Abstract
Background:
Antiretroviral (ARV) drugs are used for HIV treatment and prevention. We analyzed ARV drug use and HIV drug resistance in a cohort of young women in rural South Africa enrolled in the HPTN 068 study, which evaluated use of a cash transfer conditional on school attendance to reduce HIV incidence.
Methods:
ARV drug testing was performed using plasma samples from 2,526 young women. This included 2,526 enrollment samples (80 HIV-infected, 2,446 HIV-uninfected) and 162 seroconversion samples (first HIV-positive study visit). Testing was performed using a qualitative assay that detects 20 ARV drugs from five drug classes. HIV drug resistance testing was performed with the ViroSeq HIV-1 Genotyping System for samples that had HIV viral loads ≥400 copies/mL.
Results:
At enrollment, ARV drugs were detected in 10 (12.5%) of 80 HIV-infected young women. None of 2,446 HIV-uninfected young women had ARV drugs detected at enrollment. ARV drugs were also detected in 16 (9.9%) of 162 seroconverters. At enrollment, nine (13.4%) of 67 young women with genotyping results had HIV drug resistance; resistance was also detected in nine (6.9%) of 131 seroconverters with genotyping results.
Conclusions:
Most of the HIV-infected young women in this cohort from rural South Africa were not taking ARV drugs, suggesting they were unaware of their HIV status or were not in care. HIV drug resistance was detected in young women with both prevalent and new HIV infection.
Keywords: ARV, drug resistance, HPTN 068, young women, South Africa
INTRODUCTION
Recent research in HIV treatment and prevention supports an expanding role for use of antiretroviral (ARV) drugs.1 In HIV-infected individuals, antiretroviral therapy (ART) reduces HIV-associated morbidity and mortality,2,3 increases life expectancy,4 and reduces the risk of HIV transmission to sexual partners and infants.5–7 Early initiation of ART, regardless of CD4 cell count, is now recommended for HIV treatment, prevention of HIV transmission in serodiscordant couples, and prevention of mother-to-child transmission worldwide.8,9 ARV drug regimens are also recommended for HIV-uninfected individuals for pre-exposure prophylaxis (PrEP), post-exposure prophylaxis (PEP), and other reasons (e.g., treatment of hepatitis).1,8,10–12 Some ARV drugs are also used off-label for recreational purposes.13,14 The availability of ARV drugs, recommendations for their use in both HIV-infected and HIV-uninfected individuals, and prevalence of ARV drug use for different indications, vary geographically and among different risk groups. This highlights the need for surveillance of ARV drug use and HIV drug resistance in different populations and settings.
ARV drug testing provides an objective method for assessing ARV drug use. Methods based on liquid chromatography–tandem mass spectroscopy (LC-MS/MS) are often used to quantify the levels of ARV drugs for research studies.15–18 These assays typically measure only one or a few ARV drugs. The cost and complexity of this type of testing limit its use for large surveys of ARV drug use. Our group developed a lower-cost, high-throughput method based on high-resolution mass spectrometry that detects 20 ARV drugs from five drug classes.19 Compared to a gold standard method based on LC-MS/MS, the multidrug assay achieved 89.1% to 100% concordance for detecting ARV drugs in clinical plasma samples.20 In a second report, the multidrug assay had 100% sensitivity and specificity for detecting tenofovir (TFV) and emtricitabine (FTC) when those drugs were present at concentrations consistent with daily PrEP use.19 We have used the multi-drug assay to evaluate ARV drug use in clinical trials and cohort studies,19,21–24 including a population-level survey of >7,000 HIV-infected adults in sub-Saharan Africa24. We also used the assay to evaluate ARV drug use in a cohort of 1,806 HIV-uninfected women in the United States; in that study, ARV drugs were detected in women at two of the 10 study sites (7% in Bronx, NY; 15% in Baltimore, MD).23
Young women in sub-Saharan Africa have significantly higher rates of HIV infection and acquire HIV infection at a younger age than their male peers.25 In this study, we evaluated ARV drug use and HIV drug resistance in a large cohort of young women in rural South Africa who participated in the HIV Prevention Trials Network (HPTN) 068 study.26,27 This study enrolled both HIV-infected and HIV-uninfected young women and evaluated the effect of a conditional cash transfer on HIV incidence.26,27 The overall annual incidence in the HPTN 068 cohort was 1.8%.27 The study was conducted in the Bushbuckridge sub-district in Mpumalanga province, South Africa. Poverty, unemployment, and circular labor migration are common in this region. Resources for HIV care may be more limited in rural areas of South Africa compared to urban areas.28 Those in rural populations may also be less likely to know their HIV status and may have lower adherence to ART, compared to their urban counterparts.29–31 These issues highlight the need to evaluate ARV drug use and HIV drug resistance in young women in rural African settings.
METHODS
Study cohort
Plasma samples were obtained from HPTN 068, a phase III randomized controlled trial (NCT01233531, enrollment period: March, 2011 to December, 2012; the study ended in 2015).26,27 The HPTN 068 study enrolled 2,537 young women aged 13–20 years who were attending high school. Participants had annual study visits through their expected high school graduation date, as previously described (main study).26,27 A subset of participants had one additional follow-up visit after exiting from the main study (follow-up study). South Africa is currently implementing the largest ART program in the world,32 and many clinics are designated as ART clinics. In HPTN 068, access to care was provided by government referral to the health care system. Clinics were within walking distance in all communities and treatment was free of charge. At the start of the trial, ART was available for individuals with CD4 cell counts under 350 cells/mm3; the threshold for ART initiation was changed to 500 cells/mm3 near the end of the trial. Active linkage to care was provided in the main HPTN 068 study, using a process similar to peer navigation, where study counselors offered to provide transportation to the clinic and to accompany young women to their first HIV care visit.
Laboratory methods
HIV testing was performed at enrollment (all participants) and at annual follow-up visits for participants who were HIV uninfected at enrollment. CD4 cell count testing was performed at study site for HIV-infected participants at their first HIV-positive study visit and follow-up visits.26 All other laboratory testing described in this report was performed retrospectively at the HPTN Laboratory Center (Johns Hopkins University School of Medicine, Baltimore, MD, USA). This included HIV testing to confirm results from site testing and to confirm HIV seroconversion events,27 HIV viral load testing using the RealTime HIV-1 Viral Load assay (Abbott Molecular, Des Plaines, IL; limit of quantification: 40 copies/mL), ARV drug testing, and HIV drug resistance testing.
ARV drug testing was performed using a qualitative assay based on high-performance liquid chromatography (HPLC) coupled with high-resolution accurate mass (HRAM) mass spectrometry (MS; QExactive-Orbitrap; Thermo Scientific, San Jose, CA).19,20 This assay detects 20 ARV drugs from five drug classes; this includes nine protease inhibitors (PIs) (amprenavir, atazanavir, darunavir, indinavir, lopinavir, nelfinavir [NFV], ritonavir [RTV], saquinavir, tipranavir); six nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) (abacavir [ABC], FTC, lamivudine [3TC], stavudine [d4T], TFV, zidovudine [ZDV]); three non-nucleoside reverse transcriptase inhibitors (NNRTIs) (efavirenz [EFV], nevirapine [NVP], rilpivirine [RPV]); one CCR5 receptor antagonist (maraviroc [MVC]); and one integrase strand transfer inhibitor (raltegravir [RAL]). The lower limit of detection was 2 ng/mL for six drugs (NFV, NVP, RPV, ABC, MVC, and RTV) and 20 ng/mL for the remaining 14 drugs.
HIV genotyping was performed using the ViroSeq HIV-1 Genotyping System v2.0 (Abbott Molecular, Des Plaines, IL) for samples with HIV viral loads ≥400 copies/mL. GenBank Accession numbers: KY883695-KY883762, KY888784-KY888875, KY921717-KY921757).
Statistical methods
Associations between ARV drug detection and individual characteristics and associations between HIV drug resistance and individual characteristics were examined using logistic regression (SAS, version 9.4; SAS Institute, Cary, NC).
Ethical considerations
All participants provided written informed consent for participation in the HPTN 068 study. The study was approved by Institutional Review Boards at the University of North Carolina at Chapel Hill and the University of the Witwatersrand Human Research Ethics Committee.
RESULTS
Study cohort
HPTN 068 enrolled 2,537 young women; eight were excluded from this analysis (four did not meet enrollment criteria; four had inconclusive HIV status at enrollment, Figure 1A). At enrollment, 81 (3.2%) of the remaining 2,529 were HIV-infected and 2,448 (96.8%) were HIV-uninfected. Overall, 164 women acquired HIV infection after study enrollment (Figure 1B); 107 acquired HIV infection in the main HPTN 068 study (before their expected graduation date) and 57 acquired HIV infection in the follow-up study (after their expected graduation date).27 We evaluated ARV drug use in three groups of women in this cohort: (1) those who were HIV-infected at study enrollment, (2) those who were HIV-uninfected at study enrollment, and (3) those who acquired HIV after study enrollment (seroconverters). HIV drug resistance was evaluated in women with HIV infection, at the first HIV-positive study visit.
Figure 1. Summary of antiretroviral drug testing and HIV resistance testing.
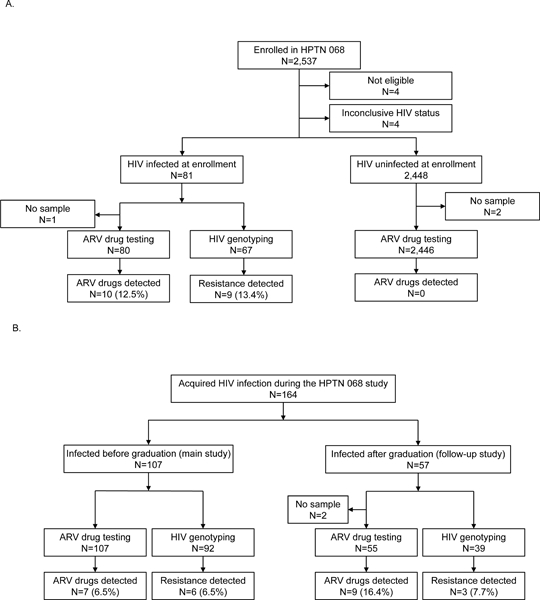
The figure shows a summary of results for antiretroviral (ARV) drug testing and HIV resistance testing. Panel A shows results from young women who enrolled in HPTN 068. Panel B shows results for participants who acquired HIV infection after study enrollment. Abbreviations: HPTN: HIV Prevention Trials Network; ARV: antiretroviral.
Detection of ARV drugs in women at study enrollment
ARV drug testing was performed using enrollment samples from 2,526 of the 2,529 women (80 from HIV-infected women; 2,446 from HIV-uninfected women, Figure 1A). ARV drugs were detected in 10 (12.5%) of 80 samples from women who were HIV-infected at enrollment; six samples had one NNRTI with one or two NRTIs, three samples had one NNRTI alone, and one sample had one NRTI alone (Table 1). ARV drugs from other ARV drug classes were not detected. The most common NNRTI detected was EFV (n=5) and the most common NRTI detected was 3TC (n=7). Five of the 10 samples with ARV drugs detected had a viral load <400 copies/mL (Table 1). No ARV drugs were detected in samples from the 2,446 HIV-uninfected women at enrollment.
Table 1.
Antiretroviral (ARV) drugs detected, HIV viral load, and HIV drug resistance in the subset of HIV-infected participants who had ARV drugs or resistance mutations detected.*
| Status | Study visit | ARV drugs detected | Viral load | Mutations detected | ||
|---|---|---|---|---|---|---|
| NNRTI | NRTI | NNRTI | NRTI | |||
| HIV infected at Enrollment (ARV drugs detected) |
Enrollment | EFV | 3TC, TFV | 68 | -- | -- |
| Enrollment | NVP | 3TC, TFV | <40 | -- | -- | |
| Enrollment | NVP | 3TC, ZDV | <40 | -- | -- | |
| Enrollment | EFV | 3TC | <40 | -- | -- | |
| Enrollment | EFV | 3TC | <40 | -- | -- | |
| Enrollment | NVP | 3TC | 9,968 | A98G, K101E, Y181C, G190A | M184V | |
| Enrollment | EFV | None | 164,089 | K103N, P225H | M184V | |
| Enrollment | EFV | None | 388,773 | K101E, K103N, P225H | M184V | |
| Enrollment | NVP | None | 7,425 | A98G, K103N, Y181C | M184V | |
| Enrollment | None | 3TC | 36,062 | V106M | A62V, M184I/V | |
| HIV infected at Enrollment (no drugs detected) |
Enrollment | None | None | 119,267 | K103N | -- |
| Enrollment | None | None | 73,392 | V106M | -- | |
| Enrollment | None | None | 1,263 | Y181C | -- | |
| Enrollment | None | None | 15,225 | G190A | -- | |
| HIV infected after enrollment (ARV drugs detected) |
Year 1 | EFV | TFV, 3TC | <40 | -- | -- |
| Year 1 | EFV | TFV, 3TC | 475 | No sample | No sample | |
| Year 1 | EFV | TFV, 3TC | 56 | -- | -- | |
| Year 1 | EFV | TFV, FTC | 4,188 | --a | --a | |
| Year 2 | EFV | None | 137 | -- | -- | |
| Year 3 | EFV | TFV, FTC | 207 | -- | -- | |
| Graduation | EFV | TFV, 3TC | <40 | -- | -- | |
| Post-Grad | EFV | TFV, FTC | 766 | K103N | --a | |
| Post-Grad | EFV | TFV, FTC | 181 | -- | -- | |
| Post-Grad | EFV | TFV, FTC | 71 | -- | -- | |
| Post-Grad | EFV | TFV, FTC | <40 | -- | -- | |
| Post-Grad | EFV | TFV, FTC | <40 | -- | -- | |
| Post-Grad | EFV | TFV, FTC | <40 | -- | -- | |
| Post-Grad | EFV | FTC | <40 | -- | -- | |
| Post-Grad | EFV | FTC | <40 | -- | -- | |
| Post-Grad | EFV | None | 85 | -- | -- | |
| HIV infected after enrollment (no drugs detected) |
Year 1 | None | None | 22,247 | K103N | -- |
| Year 2 | None | None | 3,664 | K103N | -- | |
| Year 2 | None | None | 8,860 | V179E, Y181C | D67N, K70E | |
| Year 3 | None | None | 10,159 | K103N | -- | |
| Year 3 | None | None | 94,359 | K103N | -- | |
| Graduation | None | None | 91,235 | V106M | -- | |
| Post-grad | None | None | 11,872 | K103N | -- | |
| Post-grad | None | None | 50,037 | K103N | -- | |
The table shows laboratory data from the subset of 38 HIV-infected participants who had one or more antiretroviral (ARV) drugs detected or had one or more HIV drug resistance mutations detected. This included 14 participants who were HIV-infected at study enrollment (10 with ARV drugs detected; 4 with no ARV drugs detected) and 24 participants who acquired HIV infection after enrollment (16 with ARV drugs detected; 8 with no ARV drugs detected). Samples were collected at enrollment or at the first HIV-positive visit (Study visit). Years 1, 2, and 3 indicate the visit type (years after enrollment). Graduation indicates a visit that occurred at the expected graduation date. Post-grad indicates a visit that occurred after 1–2 years after the expected graduation date. HIV genotyping was performed for the 19 samples that had HIV viral loads ≥400 copies/mL; one sample was not available for testing. Major resistance mutations (shown in bold) were detected in 18 (94.7%) of the 19 samples with genotyping results. NNRTI and NRTI resistance mutations are shown; PI resistance mutations were not detected. Accessory mutations are shown in italics. Viral load data are shown as copies/mL.
These women were at risk of having additional resistance mutations.
Abbreviations: ARV: antiretroviral; NNRTI; non-nucleoside reverse transcriptase inhibitor; NRTI: nucleoside/nucleotide reverse transcriptase inhibitor; PI: protease inhibitor; EFV: efavirenz; NVP: nevirapine; 3TC: lamivudine; TFV: tenofovir.
Detection of ARV drugs in women who acquired HIV infection after study enrollment
Overall, 164 of the women who were uninfected at enrollment acquired HIV infection during the main study or follow-up study (Figure 1B). None of the 164 women had drugs detected at enrollment (prior to HIV infection). Samples were available from the first HIV-positive study visit for 162 of the 164 women. ARV drugs were detected in 16 (9.9%) of 162 samples (7 [6.5%] of 107 women during the main study; 9 [16.4%] of 55 women infected during the follow-up study, Table 1). All 16 samples had EFV detected; 14 samples also had one or two NRTIs detected (eight had TFV+FTC; four had TFV+3TC, two had FTC alone, Table 1). Thirteen (81.3%) of the 16 samples had viral loads <400 copies/mL (Table 1).
HIV drug resistance
HIV genotyping results were obtained for 198 (93.8%) of the 211 women who had a viral load ≥400 copies/mL. This included 67 women who were HIV infected at enrollment (enrollment samples were tested, Figure 1A) and 131 women who acquired HIV infection after enrollment (the first HIV-positive sample was tested, Figure 1B). Eight (4.0%) of the 198 samples had one or more ARV drugs detected. Major resistance mutations were identified in 18 (9.1%) of the 198 samples (Table 1). This included samples from nine (13.4%) of the 67 women who were HIV-infected at enrollment and nine (6.9%) of the 131 seroconverters (Table 1). At least one NNRTI resistance mutation was detected in all 18 cases. The most common NNRTI resistance mutations detected were K103N (n=11), V106M (n=3), and Y181C (n=4). These mutations confer resistance to NVP and EFV, Y181C also confers resistance to RPV. Major PI resistance mutations were not detected in any of the samples.
Five of the 18 women with HIV drug resistance had multi-class resistance (NNRTI + NRTI resistance); all five were HIV infected at study enrollment. The following combinations of mutations were detected: K103N+M184V (n=2), K103N+Y181C+M184V, V106M+M184I/V, and Y181C+G190A+M184V. The mutation, M184V, which was detected in all five cases, confers resistance to 3TC, FTC and other NRTIs. ARV drugs were detected in all five cases. The viral loads in these cases ranged from 7,425 to 388,773 copies/mL).
Nine of the 18 women with HIV drug resistance acquired HIV infection during the HPTN 068 study; all nine of these women had resistance to NNRTIs (seven had K103N, one had V106M, one had Y181C). ARV drugs were only detected in one of the nine samples; the remaining eight seroconverters who did not have drugs detected may have been infected with resistant HIV strains. ARV drug testing also revealed that some of the newly-infected women were at risk of acquiring additional drug resistance. One woman who had a viral load of 4,188 copies/mL and did not have any resistance mutations was taking EFV, tenofovir disoproxil fumarate (TDF), and 3TC; a second woman who had a viral load of 766 copies/mL and did not have NRTI resistance mutations was taking NRTIs (TDF and FTC, along with EFV).
Factors associated with detection of ARV drugs and HIV drug resistance
We examined the association of demographic, laboratory, and clinical factors with detection of ARV drugs and HIV drug resistance. Not surprisingly, a significantly higher prevalence of ARV drugs was observed among women who had a lower viral load at their first HIV-positive visit (OR: 0.21, 95% CI: 0.11, 0.4; p<0.0001). ARV drugs were also detected more frequently in women who had two deceased parents (double orphans) compared to those had two living parents (5/18=27.8% vs. 16/153=10.5%, p=0.04). There was no significant association of ARV drug detection with age, CD4 cell count, infection group, pregnancy history, food insecurity, school attendance, depression, or alcohol use. The only factor associated with HIV drug resistance was having one parent deceased, compared to having two living parents (p=0.04). The percentage of women who had viral loads <400 copies/mL was similar for those with two living parents vs. one or no living parents (1.7 [0.5, 6.3]; p=0.45).
DISCUSSION
In this study, we characterized ARV drug use and HIV drug resistance in a large cohort of young women in rural South Africa. Previous reports have described significant differences between urban and rural areas of South Africa in ART coverage, HIV prevalence among women, and the rate of acquired drug resistance among adults failing first-line ART.33–35 This report adds to previous studies by examining these issues in school-aged girls in a rural area, by evaluating ARV drug use as well as drug resistance, and by analyzing both acquired and transmitted drug resistance.
The HPTN 068 trial enrolled young women ages 13–20 in 2011 and 2012. Previous studies have estimated the level of ART coverage in South Africa to be 66–81% (Joint United Nations Programme survey; 2011, 2013),36,37 57.8% (women aged 15–59, population survey in KwaZulu-Natal that included ARV drug testing; 2013 )38 and 31.7% (ages 15–32, population survey in Kwazulu-Natal; 2009 to 2011).24 In HPTN 068, ARV drugs were only detected in 12.5% of the participants who were infected at enrollment and 9.9% of the seroconverters. The lower frequency of ARV drug use in the HPTN 068 cohort may reflect lack of knowledge of HIV status, lower adherence to ART among adolescents and young adults, less access to ART in the study communities, or other factors.27
At the time HPTN 068 was conducted, the recommended first-line ART regimen included two NRTIs (TDF + 3TC or FTC) and a NNRTI (EFV or NVP).39 In HPTN 068, a NNRTI (EFV or NVP) alone or in combination with one or two NRTIs (TFV, 3TC, d4T, ZDV) was detected in nine of 10 participants who had ARV drugs detected at enrollment, and in all seroconverters who had ARV drugs detected at their first HIV-positive study visit. Because NRTIs have shorter half-lives compared to NNRTIs, detection of NRTIs may depend on the time between drug dosing and sample collection; we considered detection of an NNRTI alone to be consistent with ART. In the two cases where EFV was detected alone, it is also possible that the drug was being used for recreational purposes.13,14 The detection of 3TC alone in one woman suggests that she was not adherent to an ART regimen or that she was using the drug for another reason (e.g., treatment of hepatitis B virus infection).
We also assessed ARV use among >2,400 young women in HPTN 068 who were HIV uninfected. In a previous report, we found unusual patterns of ARV drug use among HIV-uninfected women in the United States (EFV and/or a PI).23 ARV drugs were not detected in any of the HIV-uninfected young women in the HPTN 068 cohort; of note, the study was performed prior to the approval of PrEP in South Africa.8
In HPTN 068, HIV drug resistance was detected in samples from 13.4% of the participants infected at enrollment and 6.9% of the seroconverters. A subset of the participants with drug resistance did not have ARV drugs detected (6.0% infected at enrollment; 6.1% of the seroconverters). Some of these participants may have taken ARV drugs in the past, or between the annual study visits. Therefore, these numbers represent a maximal estimate of transmitted drug resistance. The estimated level of transmitted drug resistance among seroconverters is categorized as moderate based on WHO guidelines.40 In a national survey conducted in South Africa, the prevalence of pre-treatment drug resistance was 9% among adults over 18 years of age (7.4% in Mpumalanga Province).41 In a study conducted in KwaZulu-Natal where the majority of participants were women, the prevalence of pre-treatment resistance was 8.7%.42 In HPTN 068, five young women had multi-class drug resistance at study enrollment. ARV drugs were detected in samples from all five young women, suggesting that the multi-class resistance was acquired due to ARV drug exposure.
In the HPTN 068 cohort, 30% of the young women who were taking ARV drugs were not virally suppressed. In a separate study, we estimated that 12.6% of the young women in HPTN 068 were viremic controllers; those young women maintained viral loads <2,000 copies/mL for at least 12 months in the absence of ARV drug use. In this study, 13 (41.9%) of the 31 young women who had viral loads <400 copies/mL at their first HIV-positive visit did not have ARV drugs detected; this indicates that low viral load is a poor surrogate marker for ARV drug use in this cohort.43
In the HPTN 068 study, double orphans were at significantly higher risk of HIV infection than those with two living parents.26 In this study, we also found that double orphans were more likely to be taking ARV drugs than women with two living parents; the reason for this difference is not clear. We also found that single orphans were more likely to have HIV drug resistance than women with two living parents; a similar trend was seen for double orphans, but this difference was not statistically significant. Previous studies have found that better relationships between caregivers and orphaned children improved the adherence to ART.44,45
The findings from this study indicate a need for improvements in HIV care. In this cohort of young women in rural South Africa, ARV drug use was relatively infrequent among young women with both prevalent and new HIV infection. Because HPTN 068 did not collect information about the availability and uptake of HIV testing in these communities, it was not possible to determine whether the low rate of ARV drug use in this cohort reflected lack of knowledge of HIV status, limited access to ART, lack of interest in HIV treatment, or other factors. Among the young women who were using ARV drugs, many were not virally suppressed and many had HIV drug resistance. This indicates a need for expanded programs for HIV/AIDS education and counseling to improve ART adherence. The relatively high frequency of drug resistance among young women with new HIV infection who were not using ARV drugs at their first HIV-positive study visit also suggests a need for broader HIV/AIDS education and ART counseling in the study communities, to reduce the rate of transmitted drug resistance.
Table 2.
Association of demographic and other factors with antiretroviral drug use and HIV drug resistance among HIV-infected women.*
| Characteristics | Detection of ARV drugs | HIV drug resistance | ||||||
|---|---|---|---|---|---|---|---|---|
| Yes (N=26) | No (N=216) | OR (95% CI) | p-value | Yes (N=18) | No (N=180) | OR (95% CI) | p-value | |
| Age (year)a,b | 18.5 (16, 21) |
19 (17,20) |
0.99 (0.79, 1.24) |
0.93 | 17.5 (16, 19) |
19 (17, 20) |
0.79 (0.61, 1.01) |
0.06 |
| Viral load (log10)a,b | 1.8 (1.6, 2.9) |
4.2 (3.7, 4.7) |
0.21 (0.11, 0.4) |
<0.0001 | 4.3 (4.0, 4.9) |
4.3 (3.9, 4.8) |
1.09 (0.54, 2.21) |
0.80 |
| CD4 cell count (cell/mm3)a,b | 484.5 (353, 754) |
559 (400, 573) |
0.98 (0.82, 1.18) |
0.86 | 414.5 (313.5, 665.5) |
534 (383.5, 731) |
0.88 (0.7, 1.11) |
0.29 |
| First HIV-positive visit | ||||||||
| Study enrollment | 10 (12.5%) | 70 (87.5%) | ref | 9 (13.4%) | 58 (86.6%) | ref | ||
| Main study | 7 (6.5%) | 100 (93.5%) | 0.49 (0.18, 1.35) |
0.17 | 6 (6.5%) | 86 (93.5%) | 0.45 (0.15, 1.33) |
0.15 |
| Follow-up study | 9 (16.4%) | 46 (83.6%) | 1.37 (0.52, 3.63) |
0.53 | 3 (7.7%) | 36 (92.3%) | 0.54 (0.14, 2.12) |
0.37 |
| Ever pregnantc | ||||||||
| Yes | 6 (12.8%) | 41 (87.2%) | 1.27 (0.48, 3.35) |
0.64 | 2 (5.1%) | 37 (94.9%) | 0.48 (0.1, 2.16) |
0.34 |
| No | 20 (10.4%) | 173 (89.6%) | ref | 16 (10.2%) | 141 (89.8%) | ref | ||
| Missing | - | 2 (100.0%) | - | 2 (100.0%) | ||||
| Orphanc | ||||||||
| Both parents alive | 16 (10.5%) | 137 (89.5%) | ref | 7 (5.8%) | 113 (94.2%) | ref | ||
| Single orphan | 2 (3.6%) | 54 (96.4%) | 0.32 (0.07, 1.43) |
0.13 | 8 (15.7%) | 43 (84.3%) | 3.00 (1.03, 8.79) |
0.04 |
| Double orphan | 5 (27.8%) | 13 (72.2%) | 3.29 (1.04, 10.44) |
0.04 | 3 (18.8%) | 13 (81.2%) | 3.73 (0.86, 16.19) |
0.08 |
| Missing data | 3 (20.0%) | 12 (80.0%) | - | 11 (100.0%) | ||||
| Food insecurityc | ||||||||
| Yes | 13 (12.6%) | 90 (87.4%) | 1.37 (0.6, 3.09) |
0.45 | 8 (9.6%) | 75 (90.4%) | 1.23 (0.45, 3.34) |
0.68 |
| No | 13 (9.6%) | 123 (90.4%) | ref | 9 (8.0%) | 104 (92.0%) | ref | ||
| Missing data | - | 3 (100.0%) | 1 (50.0%) | 1 (50.0%) | ||||
| School days missed per monthc | ||||||||
| 0–2 days | 20 (11.5%) | 154 (88.5%) | ref | 13 (9.4%) | 125 (90.6%) | ref | ||
| ≥3 days | 6 (10.2%) | 53 (89.8%) | 0.87 (0.33, 2.29) |
0.78 | 5 (9.8%) | 46 (90.2%) | 1.05 (0.35, 3.09) |
0.94 |
| Missing data | - | 9 (100.0%) | - | 9 (100.0%) | ||||
| Depressionc,d | ||||||||
| Yes | 9 (13.2%) | 59 (86.8%) | 1.30 (0.55, 3.08) |
0.55 | 5 (9.1%) | 50 (90.9%) | 1.10 (0.36, 3.33) |
0.87 |
| No | 17 (10.5%) | 145 (89.5%) | ref | 11 (8.3%) | 121 (91.7%) | ref | ||
| Missing data | - | 12 (100.0%) | 2 (18.2%) | 9 (81.8 %) | ||||
| Alcohol usee | ||||||||
| Never or less than once a month | 23 (10.4%) | 198 (89.6%) | ref | 17 (9.4%) | 163 (90.6%) | ref | ||
| Once a month or more | 3 (14.3%) | 18 (85.7%) | 1.43 (0.39, 5.25) |
0.59 | 1 (5.6%) | 17 (94.4%) | 0.56 (0.07, 4.5) |
0.59 |
The table shows the association of demographic and other factors associated with antiretroviral (ARV) drug detection and HIV drug resistance. P<0.05 is bolded.
Median (interquartile range [IQR]).
These variables were assessed at the first HIV-positive visit.
These variables were assessed at the enrollment visit.
Depression was measured using the Short Form Children’s Depression Index (CDI, 10 items). A cut-off of 7 was used, where an index ≥7 or above is depressed, and an index <7 is not depressed.
This variable was assessed during follow-up (from enrollment through the expected graduation date).
Abbreviations: ARV: antiretroviral; N: number; OR: odds ratio;CI: confidence interval, ref: reference.
ACKNOWLEDGEMENTS
The authors thank the HPTN 068 study team and study participants for their contributions to the HPTN 068 study. The authors also thank the laboratory investigators and study staff for their assistance with sample management and laboratory testing.
Source of funding:
This study was supported by the National Institute of Allergy and Infectious Diseases (NIAID) and the National Institute of Mental Health (NIMH) of the National Institutes of Health (NIH; UM1-AI068613; UM1-AI068617; UM1-AI068619; NIMH R01-MH087118; and the Carolina Population Center [NIH Center grant P2C-HD050924]).
Footnotes
Conflicts of Interest:
None of the authors has a conflict of interest or potential conflict of interest, with the following exceptions: Susan Eshleman has collaborated on research studies with investigators from Abbott Laboratories; Abbott Laboratories has provided reagents for collaborative research studies.
Contributor Information
Yinfeng Zhang, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Mariya V. Sivay, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Sarah E. Hudelson, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
William Clarke, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Autumn Breaud, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Jing Wang, Statistical Center for HIV/AIDS Research & Prevention (SCHARP), Seattle, WA, USA.
Estelle Piwowar-Manning, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Yaw Agyei, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Jessica M. Fogel, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Erica L. Hamilton, Science Facilitation Department, FHI 360, Durham, NC, USA.
Amanda Selin, Carolina Population Center, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Catherine MacPhail, School of Health and Society, University of Wollongong, Australia; MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Kathleen Kahn, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
F. Xavier Gomez-Olive, MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
James P. Hughes, Department of Biostatistics, University of Washington, Seattle, WA, USA.
Audrey Pettifor, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, NC; MRC/Wits Rural Public Health and Health Transitions Research Unit (Agincourt), School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa.
Susan H. Eshleman, Department of Pathology, Johns Hopkins University School of Medicine, Baltimore, MD.
REFERENCES
- 1.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Available at: http://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed July, 2017.
- 2.Eholie SP, Badje A, Kouame GM, et al. Antiretroviral treatment regardless of CD4 count: the universal answer to a contextual question. AIDS Res Ther 2016;13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Insight Start Study Group, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28(8):1193–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safren SA, Mayer KH, Ou SS, et al. Adherence to early antiretroviral therapy: results from HPTN 052, a phase III, multinational randomized trial of ART to prevent HIV-1 sexual transmission in serodiscordant couples. J Acquir Immune Defic Syndr 2015;69(2):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bispo S, Chikhungu L, Rollins N, Siegfried N, Newell ML. Postnatal HIV transmission in breastfed infants of HIV-infected women on ART: a systematic review and meta-analysis. J Int AIDS Soc 2017;20(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO expands recommendation on oral pre-exposure prophylaxis of HIV infection (PrEP). Available at: http://www.who.int/hiv/pub/prep/policy-brief-prep-2015/en/. Accessed November, 2017.
- 9.Department of Health, South Africa. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Available at: https://aidsfree.usaid.gov/sites/default/files/tx_south-africa_pmtct_2015.pdf. Accessed December, 2017.
- 10.Kalapila AG, Marrazzo J. Antiretroviral therapy for prevention of Human Immunodeficiency Virus infection. Med Clin North Am. 2016;100(4):927–950. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Guidelines on post-exposure prophylaxis for HIV and the use of co-trimoxazole prophylaxis for HIV-related infections among adults, adolescents and children. Available at: http://www.who.int/hiv/pub/guidelines/arv2013/arvs2013upplement_dec2014/en/. Accessed November, 2017.
- 12.U.S. Food and Drug Administration. Hepatitis B and C Treatments. Available at: http://www.fda.gov/ForPatients/Illness/HepatitisBC/ucm408658.htm. Accessed January, 2017.
- 13.Gatch MB, Kozlenkov A, Huang RQ, et al. The HIV antiretroviral drug efavirenz has LSD-like properties. Neuropsychopharmacol 2013;38(12):2373–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rough K, Dietrich J, Essien T, et al. Whoonga and the abuse and diversion of antiretrovirals in Soweto, South Africa. AIDS Behav 2014;18(7):1378–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blum MR, Chittick GE, Begley JA, Zong J. Steady-state pharmacokinetics of emtricitabine and tenofovir disoproxil fumarate administered alone and in combination in healthy volunteers. J Clin Pharmacol 2007;47(6):751–759. [DOI] [PubMed] [Google Scholar]
- 16.Donnell D, Baeten JM, Bumpus NN, et al. HIV protective efficacy and correlates of tenofovir blood concentrations in a clinical trial of PrEP for HIV prevention. J Acquir Immune Defic Syndr 2014;66(3):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendrix CW, Andrade A, Bumpus NN, et al. Dose frequency ranging pharmacokinetic study of tenofovir-emtricitabine after directly observed dosing in healthy volunteers to establish adherence benchmarks (HPTN 066). AIDS Res Hum Retroviruses. 2016;32(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hendrix CW, Chen BA, Guddera V, et al. MTN-001: randomized pharmacokinetic cross-over study comparing tenofovir vaginal gel and oral tablets in vaginal tissue and other compartments. PLoS One. 2013;8(1):e55013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Clarke W, Marzinke MA, et al. Evaluation of a multidrug assay for monitoring adherence to a regimen for HIV preexposure prophylaxis in a cinical study, HIV Prevention Trials Network 073. Antimicrob Agents Chemother. 2017;61(7): e02743–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marzinke MA, Breaud A, Parsons TL, et al. The development and validation of a method using high-resolution mass spectrometry (HRMS) for the qualitative detection of antiretroviral agents in human blood. Clin Chim Acta. 2014;433:157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marzinke MA, Clarke W, Wang L, et al. Nondisclosure of HIV status in a clinical trial setting: antiretroviral drug screening can help distinguish between newly diagnosed and previously diagnosed HIV infection. Clin Infect Dis 2014;58(1):117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen I, Connor MB, Clarke W, et al. Antiretroviral drug use and HIV drug resistance among HIV-infected black men who have sex with men: HIV Prevention Trials Network 061. J Acquir Immune Defic Syndr 2015;69(4):446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen I, Clarke W, Ou SS, et al. Antiretroviral drug use in a cohort of HIV-uninfected women in the United States: HIV Prevention Trials Network 064. PLoS One. 2015;10(10):e0140074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fogel JM, Clarke W, Kulich M, et al. Antiretroviral drug use in a cross-sectional population survey in Africa: NIMH project accept (HPTN 043). J Acquir Immune Defic Syndr 2017;74(2):158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharsany AB, Karim QA. HIV infection and AIDS in sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J 2016;10:34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pettifor A, MacPhail C, Selin A, et al. HPTN 068: a randomized control trial of a conditional cash transfer to reduce HIV infection in young women in South Africa-study design and baseline results. AIDS Behav 2016;20(9):1863–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettifor A, MacPhail C, Hughes JP, et al. The effect of a conditional cash transfer on HIV incidence in young women in rural South Africa (HPTN 068): a phase 3, randomised controlled trial. Lancet Glob Health. 2016;4(12):e978–e988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaede B, Versteeg M. The state of the right to health in rural South Africa. SAHR; Available at: http://www.rhap.org.za/wp-content/uploads/2014/02/Chap-9-State-of-right-Rural-Health-pgs-99-106.pdf. Accessed May, 2018. [Google Scholar]
- 29.Peltzer K, Matseke G, Mzolo T, Majaja M. Determinants of knowledge of HIV status in South Africa: results from a population-based HIV survey. BMC Public Health. 2009;9:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makusha T, Mabaso M, Richter L, Desmond C, Jooste S, Simbayi L. Trends in HIV testing and associated factors among men in South Africa: evidence from 2005, 2008 and 2012 national population-based household surveys. Public Health. 2017;143:1–7. [DOI] [PubMed] [Google Scholar]
- 31.Peltzer K, Friend-du Preez N, Ramlagan S, Anderson J. Antiretroviral treatment adherence among HIV patients in KwaZulu-Natal, South Africa. BMC Public Health. 2010;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Department of Health, South Africa, and South African National AIDS Council. South African HIV and TB Investment Case, Summary Report Phase 1. Available at: http://sanac.org.za/wp-content/uploads/2016/03/1603-Investment-Case-Report-LowRes-18-Mar.pdf. Accessed May, 2018.
- 33.Tromp N, Michels C, Mikkelsen E, Hontelez J, Baltussen R. Equity in utilization of antiretroviral therapy for HIV-infected people in South Africa: a systematic review. Int J Equity Health. 2014;13:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karim QA, Kharsany AB, Frohlich JA, et al. Stabilizing HIV prevalence masks high HIV incidence rates amongst rural and urban women in KwaZulu-Natal, South Africa. Int J Epidemiol. 2011;40(4):922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossouw TM, Nieuwoudt M, Manasa J, et al. HIV drug resistance levels in adults failing first-line antiretroviral therapy in an urban and a rural setting in South Africa. HIV Med 2017;18(2):104–114. [DOI] [PubMed] [Google Scholar]
- 36.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global report: UNAIDS report on the global AIDS epidemic 2012. Available at: http://files.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_UNAIDS_Global_Report_2012_with_annexes_en.pdf. Accessed June, 2017.
- 37.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global report: UNAIDS report on the global AIDS epidemic 2013. Available at: http://files.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf. Accessed September, 2017.
- 38.Huerga H, Shiferie F, Grebe E, et al. A comparison of self-report and antiretroviral detection to inform estimates of antiretroviral therapy coverage, viral load suppression and HIV incidence in Kwazulu-Natal, South Africa. BMC Infect Dis. 2017;17(1):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.South African National Department of Health. Clinical guidelines for the managements of HIV & AIDS in adults and adolescents. Available at: http://www.who.int/hiv/pub/guidelines/south_africa_art.pdf. Accessed May, 2017.
- 40.Bennett DE, Myatt M, Bertagnolio S, Sutherland D, Gilks CF. Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antivir Ther. 2008;13 Suppl 2:25–36. [PubMed] [Google Scholar]
- 41.Steegen K, Carmona S, Bronze M, et al. Moderate levels of pre-treatment HIV-1 antiretroviral drug resistance detected in the first South African national survey. PLoS One. 2016;11(12):e0166305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anne D, Collins CI, Siva D, Anne-Geneviève M, Vincent C, Tulio de O, François D, Deenan P. Prevalence and impact of pretreatment drug resistance in the ANRS 12249 TasP trial. 2017 Conf. on Retroviruses and Opportunistic Infections Seattle, Washington: February 2017. [Google Scholar]
- 43.Sivay MV, Zhang Y, Wang J, Fogel JM, Piwowar-Manning E, Clarke W, Hamilton EL, Kahn K, Selin A, Gomez-Olive FX, MacPhail C, Hughes J, Pettifor A, Eshleman SH. Natural control of HIV infection in a cohort of young women in South Africa (HPTN 068). 2018 Conf. on Retroviruses and Opportunistic Infections. Boston, MA: February 2018. [Google Scholar]
- 44.Gichane MW, Sullivan KA, Shayo AM, et al. Caregiver role in HIV medication adherence among HIV-infected orphans in Tanzania. AIDS Care. 2017:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kikuchi K, Poudel KC, Muganda J, et al. High risk of ART non-adherence and delay of ART initiation among HIV positive double orphans in Kigali, Rwanda. PLoS One. 2012;7(7):e41998. [DOI] [PMC free article] [PubMed] [Google Scholar]


