4-TB90-BUWH-27JX. [DOI] [PubMed] [Google Scholar]
- Lok A, Mocquard J, Bourcier J, Redelsperger L, Bonnet A, Chauvin C, Moreau P. Subcutaneous bortezomib incorporated into the bortezomib-thalidomide-dexamethasone regimen as part of front-line therapy in the context of autologous stem cell transplantation for multiple myeloma. Haematologica. 2014;99(3):e33–34. doi: 10.3324/haematol.2013.100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Wang J, Ling D. Surface Engineering of Nanoparticles for Targeted Delivery to Hepatocellular Carcinoma. Small. 2018;14(5) doi: 10.1002/smll.201702037. [DOI] [PubMed] [Google Scholar]
- Lyman GH. Impact of venous thromboembolism on survival in patients with advanced cancer: an unmet clinical need. Intern Emerg Med. 2014;9(5):497–499. doi: 10.1007/s11739-014-1087-2. [DOI] [PubMed] [Google Scholar]
- Maeda H, Tsukigawa K, Fang J. A Retrospective 30 Years After Discovery of the Enhanced Permeability and Retention Effect of Solid Tumors: Next-Generation Chemotherapeutics and Photodynamic Therapy–Problems, Solutions, and Prospects. Microcirculation. 2016;23(3):173–182. doi: 10.1111/micc.12228. [DOI] [PubMed] [Google Scholar]
- Manier S, Kawano Y, Bianchi G, Roccaro AM, Ghobrial IM. Cell autonomous and microenvironmental regulation of tumor progression in precursor states of multiple myeloma. Curr Opin Hematol. 2016;23(4):426–433. doi: 10.1097/MOH.0000000000000259. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Ocio EM, Paiva B, Rosinol L, Martinez-Lopez J, Blade J, San Miguel JF. Treatment for patients with newly diagnosed multiple myeloma in 2015. Blood Rev. 2015;29(6):387–403. doi: 10.1016/j.blre.2015.06.001. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Oriol A, Rosinol L, de Arriba F, Puig N, Martin J, San Miguel JF. Bendamustine, bortezomib and prednisone for the treatment of patients with newly diagnosed multiple myeloma: results of a prospective phase 2 Spanish/PETHEMA trial. Haematologica. 2015;100(8):1096–1102. doi: 10.3324/haematol.2015.124818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Rekhtman G, Arnulf B. Subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma: subanalysis of patients with renal impairment in the phase III MMY-3021 study. Haematologica. 2015;100(5):e207–210. doi: 10.3324/haematol.2014.118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan GJ, Walker BA, Davies FE. The genetic architecture of multiple myeloma. Nat Rev Cancer. 2012;12(5):335–348. doi: 10.1038/nrc3257. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. doi: 10.1038/nature12984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu CF, Shen J, Liang J, Zheng HS, Xiong Y, Wei YH, Li F. Targeted drug delivery for tumor therapy inside the bone marrow. Biomaterials. 2018;155:191–202. doi: 10.1016/j.biomaterials.2017.11.029. [DOI] [PubMed] [Google Scholar]
- Muchtar E, Kumar SK, Magen H, Gertz MA. Diagnosis and management of smoldering multiple myeloma: the razor's edge between clonality and cancer. Leuk Lymphoma. 2018;59(2):288–299. doi: 10.1080/10428194.2017.1334124. [DOI] [PubMed] [Google Scholar]
- Munshi NC, Anderson KC. New strategies in the treatment of multiple myeloma. Clin Cancer Res. 2013;19(13):3337–3344. doi: 10.1158/1078-0432.CCR-12-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NK, Duan B, Butcher JT, Mazumder A, Narayanan BA. Characterization of multiple myeloma clonal cell expansion and stromal Wnt/beta-catenin signaling in hyaluronic acid-based 3D hydrogel. In Vivo. 2014;28(1):67–73. [PubMed] [Google Scholar]
- Nesi-Reis V, Lera-Nonose D, Oyama J, Paula Silva-Lalucci MP, Demarchi IG, Aristides SMA, Lonardoni MVC. Contribution of Photodynamic Therapy in Wound Healing: A Systematic Review. Photodiagnosis Photodyn Ther. 2017 doi: 10.1016/j.pdpdt.2017.12.015. [DOI] [PubMed] [Google Scholar]
- Nishihori T, Shain K. Insights on Genomic and Molecular Alterations in Multiple Myeloma and Their Incorporation towards Risk-Adapted Treatment Strategy: Concise Clinical Review. Int J Genomics. 2017;2017:6934183. doi: 10.1155/2017/6934183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo A, Rajkumar SV, Dimopoulos MA, Richardson PG, San Miguel J, Barlogie B, International Myeloma Working, G Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22(2):414–423. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- Pan D, Pham CT, Weilbaecher KN, Tomasson MH, Wickline SA, Lanza GM. Contact-facilitated drug delivery with Sn2 lipase labile prodrugs optimize targeted lipid nanoparticle drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8(1):85–106. doi: 10.1002/wnan.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111(8):3923–3930. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Sykes DB, Scadden DT. The hematopoietic stem cell niche. Front Biosci (Landmark Ed) 2012;17:30–39. doi: 10.2741/3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Liu R, Marik J, Wang X, Takada Y, Lam KS. Combinatorial chemistry identifies high-affinity peptidomimetics against alpha4beta1 integrin for in vivo tumor imaging. Nat Chem Biol. 2006;2(7):381–389. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]
- Pol J, Vacchelli E, Aranda F, Castoldi F, Eggermont A, Cremer I, Galluzzi L. Trial Watch: Immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4(4):e1008866. doi: 10.1080/2162402X.2015.1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaghello L, Vacca A, Pistoia V, Ribatti D. Cancer associated fibroblasts in hematological malignancies. Oncotarget. 2015;6(5):2589–2603. doi: 10.18632/oncotarget.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, Miguel JF. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. [Review] Lancet Oncol. 2014;15(12):e538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Rosinol L, Hussein M, Catalano J, Jedrzejczak W, Lucy L, Blade J. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. J Clin Oncol. 2008;26(13):2171–2177. doi: 10.1200/JCO.2007.14.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramdass B, Chowdhary A, Koka PS. Hematological malignancies: disease pathophysiology of leukemic stem cells. J Stem Cells. 2013;8(3–4):151–187. doi: jsc.2014.8.3/4.151. [PubMed] [Google Scholar]
- Rankin EB, Narla A, Park JK, Lin S, Sakamoto KM. Biology of the bone marrow microenvironment and myelodysplastic syndromes. Mol Genet Metab. 2015;116(1–2):24–28. doi: 10.1016/j.ymgme.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Vacca A. Multiple myeloma as a model for the role of bone marrow niches in the control of angiogenesis. Int Rev Cell Mol Biol. 2015;314:259–282. doi: 10.1016/bs.ircmb.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Ribourtout B, Zandecki M. Plasma cell morphology in multiple myeloma and related disorders. Morphologie. 2015;99(325):38–62. doi: 10.1016/j.morpho.2015.02.001. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Baz R, Wang M, Jakubowiak AJ, Laubach JP, Harvey RD, Lonial S. Phase 1 study of twice-weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood. 2014;124(7):1038–1046. doi: 10.1182/blood-2014-01-548826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS, Anderson KC. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. [Clinical Trial, Phase I Clinical Trial, Phase II Multicenter Study Research Support, Non-U.S. Gov’t] Blood. 2010;116(5):679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios-Tamayo R, Martin-Garcia A, Alarcon-Payer C, Sanchez-Rodriguez D, de la Guardia A, Garcia Collado CG, Cabeza Barrera J. Pomalidomide in the treatment of multiple myeloma: design, development and place in therapy. Drug Des Devel Ther. 2017;11:2399–2408. doi: 10.2147/DDDT.S115456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385(9983):2197–2208. doi: 10.1016/S0140-6736(14)60493-1. [DOI] [PubMed] [Google Scholar]
- Rundle P. Photodynamic Therapy for Eye Cancer. Biomedicines. 2017;5(4) doi: 10.3390/biomedicines5040069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Miguel J. Multiple myeloma: a model for scientific and clinical progress. Hematology Am Soc Hematol Educ Program. 2014;2014(1):1–7. doi: 10.1182/asheducation-2014.1.1. [DOI] [PubMed] [Google Scholar]
- Sanna V, Pala N, Sechi M. Targeted therapy using nanotechnology: focus on cancer. Int J Nanomedicine. 2014;9:467–483. doi: 10.2147/IJN.S36654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M, Bendas G. Contribution of very late antigen-4 (VLA-4) integrin to cancer progression and metastasis. Cancer Metastasis Rev. 2015;34(4):575–591. doi: 10.1007/s10555-014-9545-x. [DOI] [PubMed] [Google Scholar]
- Shallis RM, Terry CM, Lim SH. The multi-faceted potential of CD38 antibody targeting in multiple myeloma. Cancer Immunol Immunother. 2017;66(6):697–703. doi: 10.1007/s00262-017-1990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Li M, Wu S, Yang F, Di W, Pan M, Dou J. Enhancing the anti-multiple myeloma efficiency in a cancer stem cell xenograft model by conjugating the ABCG2 antibody with microbubbles for a targeted delivery of ultrasound mediated epirubicin. Biochem Pharmacol. 2017;132:18–28. doi: 10.1016/j.bcp.2017.02.014. [DOI] [PubMed] [Google Scholar]
- Siegel DS, Martin T, Wang M, Vij R, Jakubowiak AJ, Lonial S, Jagannath S. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood. 2012;120(14):2817–2825. doi: 10.1182/blood-2012-05-425934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soodgupta D, Pan D, Cui G, Senpan A, Yang X, Lu L, Tomasson MH. Small Molecule MYC Inhibitor Conjugated to Integrin-Targeted Nanoparticles Extends Survival in a Mouse Model of Disseminated Multiple Myeloma. Mol Cancer Ther. 2015;14(6):1286–1294. doi: 10.1158/1535-7163.MCT-14-0774-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanick JF, Ashley JD, Bilgicer B. Enhanced cellular uptake of peptide-targeted nanoparticles through increased peptide hydrophilicity and optimized ethylene glycol peptide-linker length. ACS Nano. 2013;7(9):8115–8127. doi: 10.1021/nn4033954. [DOI] [PubMed] [Google Scholar]
- Tamura H. Immunopathogenesis and immunotherapy of multiple myeloma. Int J Hematol. 2018 doi: 10.1007/s12185-018-2405-7. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Liu Z, Tang TC, Zheng Q, Francis S, Wang TW, Thompson JE. Modulation of eIF5A expression using SNS01 nanoparticles inhibits NF-kappaB activity and tumor growth in murine models of multiple myeloma. Mol Ther. 2012;20(7):1305–1314. doi: 10.1038/mt.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenreiro MM, Correia ML, Brito MA. Endothelial progenitor cells in multiple myeloma neovascularization: a brick to the wall. Angiogenesis. 2017;20(4):443–462. doi: 10.1007/s10456-017-9571-8. [DOI] [PubMed] [Google Scholar]
- Terpos E, Ntanasis-Stathopoulos I, Gavriatopoulou M, Dimopoulos MA. Pathogenesis of bone disease in multiple myeloma: from bench to bedside. Blood Cancer J. 2018;8(1):7. doi: 10.1038/s41408-017-0037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran S, DeGiovanni PJ, Piel B, Rai P. Cancer nanomedicine: a review of recent success in drug delivery. Clin Transl Med. 2017;6(1):44. doi: 10.1186/s40169-017-0175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usmani SZ, Rodriguez-Otero P, Bhutani M, Mateos MV, Miguel JS. Defining and treating high-risk multiple myeloma. Leukemia. 2015;29(11):2119–2125. doi: 10.1038/leu.2015.209. [DOI] [PubMed] [Google Scholar]
- Usmani SZ, Weiss BM, Plesner T, Bahlis NJ, Belch A, Lonial S, Nahi H. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. [Clinical Trial, Phase I Clinical Trial, Phase II Multicenter Study Research Support, Non-U.S. Gov’t] Blood. 2016;128(1):37–44. doi: 10.1182/blood-2016-03-705210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Donk NW, Janmaat ML, Mutis T, Lammerts van Bueren JJ, Ahmadi T, Sasser AK, Parren PW. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol Rev. 2016;270(1):95–112. doi: 10.1111/imr.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Donk NW, Lokhorst HM. New developments in the management and treatment of newly diagnosed and relapsed/refractory multiple myeloma patients. Expert Opin Pharmacother. 2013;14(12):1569–1573. doi: 10.1517/14656566.2013.805746. [DOI] [PubMed] [Google Scholar]
- Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–157. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Wu S, He X, Li M, Shi F, Wu D, Pan M, Dou J. MiRNA-34a overexpression inhibits multiple myeloma cancer stem cell growth in mice by suppressing TGIF2. Am J Transl Res. 2016;8(12):5433–5443. [PMC free article] [PubMed] [Google Scholar]
- Yhee JY, Son S, Lee H, Kim K. Nanoparticle-Based Combination Therapy for Cancer Treatment. Curr Pharm Des. 2015;21(22):3158–3166. doi: 10.2174/1381612821666150531165059. [DOI] [PubMed] [Google Scholar]
- Youssef Z, Vanderesse R, Colombeau L, Baros F, Roques-Carmes T, Frochot C, Gazzali AM. The application of titanium dioxide, zinc oxide, fullerene, and graphene nanoparticles in photodynamic therapy. Cancer Nanotechnol. 2017;8(1):6. doi: 10.1186/s12645-017-0032-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu VW, Scadden DT. Hematopoietic Stem Cell and Its Bone Marrow Niche. Curr Top Dev Biol. 2016;118:21–44. doi: 10.1016/bs.ctdb.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonder JA, Mohrbacher AF, Singhal S, van Rhee F, Bensinger WI, Ding H, Singhal AK. A phase 1, multicenter, open-label, dose escalation study of elotuzumab in patients with advanced multiple myeloma. Blood. 2012;120(3):552–559. doi: 10.1182/blood-2011-06-360552. [DOI] [PMC free article] [PubMed] [Google Scholar]


